Colorectal cancer is one of the leading causes of morbidity and mortality today. Knowledge of its pathogenesis has made it possible to advance the development of different therapeutic strategies. However, the appearance of drug resistance constitutes one of the main causes of treatment failure. Bioactive compounds of vegetable origin are being studied as a new strategy to improve antitumor treatment, due to their ability to regulate the pathways involved in the development of carcinogenesis or processes that are decisive in its evolution, including multidrug resistance. In vitro and in vivo studies of these substances in combination with cytotoxic drugs have shown that they reduce resistance and increase therapeutic efficacy. The objective of this review is to summarize the knowledge that is described in the scientific literature on the antitumor and chemo-sensitizing capacity of vegetable-derived biomolecules such as polyphenols, flavonoids, and terpenes. These compounds may hold a promising future in improving the treatment of colorectal cancer.
- vegetal compounds
- colorectal cancer
- cancer
- vegetal extracts
- cancer treatment
- systematic review
21. Colorectal Cancer: Resistant Mechanisms
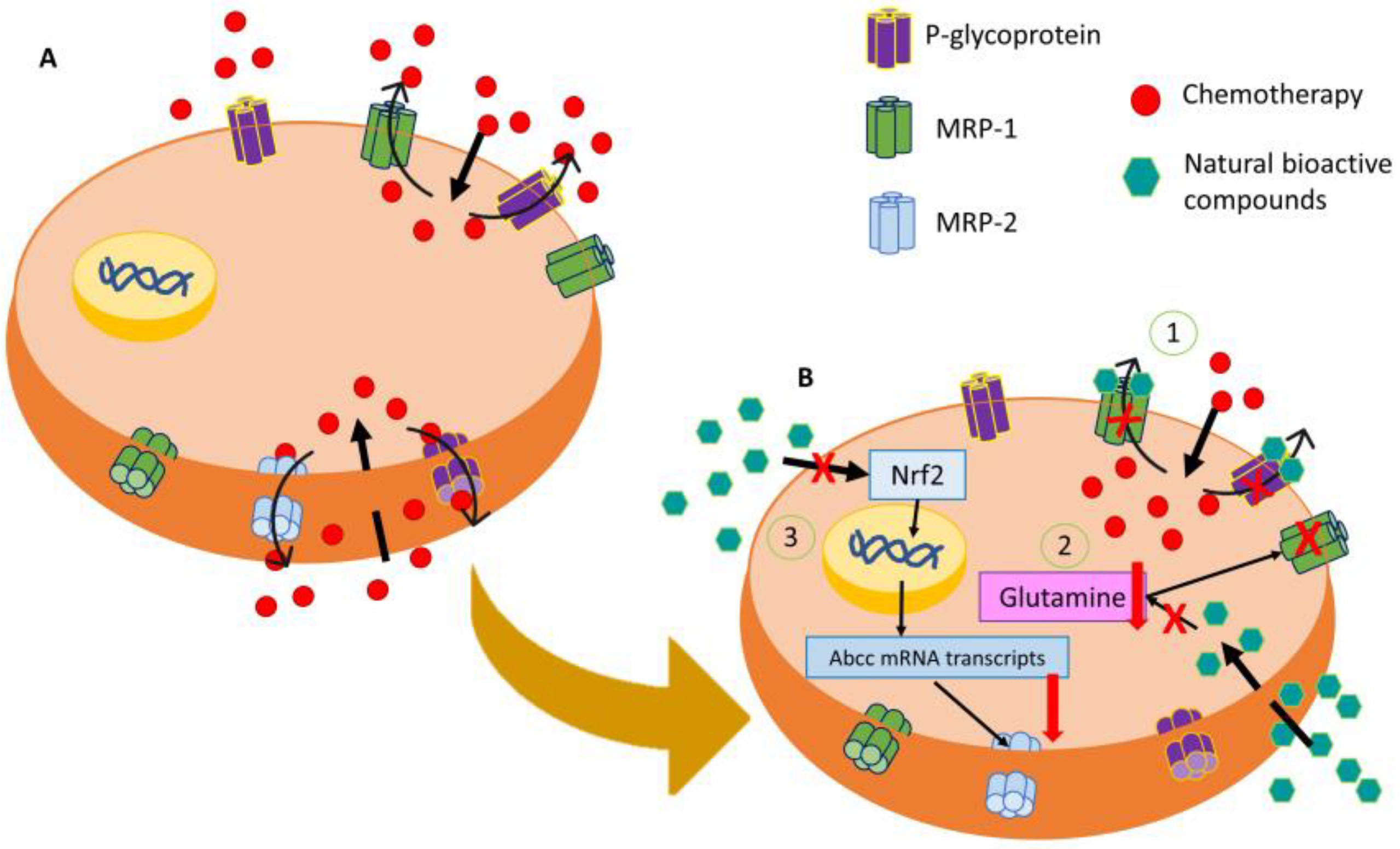
2. Antitumor Potential of Natural Products for Colorectal Cancer
| Fam. | Comp. | Sinergy | In Vitro | In Vivo | Clinical Trials | Refs. |
|---|---|---|---|---|---|---|
| Polyphenols | Resveratrol | 5-FU, OXA | Apoptosis and decreased AC (VEGF inhibition). IC50 of 10 mg/mL in HT-29 (72 h). | 150 or 300 ppm doses prevented cancerous lesions and induced apoptosis via BAX | Safe intake up to a 1g/day dose. Limited BAV (2–29%). | [20,47,49,2350,52,][2454,][2555,]57,58][[26][27][28][29][30][31] |
| Curcumin | DOX, 5-FU, OXA | G1-CCA and apoptosis at 20 µM. Reduced cell migration and invasion. | 300 mg/kg dose prevented precancerous lesions and decreased tumor size. | Low absorption and BAV. Increased tumor apoptosis. | [69,]70,71,[72,73,32][33][34]74,75,76,81][21[35][36][37][38][39] | |
