Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Karen Khachatryan and Version 2 by Jason Zhu.
An up-to-date overview of the current state of the art of polysaccharide-based spherical particles as carriers of active/bioactive substances, with a particular emphasis on their applications in the food industry, is provided. Owing to the rapid advances in nanotechnology, much effort has been dedicated to the synthesis and potential uses of these particles.
- nanocapsules
- micelles
- liposomes
- nanotechnology
- functional food
- food fortification
- natural preservatives
1. Introduction
The prevalent spherical nanostructures used as carriers for bioactive compounds are nanocapsules, micelles, inverted micelles, and liposomes (Figure 1).
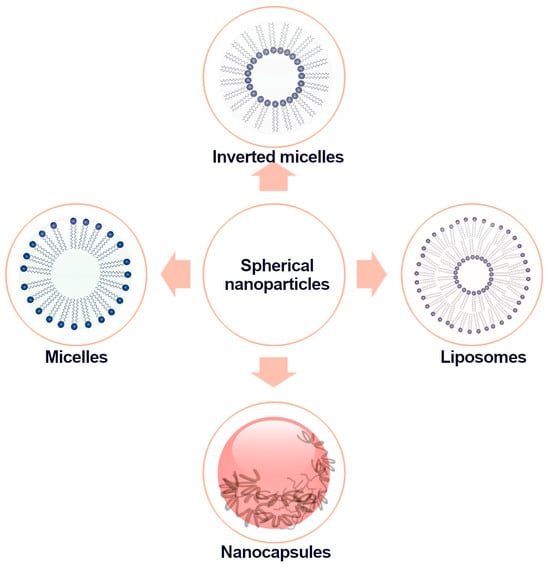
Figure 1.
Schematic diagram of the structure of spherical nanoparticles.
Polysaccharides are a promising biomaterial for developing nutrient delivery systems due to their high stability, hydrophilicity, biodegradability, non-toxicity, and biocompatibility, coupled with their versatile chemical functions. Particle size, shape, and potential have a crucial impact on nutrient uptake by spherical nanoparticles, which are obtained primarily through hydrophobic interactions, hydrogen bonds, and electrostatic interactions [1][47]. The shape and size of a nanoparticle have an impact on the biological processes of living organisms. The size determines the potential for intracellular transport such as phagocytosis, endocytosis, and transportation through blood vessels, which, in turn, influences permeation through cell membranes, tissues, and organs. According to scientific reports, it is advised that the nanoparticle size should not be too small. It is established that renal excretion ranges from 6 to 8 nm. Additionally, particles smaller than 6 nm may be coated with serum proteins in the bloodstream, resulting in a larger hydrodynamic diameter, which can hinder renal excretion. Nanoparticles larger than 100 nm are likewise captured by macrophages in the lung, spleen, or liver [2][48]. Moreover, nanoparticles’ high surface area-to-volume ratio has an impact on the quick release potential of the core substance or drug.
Recent scientific research indicates that spherical polysaccharide nanoparticles possess the potential to be utilised as functional biomaterials in the food industry, particularly for the encapsulation of hydrophobic natural phytochemicals (such as zein-Mesona quinensis) [1][3][47,49]. The capsule core wall includes polysaccharides that act as a surface decorator. This can potentially improve cellular uptake and increase the anticancer effectiveness of nanomaterials [4][50]. The polysaccharide used affects the potential of the particles, which further influences their applicability and usage. For instance, chitosan, currently the most widely used polysaccharide, is capable of electrostatic interaction with negatively charged mucosal surfaces, resulting in favourable mucoadhesive properties. Furthermore, the presence of a positively charged NH3+ group in chitosan allows for interaction with negatively charged proteoglycans on the cell surface, leading to improved absorption of the nanoparticle core in the gut by promoting the opening up of connections between epithelial cells [5][6][51,52]. Depending on the utilisation and characteristics of the polysaccharide, it can be determined how the active ingredient of the nanocapsule’s core will impact the consumer within the product. For example, dextran possesses multiple hydroxylated groups, making it easily conjugated to proteins [5][51].