| Kaempferol | DOX, 5-FU, OXA | G1 and G2/M-CCA and apoptosis induction. Decreased AC at 0.1 µM in MDA cell line. | Decreased AC and MC. | Low toxicity and BAV (2%). | [82,83,84,85,[86,87,40][88,41][89,90][22]42][43][44][45][46][47] | |
| Flavonoids | Quercetin | DOX | CCA, apoptosis and decreased AC and MC. CYT in up to 300 µM doses in HCT-15, RKO, CT26, MC38, and HT29 cells | 10 and 50 mg/kg reduced precancerous lesions and tumor size. Decreased MC and CHR. | Not performed. | [24,92,93,94,95,96,97][48][49][50][51][52][53][54] |
| EGCG | 5-FU, IRI, CPT, OXA | S and G2-CCA, apoptosis, CYT IC50 between 74.6 and 112.1 in CRC cell lines SW480, SW620, and LS411N. Decreased MC. | 30 mg/kg for 2 weeks decreased MC, tumor growth and induced apoptosis | 150 mg twice a day of green tea extract did not show any effect in CRC development risk | [18,99,100,101,102,103,104,105,106,107][55][56][57][58][59][60][61][62][63][64] | |
| Terpenes | Geraniol | 5-FU | S-CCA. CYT IC30 of 200 µM for 7 days treatment in Caco-2 cell line. | 250 mg/kg for 4 weeks prevented CRC precancerous lesions. Reduced tumor growth and apoptosis induction. | Not performed. | [116,117,118][65][66][67] |
| Panaxadiol | 5-FU, IRI | CYT IC50 lower than 10 µM in HCT116 cell line (72 h). Decreased AC (VEGF inhibition) | 30 mg/kg for 3 weeks reduced tumor growth and AC. | Not performed. | [119,123,124,125][68][69][70][71] | |
| Rg3 | 5-FU | CYT IC50 100–200 µM in HT-29 cell line (48 h). Induction of apoptosis and AC via AMPK dysregulation. | 25 mg/kg for 12 days reduced tumor vascularization and decreased CHR to 5-FU and OXA. | Not performed. | [121,122,125][71][72][73] |
References
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233.
- Wang, X.; Zhang, H.; Chen, X. Drug Resistance and Combating Drug Resistance in Cancer. Cancer Drug Resist. 2019, 2, 141–160.
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug Resistance in Cancer: An Overview. Cancers 2014, 6, 1769–1792.
- Linn, S.C.; Giaccone, G. MDR1/P-Glycoprotein Expression in Colorectal Cancer. Eur. J. Cancer 1995, 31A, 1291–1294.