2. Nanocapsules
2.1. Design, Shape, and Size
The process of encapsulation entails enclosing or coating the active substance (core) with a carrier material (such as wall material, coating, external phase, or membrane) in order to generate capsules or particles at either a macrometric or nanometric scale. The encapsulation process produces macrocapsules (measuring more than 1000 μm), micro-capsules (measuring 1–1000 μm), and nanocapsules (measuring less than 1 μm), which have a profound impact on enhancing product effectiveness [7][53]. Nanoencapsulation has become increasingly popular in recent times as a result of nanocapsules’ distinct characteristics, including enhanced bioavailability, high encapsulation efficiency, and loading capacity (due to their larger surface area), as well as their sustained release profile and ability to mask undesirable flavours. Additionally, nanocapsules offer improved stability, reduced particle size, and a narrower particle distribution [8][54]. The shape and size of the capsules are determined by the methodology and the polysaccharide properties. For instance, the nanocapsules resulting from spray drying exhibit diverse diameters and spherical forms. Moreover, they possess a concave structure due to speedy moisture loss following cooling [9][55].
Nanocapsule core–shell structures can be categorized into inorganic/inorganic, organic/organic, inorganic/organic, and organic/inorganic based on the type of material and coating. The properties of core–shell microparticles, including morphology, size, and structure, greatly influence their application in the food industry [10][56].
2.2. Methods of Obtaining, Factors Affecting Stability, and Properties
Two methods are employed in the creation of encapsulated systems: top-down and bottom-up. The top-down technique involves reducing the size of large structures by shaping them with external mechanical forces. Techniques used under this approach include the emulsification technique and solvent evaporation. The bottom-up approach involves particle association to develop large particles. Techniques such as spray drying, blanketing, and electrospinning have been used based on this approach [7][53].
Various techniques can be employed depending on factors such as the core composition, thermolability, coating type, and size parameters required for capsule application. These techniques include pulverisation, coacervation, lyophilisation, molecular inclusion, and the extrusion method, which involves passing bioactive compound-containing polysaccharide solutions through a syringe into a gelling solution. The molecular inclusion technique entails trapping apolar molecules within an apolar cavity through non-covalent bonds, whereas the coacervation process includes the separation of a substance into two phases by adding a third component. Polysaccharide materials can be applied through fluid bed coating, which involves suspending particles in air. Other techniques including vacuum drying, electrospinning, and emulsification are also available. Emulsification requires an emulsifier to stabilise the system [9][11][12][55,57,58]. The Vallejo-Castillo et al. [13][59] study effectively utilised the extrusion technique, employing both in situ and two-step encapsulation, to produce capsules composed of alginate and pectin (55:45 ratio) containing a core of papaya fruit extract and gallic acid (a model polyphenol). Barbosa et al. [14][60] adopted the coacervation technique in their study, using carboxymethylcellulose and lactoferrin as the wall material to encapsulate β-carotene in sachia inchi oil. The formation of complexes between the biopolymers occurred in two stages. In the first stage, electrostatic interactions dominated, and in the second stage, hydrophobic and hydrogen interactions took over.
Currently, the electrospinning technique is a frequently employed method due to its high encapsulation process efficiency, as well as its capacity to maintain compound stability during storage, based on Lamarra et al.’s techniques [15][61]. In addition, the electrospinning technique does not entail high-temperature requirements, which is crucial when working with bioactive compounds that are typically thermolabile. The electrospinning process for encapsulation is primarily influenced by the extent of chain entanglement, the chemical composition of the polysaccharide utilised, its molecular weight, and the appropriate concentration required to enable adequate molecular proximity, which allows a chain to bind to the next one [16][62]. Empirical research has indicated that polysaccharides like starch, recognized by their unique attributes, play a significant role in this process. Polysaccharides, such as alginate, methyl cellulose, pullulan, or dextran, exhibit low shear thinning and are, therefore, not as efficient in encapsulation as those with an anionic group in their structure. This is due to their insufficient entanglement of polymer chains and limited viscosity. As a result, when applying high voltages during electrospinning, such solutions are not able to form continuous streams for fibre formation, as evidenced by Balik et al. [17][63] and Ma et al. [18][64]. The benefits of electrospinning and electrospraying encompass several crucial features, including low energy costs, control over encapsulation morphology and physical properties, high encapsulation efficiency (increased surface area-to-area ratio), efficacy for components that are poorly soluble in water, encapsulation of hydrophilic and lipophilic bioactive compounds, compatibility with animal and plant polymers, ability to use both water and organic solvents, preservation of the functional and structural stability of encapsulated biomolecules, uniform size distribution, and reduction in aggregation [19][65].