- Yuan, Z.; Liang, X.; Zhan, Y.; Wang, Z.; Xu, J.; Qiu, Y.; Wang, J.; Cao, Y.; Le, V.-M.; Ly, H.-T.; et al. Targeting CD133 Reverses Drug-Resistance via the AKT/NF-ΚB/MDR1 Pathway in Colorectal Cancer. Br. J. Cancer 2020, 122, 1342–1353.
- Cao, D.; Qin, S.; Mu, Y.; Zhong, M. The Role of MRP1 in the Multidrug Resistance of Colorectal Cancer. Oncol. Lett. 2017, 13, 2471–2476.
- Hu, T.; Li, Z.; Gao, C.-Y.; Cho, C.H. Mechanisms of Drug Resistance in Colon Cancer and Its Therapeutic Strategies. World J. Gastroenterol. 2016, 22, 6876–6889.
- Wang, Z.; Sun, X.; Feng, Y.; Wang, Y.; Zhang, L.; Wang, Y.; Fang, Z.; Azami, N.L.B.; Sun, M.; Li, Q. Dihydromyricetin Reverses MRP2-Induced Multidrug Resistance by Preventing NF-ΚB-Nrf2 Signaling in Colorectal Cancer Cell. Phytomedicine 2021, 82, 153414.
- Asano, T. Drug Resistance in Cancer Therapy and the Role of Epigenetics. J. Nippon. Med. Sch. 2020, 87, 244–251.
- Das, P.K.; Islam, F.; Lam, A.K. The Roles of Cancer Stem Cells and Therapy Resistance in Colorectal Carcinoma. Cells 2020, 9, 1392.
- Neophytou, C.M.; Trougakos, I.P.; Erin, N.; Papageorgis, P. Apoptosis Deregulation and the Development of Cancer Multi-Drug Resistance. Cancers 2021, 13, 4363.
- Prasetyanti, P.R.; Medema, J.P. Intra-Tumor Heterogeneity from a Cancer Stem Cell Perspective. Mol. Cancer 2017, 16, 41.
- Phi, L.T.H.; Sari, I.N.; Yang, Y.G.; Lee, S.H.; Jun, N.; Kim, K.S.; Lee, Y.K.; Kwon, H.Y. Cancer Stem Cells (CSCs) in Drug Resistance and Their Therapeutic Implications in Cancer Treatment. Stem. Cells Int. 2018, 2018, 5416923.
- Burrell, R.A.; Swanton, C. Tumour Heterogeneity and the Evolution of Polyclonal Drug Resistance. Mol. Oncol. 2014, 8, 1095–1111.
- Junttila, M.R.; de Sauvage, F.J. Influence of Tumour Micro-Environment Heterogeneity on Therapeutic Response. Nature 2013, 501, 346–354.
- Ortíz, R.; Quiñonero, F.; García-Pinel, B.; Fuel, M.; Mesas, C.; Cabeza, L.; Melguizo, C.; Prados, J. Nanomedicine to Overcome Multidrug Resistance Mechanisms in Colon and Pancreatic Cancer: Recent Progress. Cancers 2021, 13, 2058.
- Gallego-Jara, J.; Lozano-Terol, G.; Sola-Martínez, R.A.; Cánovas-Díaz, M.; Puente, T.d.D. A Compressive Review about Taxol®: History and Future Challenges. Molecules 2020, 25, 5986.
- Yuan, M.; Zhang, G.; Bai, W.; Han, X.; Li, C.; Bian, S. The Role of Bioactive Compounds in Natural Products Extracted from Plants in Cancer Treatment and Their Mechanisms Related to Anticancer Effects. Oxid. Med. Cell Longev. 2022, 2022, 1429869.
- Elshaer, M.; Chen, Y.; Wang, X.J.; Tang, X. Resveratrol: An Overview of Its Anti-Cancer Mechanisms. Life Sci. 2018, 207, 340–349.
- Weng, W.; Goel, A. Curcumin and Colorectal Cancer: An Update and Current Perspective on This Natural Medicine. Semin. Cancer Biol. 2022, 80, 73–86.
- Yin, T.F.; Wang, M.; Qing, Y.; Lin, Y.M.; Wu, D. Research Progress on Chemopreventive Effects of Phytochemicals on Colorectal Cancer and Their Mechanisms. World J. Gastroenterol. 2016, 22, 7068.