In terms of the encapsulation process, chemical (polymerisation, interaction, in-situ emulsion) and physical (physical-chemical, e.g., layer-by-layer adsorption, sol–gel encapsulation; physical-mechanical, e.g., spray-drying, electro-spraying–coaxial electrohydrodynamic atomisation (CEHDA)) methods can be distinguished. The layer-by-layer technique typically leads to the formation of core–shell capsules and operates on the principle of bottom-up assembly. This technique provides control over the physical properties of the coating and enables the customisation of the size and properties of the resulting capsules [10][56].
In their research, Tan et al. [20][66] implemented a layer-by-layer approach to enclose anthocyanins in yeast capsules, which were progressively layered with oppositely charged polysaccharides. Chitosan, a positively charged polysaccharide, was complexed with chondroitin sulphate, a negatively charged polysaccharide. This complex was used to coat the yeast surface, which subsequently promoted the formation of anthocyanin-containing capsules.
Obtaining core–shell structures via convective techniques presents various challenges, such as limited control of morphology, polydispersity, and low reproducibility. State-of-the-art techniques, like electrospraying, electrospinning, or microfluidics, offer a promising solution to these difficulties. These techniques provide more precise microscale flow control, resulting in uniform capsules that are biologically and chemically compatible. These methods are applicable to an extensive variety of coating or core materials [10][56]. The process of encapsulation typically entails active components that are sensitive to various external factors (such as light, temperature, oxygen, pH, and enzymes), as noted by Hu et al. [21][67] and Maleki et al. [22][68]. Among the most frequently encapsulated active compounds are bioactive compounds from plants (polyphenols, anthocyanins, carotenoids), which are recognised for their therapeutic and/or preventative effects on cardiovascular diseases, metabolic diseases, degenerative diseases, urinary tract infections, gastric ulcers, and some types of cancer [7][23][53,69]. Consequently, to improve the quality of bioactive compounds whilst enhancing their quantity, it is necessary to take proper measures.
3. Micelles
3.1. Design, Shape, and Size
Micelles, which are formed through the self-organisation of amphiphilic polymers in aqueous solutions, are nanosized aggregates. Due to their unique properties, they are a suitable matrix for encapsulation, particularly in the context of polysaccharide micelles. Polysaccharides exhibit hydrophilic characteristics, and their hydroxyl, carboxyl, and amino groups can react with hydrophobic compounds to generate amphiphilic polysaccharides [24][70].
Micelles possess a hydrophobic core and a hydrophilic shell, making them an exceptional carrier for hydrophobic functional food ingredients. The solubility of hydrophobic ingredients increases due to the hydrophilic coating of micelles, while hydrophobic ingredients can be adsorbed or bound by the hydrophobic core of micelles. The hydrophilic coating encapsulates and stabilises the hydrophobic core of the chain (in aqueous solutions). Therefore, amphiphilic di- and tri-block copolymers exceeding 100 nm in size may still be classified as micelles according to Malekhosseini et al. [25][71] and Lu et al. [26][72].
To form a micelle through self-organisation, hydrophobic molecules attached to the polysaccharide backbone are commonly utilised. Polysaccharides, namely chitosan, dextrin, starch, and maltodextrin [27][73], are often used in food products. Chitosan-based micelles can be created by adjusting chitosan with hydrophobic groups such as polylactic acid, polycaprolactone, palmitic anhydride, and stearic acid. The chitosan-based polymer that has been modified can naturally produce spherical micelles in aqueous solutions with a size ranging from 20 to 500 nm [27][28][29][73,74,75]. To form dextrin-based micelles, the amphiphilic properties of dextrin are modified via techniques such as the Maillard reaction [30][31][76,77] and esterification [32][33][78,79]. Amphiphilic polymers are produced by hydrophobic modification of dextrans to create functional delivery systems for bioactive ingredients. The introduction of maltodextrin and spray drying, when compared to micelles acquired through acid, freeze-drying, or heat treatment, achieved a significantly higher level of stability in curcumin micelles [27][73]. Starch-based micelles form because of alterations that entail the binding of hydrophobic groups. Such changes attribute amphiphilic properties to the starch. Modification by OSA (octenyl succinic anhydride) esterification is currently the most commonly used method for the hydrophobic modification of starch [8][27][54,73]; in addition, acetic acid anhydride [34][80] and, in order to synthesise an amphiphilic conjugate (curcumin–hydrophobic component, hydroxyethyl starch–hydrophilic component), an acid-labile ester linker were used [35][81].