- Chen, A.Y.; Chen, Y.C. A Review of the Dietary Flavonoid, Kaempferol on Human Health and Cancer Chemoprevention. Food Chem. 2013, 138, 2099–2107.
- Huang, L.; Zhang, S.; Zhou, J.; Li, X. Effect of Resveratrol on Drug Resistance in Colon Cancer Chemotherapy. RSC Adv. 2019, 9, 2572–2580.
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589.
- Fuggetta, M.P.; Lanzilli, G.; Tricarico, M.; Cottarelli, A.; Falchetti, R.; Ravagnan, G.; Bonmassar, E. Effect of Resveratrol on Proliferation and Telomerase Activity of Human Colon Cancer Cells in Vitro. J. Exp. Clin. Cancer Res. 2006, 25, 189–193.
- Rotelli, M.T.; Bocale, D.; de Fazio, M.; Ancona, P.; Scalera, I.; Memeo, R.; Travaglio, E.; Zbar, A.P.; Altomare, D.F. IN-VITRO Evidence for the Protective Properties of the Main Components of the Mediterranean Diet against Colorectal Cancer: A Systematic Review. Surg Oncol. 2015, 24, 145–152.
- Saud, S.M.; Li, W.; Morris, N.L.; Matter, M.S.; Colburn, N.H.; Kim, Y.S.; Young, M.R. Resveratrol Prevents Tumorigenesis in Mouse Model of Kras Activated Sporadic Colorectal Cancer by Suppressing Oncogenic Kras Expression. Carcinogenesis 2014, 35, 2778–2786.
- Aires, V.; Limagne, E.; Cotte, A.K.; Latruffe, N.; Ghiringhelli, F.; Delmas, D. Resveratrol Metabolites Inhibit Human Metastatic Colon Cancer Cells Progression and Synergize with Chemotherapeutic Drugs to Induce Cell Death. Mol. Nutr. Food Res. 2013, 57, 1170–1181.
- Cottart, C.H.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Beaudeux, J.L. Resveratrol Bioavailability and Toxicity in Humans. Mol. Nutr. Food Res. 2010, 54, 7–16.
- Abdel Latif, Y.; El-Bana, M.; Hussein, J.; El-Khayat, Z.; Farrag, A.R. Effects of Resveratrol in Combination with 5-Fluorouracil on N-Methylnitrosourea-Induced Colon Cancer in Rats. Comp. Clin. Path 2019, 28, 1351–1362.
- Buhrmann, C.; Yazdi, M.; Popper, B.; Shayan, P.; Goel, A.; Aggarwal, B.B.; Shakibaei, M. Resveratrol Chemosensitizes TNF-β-Induced Survival of 5-FU-Treated Colorectal Cancer Cells. Nutrients 2018, 10, 888.
- Jaiswal, A.S.; Marlow, B.P.; Gupta, N.; Narayan, S. Beta-Catenin-Mediated Transactivation and Cell-Cell Adhesion Pathways Are Important in Curcumin (Diferuylmethane)-Induced Growth Arrest and Apoptosis in Colon Cancer Cells. Oncogene 2002, 21, 8414–8427.
- Basbinar, Y.; Calibasi-Kocal, G.; Pakdemirli, A.; Bayrak, S.; Ozupek, N.M.; Sever, T.; Ellidokuz, H.; Yigitbasi, T. Curcumin Effects on Cell Proliferation, Angiogenesis and Metastasis in Colorectal Cancer. JBUON 2019, 24, 1482–1487.
- Pricci, M.; Girardi, B.; Giorgio, F.; Losurdo, G.; Ierardi, E.; di Leo, A. Curcumin and Colorectal Cancer: From Basic to Clinical Evidences. Int. J. Mol. Sci. 2020, 21, 2364.
- Tunstall, R.G.; Sharma, R.A.; Perkins, S.; Sale, S.; Singh, R.; Farmer, P.B.; Steward, W.P.; Gescher, A.J. Cyclooxygenase-2 Expression and Oxidative DNA Adducts in Murine Intestinal Adenomas: Modification by Dietary Curcumin and Implications for Clinical Trials. Eur. J. Cancer 2006, 42, 415–421.
- Yin, J.; Wang, L.; Wang, Y.; Shen, H.; Wang, X.; Wu, L. Curcumin Reverses Oxaliplatin Resistance in Human Colorectal Cancer via Regulation of TGF-β/Smad2/3 Signaling Pathway. Onco. Targets Ther. 2019, 12, 3893–3903.
- Zheng, X.; Yang, X.; Lin, J.; Song, F.; Shao, Y. Low Curcumin Concentration Enhances the Anticancer Effect of 5-Fluorouracil against Colorectal Cancer. Phytomedicine 2021, 85, 153547.
- Hosseini, S.A.; Zand, H.; Cheraghpour, M. The Influence of Curcumin on the Downregulation of MYC, Insulin and IGF-1 Receptors: A Possible Mechanism Underlying the Anti-Growth and Anti-Migration in Chemoresistant Colorectal Cancer Cells. Medicina 2019, 55, 90.
- Gupta, A.; Sood, A.; Dhiman, A.; Shrimali, N.; Singhmar, R.; Guchhait, P.; Agrawal, G. Redox Responsive Poly(Allylamine)/Eudragit S-100 Nanoparticles for Dual Drug Delivery in Colorectal Cancer. Biomater. Adv. 2022, 143, 213184.
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Gondal, T.A.; Saeed, F.; Imran, A.; Shahbaz, M.; Fokou, P.V.T.; Arshad, M.U.; Khan, H.; et al. Kaempferol: A Key Emphasis to Its Anticancer Potential. Molecules 2019, 24, 2277.
- Schindler, R.; Mentlein, R. Flavonoids and Vitamin E Reduce the Release of the Angiogenic Peptide Vascular Endothelial Growth Factor from Human Tumor Cells. J. Nutr. 2006, 136, 1477–1482.
- Barve, A.; Chen, C.; Hebbar, V.; Desiderio, J.; Saw, C.L.-L.; Kong, A.-N. Metabolism, Oral Bioavailability and Pharmacokinetics of Chemopreventive Kaempferol in Rats. Biopharm. Drug Dispos. 2009, 30, 356–365.
- Cho, H.J.; Park, J.H.Y. Kaempferol Induces Cell Cycle Arrest in HT-29 Human Colon Cancer Cells. J. Cancer Prev. 2013, 18, 257–263.
- Riahi-Chebbi, I.; Souid, S.; Othman, H.; Haoues, M.; Karoui, H.; Morel, A.; Srairi-Abid, N.; Essafi, M.; Essafi-Benkhadir, K. The Phenolic Compound Kaempferol Overcomes 5-Fluorouracil Resistance in Human Resistant LS174 Colon Cancer Cells. Sci. Rep. 2019, 9, 195.
- Li, Q.; Wei, L.; Lin, S.; Chen, Y.; Lin, J.; Peng, J. Synergistic Effect of Kaempferol and 5-fluorouracil on the Growth of Colorectal Cancer Cells by Regulating the PI3K/Akt Signaling Pathway. Mol. Med. Rep. 2019, 20, 728–734.
- Park, J.; Lee, G.E.; An, H.J.; Lee, C.J.; Cho, E.S.; Kang, H.C.; Lee, J.Y.; Lee, H.S.; Choi, J.S.; Kim, D.J.; et al. Kaempferol Sensitizes Cell Proliferation Inhibition in Oxaliplatin-Resistant Colon Cancer Cells. Arch. Pharm. Res. 2021, 44, 1091–1108.
- Meena, D.; Vimala, K.; Kannan, S. Combined Delivery of DOX and Kaempferol Using PEGylated Gold Nanoparticles to Target Colon Cancer. J. Clust. Sci. 2022, 33, 173–187.
- Zhou, Y.; Zhang, J.; Wang, K.; Han, W.; Wang, X.; Gao, M.; Wang, Z.; Sun, Y.; Yan, H.; Zhang, H.; et al. Quercetin Overcomes Colon Cancer Cells Resistance to Chemotherapy by Inhibiting Solute Carrier Family 1, Member 5 Transporter. Eur. J. Pharmacol. 2020, 881, 173185.
- Tang, S.M.; Deng, X.T.; Zhou, J.; Li, Q.P.; Ge, X.X.; Miao, L. Pharmacological Basis and New Insights of Quercetin Action in Respect to Its Anti-Cancer Effects. Biomed Pharm. 2020, 121, 109604.
- Reyes-Farias, M.; Carrasco-Pozo, C. The Anti-Cancer Effect of Quercetin: Molecular Implications in Cancer Metabolism. Int. J. Mol. Sci. 2019, 20, 3177.