To effectively use micelles in a biological setting, it is essential to consider factors such as particle size and shape, critical micelle concentration (CMC), surface potential, and loading efficiency to predict micelle behaviour in a specific environment. The size and percentage of micelle aggregation is dependent on the volume fraction of the hydrophobic block, the degree to which the CMC is exceeded, the mixing speed during micelle synthesis, and the substrate concentration [24][70]. The dimensions and morphology of micelles can be investigated through DLS (dynamic light scattering), TEM (transmission electron microscopy), and AFM (atomic force microscopy). The self-assembly of amphiphilic molecules leads to an array of nanostructured aggregates exhibiting distinct geometric configurations. While surfactants are inclined to form spherical micelles, they can also produce ellipsoidal and cylindrical micelles. Moreover, micelles can adopt vesicular configurations [36][83]. Polysaccharide chains can adopt rod, helical, or helical conformations, which have a significant impact on the movement of hydrophobic molecules within the chains and the process of self-organisation [24][70]. The definition of surfactants is closely linked to the micelle’s shape, but measuring the degree of packing proves arduous due to the absence of a rigid boundary. The final shape of the self-aggregates is determined by the microphase separation of the amphiphilic block copolymers, as well as the critical packing parameter (CPP). CPP is computed based on factors such as chain volume, length, and cross-sectional area per molecule at the aggregate interface. Spherical micelles are formed when CPP is less than or equal to 1/3, cylindrical micelles are formed when 1/3 < CPP ≤ 1/2, vesicular micelles are formed when 1/2 < CPP < 1, bilayers (lamellae) are formed when CPP equals 1, and inverted micelles are formed when CPP is greater than 1 [24][36][37][70,83,84]. Nevertheless, pure contact potentials do not always determine the interactions that drive aggregation, particularly with ionic amphiphiles where Coulomb forces mediated by electrolytes dominate intermolecular forces. In addition, electrostatic interactions have been attributed to the emergence of quasi-crystalline Franck–Kasper phases in ionic surfactants. Thus, in such cases, theoretical models are employed, which consider the free energy contribution that results in hydrophilic head-to-head repulsion and the translational entropy of surfactant monomers, counterions, and aggregates, as well as the hydrophobic contribution responsible for tail packing [27][38][73,85].
3.2. Methods of Obtaining
Micelles are created through the self-organisation of amphiphilic polymers in aqueous solutions. Conversely, amphiphilic polymers exist as unimers at low concentrations. When the concentration of amphiphilic polymers is close to the critical micelle concentration (CMC), unimers aggregate to form micelles [25][26][39][71,72,86]. The formation of micelles and reverse micelles depends on environmental and structural factors, with their creation predominantly influenced by intermolecular bonds including hydrogen bonds, hydrophobic interactions (amphiphilic micelles), and electrostatic interactions (polyionic micelles). The reduction in interfacial free energy is the primary driver of micellization and its stabilisation. Hydrophobic interaction prevails as the primary mechanism for reducing the interfacial free energy of a block copolymer in aqueous environments. In cases of micellization, the separated core-forming molecules necessitate additional proximity forces to further stabilise the micelles [24][70].
There are two primary approaches for creating micelles: physical and chemical. The latter involves the formation of a reversible bond between the amphiphilic polymer and the hydrophobic functional component, which enables the encapsulation of the hydrophobic substance into the micelle’s core. Unfortunately, this technique is unfit for implementation in the food industry due to the creation of new chemical bonds. In contrast, the physical method involves the self-organisation of the amphiphilic polymer in a solution, with the hydrophobic functional component being encapsulated within the core via hydrophobic interactions and/or hydrogen bonds. Physical methods for the preparation of polymer nanoparticles include direct dissolution, solvent evaporation, dialysis, film hydration, ultrasound-assisted, nanoprecipitation, polymerisation-induced self-assembly (PISA), and oil-in-water emulsion methods [24][25][27][70,71,73]. The direct dissolution method is typically utilised for polymers with high water solubility. Micelles are formed when the concentration of polymer exceeds CMC. The polymer self-assembles with gentle agitation in water [40][87]. In the solvent evaporation method, volatile organic solvents are utilised to enhance the solubility of hydrophobic polymer chains and hydrophobic functional components [27][73]. The dialysis technique is employed to prepare micelles for poorly soluble polymers and/or functional components. It entails transferring the material and polymer from a solvent in which they can be jointly dissolved to a solvent selective for the