- Kee, J.Y.; Han, Y.H.; Kim, D.S.; Mun, J.G.; Park, J.; Jeong, M.Y.; Um, J.Y.; Hong, S.H. Inhibitory Effect of Quercetin on Colorectal Lung Metastasis through Inducing Apoptosis, and Suppression of Metastatic Ability. Phytomedicine 2016, 23, 1680–1690.
- Srivastava, N.S.; Srivastava, R.A.K. Curcumin and Quercetin Synergistically Inhibit Cancer Cell Proliferation in Multiple Cancer Cells and Modulate Wnt/β-Catenin Signaling and Apoptotic Pathways in A375 Cells. Phytomedicine 2019, 52, 117–128.
- Neamtu, A.A.; Maghiar, T.A.; Alaya, A.; Olah, N.K.; Turcus, V.; Pelea, D.; Totolici, B.D.; Neamtu, C.; Maghiar, A.M.; Mathe, E. A Comprehensive View on the Quercetin Impact on Colorectal Cancer. Molecules 2022, 27, 1973.
- Chang, C.E.; Hsieh, C.M.; Huang, S.C.; Su, C.Y.; Sheu, M.T.; Ho, H.O. Lecithin-Stabilized Polymeric Micelles (LsbPMs) for Delivering Quercetin: Pharmacokinetic Studies and Therapeutic Effects of Quercetin Alone and in Combination with Doxorubicin. Sci. Rep. 2018, 8, 17640.
- Wu, W.; Dong, J.; Gou, H.; Geng, R.; Yang, X.; Chen, D.; Xiang, B.; Zhang, Z.; Ren, S.; Chen, L.; et al. EGCG Synergizes the Therapeutic Effect of Irinotecan through Enhanced DNA Damage in Human Colorectal Cancer Cells. J. Cell Mol. Med. 2021, 25, 7913–7921.
- Almatrood, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydh, F.A.; Alsahl, M.A.; Rahmani, A.H. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules 2020, 25, 3146.
- Luo, K.W.; Xia, J.; Cheng, B.H.; Gao, H.C.; Fu, L.W.; Luo, X. le Tea Polyphenol EGCG Inhibited Colorectal-Cancer-Cell Proliferation and Migration via Downregulation of STAT3. Gastroenterol. Rep. 2020, 9, 59–70.
- Wubetu, G.Y.; Shimada, M.; Morine, Y.; Ikemoto, T.; Ishikawa, D.; Iwahashi, S.; Yamada, S.; Saito, Y.; Arakawa, Y.; Imura, S. Epigallocatechin Gallate Hinders Human Hepatoma and Colon Cancer Sphere Formation. J. Gastroenterol. Hepatol. 2016, 31, 256–264.
- Toden, S.; Tran, H.M.; Tovar-Camargo, O.A.; Okugawa, Y.; Goel, A. Epigallocatechin-3-Gallate Targets Cancer Stem-like Cells and Enhances 5-Fluorouracil Chemosensitivity in Colorectal Cancer. Oncotarget 2016, 7, 16158–16171.
- La, X.; Zhang, L.; Li, Z.; Li, H.; Yang, Y. (-)-Epigallocatechin Gallate (EGCG) Enhances the Sensitivity of Colorectal Cancer Cells to 5-FU by Inhibiting GRP78/NF-ΚB/MiR-155-5p/MDR1 Pathway. J. Agric. Food Chem. 2019, 67, 2510–2518.
- Maruyama, T.; Murata, S.; Nakayama, K.; Sano, N.; Ogawa, K.; Nowatari, T.; Tamura, T.; Nozaki, R.; Fukunaga, K.; Ohkohchi, N. (-)-Epigallocatechin-3-Gallate Suppresses Liver Metastasis of Human Colorectal Cancer. Oncol. Rep. 2014, 31, 625–633.
- Hu, F.; Wei, F.; Wang, Y.; Wu, B.; Fang, Y.; Xiong, B. EGCG Synergizes the Therapeutic Effect of Cisplatin and Oxaliplatin through Autophagic Pathway in Human Colorectal Cancer Cells. J. Pharmacol. Sci. 2015, 128, 27–34.