hydrophilic chains of the polymer. Under the influence of the selective solvent, hydrophobic polymer chains combine gradually, leading to the emergence of micelle cores. Concurrently, the hydrophobic functional component transfers to the micelle core. Dimethylsulfoxide, ethanol, acetone, and tetrahydrofuran are among the usual solvents employed in this method [41][88]. The cellular structure of micelles acquired using the dialysis method is more stable and compact in comparison to the solvent method. However, this technique results in significant losses and requires an extended duration [27][73]. Conversely, the film hydration method utilises volatile organic solvents to dissolve hydrophobic functional components and copolymers. Evaporation eliminates these components in a subsequent step to produce a lean polymer film. Subsequently, polymer micelles are formed upon dissolving the polymer film in water, into which hydrophobic components are loaded [42][89]. The technique for obtaining micelles assisted by ultrasound is generally reserved for polymers with favourable water solubility, as ultrasound alone is insufficient for encapsulating hydrophobic components and hydrophilic polymers [40][87]. The encapsulation of water-insoluble components through the oil-in-water emulsion method is a convenient technique for preparing micelles. First, the hydrophobic components and polymer dissolve in water-insoluble organic solvents before being added to the aqueous solution with vigorous mixing (homogenisation, ultrasound), forming an oil-in-water emulsion. The resulting outer phase is a continuous aqueous phase, the inner phase is an organic phase, and the polymer self-organises to create a micelle structure [43][90]. The categorisation of micelles formed by standard block polymers is determined by analysing the interactions that cause core segments to aggregate from a solution of water. The categories are amphiphilic micelles (caused by hydrophobic interactions), polyionic micelles (caused by electrostatic interactions), and metal complexing micelles [24][70].
3.3. Factors Influencing Stability and Properties
The stability of the micelles is influenced by the structural parameters of the polysaccharides and environmental factors like pH, solution temperature, and salt ions. Specifically, intermolecular interactions such as electrostatic, hydrogen bonds, and hydrophobic interactions [44][82] play a significant role. The size and hydrophobicity of the polymer particles’ hydrophobic region directly determine the stability of the micelles. The efficiency of micelle formation is impacted by the degree of substitution in hydrophobic polymer groups and the hydrophobic chain length. Reduction in temperature results in a decrease in micelle size and may be attributed to improved hydrophobic interactions between amphiphilic polymer chains or the weakened hydrogen bonds of water molecules to polymer hydrophilic chains. Furthermore, the temperature can impact the charge and storage of functional elements within micelles, while the existence of other ions enhances micelle stability. An increase in ionic strength results in a greater aggregation of micelles, as the surface charge of the micelles correspondingly diminishes [45][91]. Additionally, the solubility of hydrophobic compounds is affected by ionic strength. As the concentration of salt ions increases, the solubility of the hydrophobic component in cationic surfactant micelles also increases. This phenomenon arises due to electrostatic repulsion between the polar main groups of the surfactants, which impacts the conversion of spherical micelles into elongated micelles [46][92]. Micelle stability impacts the encapsulation process and delivery of functional ingredients. The stability of micelles is dictated by the dendritic architecture and the molecular weight of the polysaccharides [44][82]. Dendritic-polysaccharide micelles can be classified based on their stiffness, and this polysaccharide-dendritic synchronisation influences the stability and compactness of micelles, as well as the kinetics of the intracellular release of biomolecules [44][82]. Better stability of dendritic-polysaccharide micelles is related to the intrinsic stiffness and globular shape of the dendrimers. Lower polydispersity and longer micelle stability were observed when the molecular weight of charged polysaccharides such as chitosan, alginate, and hyaluronic acid was decreased, which is due to the high rigidity of these polysaccharides. Lopez-Blanco et al. [44][82] conducted a study which demonstrated the possibility of preparing charged polysaccharide PIC (dendritic-polysaccharide polyion complex) micelles using PEG-dendritic block copolymers. The polysaccharides used were chitosan, alginate, and hyaluronic acid, and the resulting micelles were found to be more stable than those produced by linear copolymers [27][73]. Micelles have garnered significant interest owing to their distinct features, namely small size, effective solubilisation ability, high encapsulation efficacy, and precision-targeted release of hydrophobic bioactive agents. Thus, micelles serve as critical agents for enclosing functional ingredients to enhance their bioavailability and stability [47][93].
4. Inverted Micelles
In an apolar environment, amphiphiles spontaneously form inverted micelles, which are polar at the core and hydrophobic on the outer surface. While the literature uses “inverted microemulsion” and “inverted micelle” interchangeably, they represent two distinct colloidal systems that differ in thermodynamic stability. Reverse micelles exhibit an organized structure, as opposed to reverse microemulsions, because of the petite water droplets that make up the surfactant monolayer [48][94].
Inverted micelles are surfactant aggregates with a water molecule at the core in a non-polar solution. The size and structure of these micelles depend on various factors such as the quantity of water added, expressed as mole [water]/mol [surfactant], the type and amount of apolar solvent used, the structure of the encapsulated polar solvent, the temperature at which the process of inverted micelle formation occurs, and even the quantity of water employed [48][94]. Water molecules enclosed within inverted micelles are immobilised due to local water interactions with the counter ions and dipoles of the surfactant core groups. Inverted micelles possess cores that resemble the physiological environment (water), which allows them to protect encapsulated biomolecules from denaturation [48][94].
Due to the ability of the aqueous cores of inverted micelles to encapsulate biomolecules and then release the trapped compounds, they have become useful in the food industry. In particular, they are utilised for extracting, separating, and purifying food compounds such as enzymes, peptides, proteins, and edible oils. They are also used for protecting food enzymes and antioxidants, developing analytical and detection media, and creating multifunctional nanomaterials [48][49][94,95]. Given that most inverted micelles are made of undesirable components (e.g., iso-octane, sodium bis-[2-ethylhexyl] sulfosuccinate), the development of secure inverted micelles employing polysaccharides in non-polar media, such as ethanol, glycerol, or vegetable oils, presents a promising solution for the use of inverted micelles in food applications. Nonetheless, the utilisation of inverted micelles presents several benefits, such as the ability to retrieve surfactants and non-polar solvents, which consequently reduces cost [48][94]. Additionally, inverted polysaccharide micelles have practical implications for the food industry in serving as nanocarriers for the encapsulation, targeted delivery, and controlled release of hydrophilic food compounds within fat-based food products such as spreads, cocoa butter, and vegetable oils [24][70]. In the context of food applications, including the detection of food and the inhibition of edible oil oxidation in non-aqueous foods, the synthesising of inverted micelles with biodegradable polysaccharides is an area of great interest that necessitates further research [24][70].
5. Liposomes
In recent years, liposomes have become increasingly popular and the subject of numerous research papers due to their distinctive properties. Liposomes have gained attention due to their potential applications in drug delivery, cosmetics, and food industries. Their unique properties make them an attractive option for targeted drug delivery due to their ability to encapsulate hydrophilic and hydrophobic drugs, improve drug solubility, and protect drugs from degradation. The term ‘liposome’ is derived from the Greek words ‘Lipos’ meaning fat and ‘Soma’ meaning body [50][96]. In 1965, Alec D. Bangham, a British biophysicist, discovered them [51][97]. The scientific literature has introduced the concept of nanoliposomes, defined as lipid bilayer vesicles which possess and maintain a nanometric size that fluctuates during storage and use, are recognised as a novel technology that offers health benefits by encapsulating bioactive ingredients with enhanced functional properties [52][98]. Overall, these structures display comparable characteristics; nevertheless, nanoliposomes present superior properties such as increased surface area, enhanced solubility, and precise release in contrast to liposomes [53][99]. Liposomes are defined as spherical vesicular structures made up of phospholipids, where a lipophilic bilayer is sandwiched between two hydrophilic layers. The efficacy and potential of liposomes as a drug delivery system for diverse active agents have been thoroughly researched and verified in the peer-reviewed scientific literature [54][55][100,101]. The purpose of liposomal encapsulation is to safeguard delicate bioactive elements and improve their effectiveness by lessening the influence of negative environmental elements and hindering the contact of enclosed ingredients with detrimental external agents [56][102].
The characteristics of liposomes are impacted by their ultimate organisation, and their structure and physicochemical properties are determined by elements such as lipid type, morphology, size, concentration, and charge [57][103]. There is a plethora of classification systems for liposomes described in the literature. These objects can be classified according to the number of layers that compose them, which include monolayer, multilayer, oligolamellar, and multilamellar vesicles [58][59][104,105]. On the other hand, membrane size and structure form the basis for another widely used classification system. An alternative system is in use, which classifies compounds based on their intracellular mechanism of action and lipid composition, as well as their mode of preparation [52][98]. Liposomes present a promising approach to encapsulating different types of essential oils and fatty acids [60][106].