- Wang, R.; Huang, J.; Chen, J.; Yang, M.; Wang, H.; Qiao, H.; Chen, Z.; Hu, L.; Di, L.; Li, J. Enhanced Anti-Colon Cancer Efficacy of 5-Fluorouracil by Epigallocatechin-3- Gallate Co-Loaded in Wheat Germ Agglutinin-Conjugated Nanoparticles. Nanomedicine 2019, 21, 102068.
- Seufferlein, T.; Ettrich, T.J.; Menzler, S.; Messmann, H.; Kleber, G.; Zipprich, A.; Frank-Gleich, S.; Algül, H.; Metter, K.; Odemar, F.; et al. Green Tea Extract to Prevent Colorectal Adenomas, Results of a Randomized, Placebo-Controlled Clinical Trial. Am. J. Gastroenterol. 2022, 117, 884–894.
- Vieira, A.; Heidor, R.; Cardozo, M.T.; Scolastici, C.; Purgatto, E.; Shiga, T.M.; Barbisan, L.F.; Ong, T.P.; Moreno, F.S. Efficacy of Geraniol but Not of β-Ionone or Their Combination for the Chemoprevention of Rat Colon Carcinogenesis. Braz. J. Med. Biol. Res. 2011, 44, 538–545.
- Carnesecchi, S.; Bras-Gonçalves, R.; Bradaia, A.; Zeisel, M.; Gossé, F.; Poupon, M.F.; Raul, F. Geraniol, a Component of Plant Essential Oils, Modulates DNA Synthesis and Potentiates 5-Fluorouracil Efficacy on Human Colon Tumor Xenografts. Cancer Lett. 2004, 215, 53–59.
- Carnesecchi, S.; Langley, K.; Exinger, F.; Gosse, F.; Raul, F. Geraniol, a Component of Plant Essential Oils, Sensitizes Human Colonic Cancer Cells to 5-Fluorouracil Treatment. J. Pharmacol. Exp. Ther. 2002, 301, 625–630.
- Gao, J.L.; Lv, G.Y.; He, B.C.; Zhang, B.Q.; Zhang, H.; Wang, N.; Wang, C.Z.; Du, W.; Yuan, C.S.; He, T.C. Ginseng Saponin Metabolite 20(S)-Protopanaxadiol Inhibits Tumor Growth by Targeting Multiple Cancer Signaling Pathways. Oncol. Rep. 2013, 30, 292–298.
- Wang, C.Z.; Zhang, Z.; Wan, J.Y.; Zhang, C.F.; Anderson, S.; He, X.; Yu, C.; He, T.C.; Qi, L.W.; Yuan, C.S. Protopanaxadiol, an Active Ginseng Metabolite, Significantly Enhances the Effects of Fluorouracil on Colon Cancer. Nutrients 2015, 7, 799–814.
- Du, G.-J.; Wang, C.-Z.; Zhang, Z.-Y.; Wen, X.-D.; Somogyi, J.; Calway, T.; He, T.-C.; Du, W.; Yuan, C.-S. Caspase-Mediated pro-Apoptotic Interaction of Panaxadiol and Irinotecan in Human Colorectal Cancer Cells. J. Pharm. Pharmacol. 2012, 64, 727–734.
- Hong, S.; Cai, W.; Huang, Z.; Wang, Y.; Mi, X.; Huang, Y.; Lin, Z.; Chen, X. Ginsenoside Rg3 Enhances the Anticancer Effect of 5-FU in Colon Cancer Cells via the PI3K/AKTpathway. Oncol. Rep. 2020, 44, 1333–1342.
- Yuan, H.D.; Quan, H.Y.; Zhang, Y.; Kim, S.H.; Chung, S.H. 20(S)-Ginsenoside Rg3-Induced Apoptosis in HT-29 Colon Cancer Cells Is Associated with AMPK Signaling Pathway. Mol. Med. Rep. 2010, 3, 825–831.
- Tang, Y.C.; Zhang, Y.; Zhou, J.; Zhi, Q.; Wu, M.Y.; Gong, F.R.; Shen, M.; Liu, L.; Tao, M.; Shen, B.; et al. Ginsenoside Rg3 Targets Cancer Stem Cells and Tumor Angiogenesis to Inhibit Colorectal Cancer Progression in Vivo. Int. J. Oncol. 2018, 52, 127–138.
- Maleki Dana, P.; Sadoughi, F.; Asemi, Z.; Yousefi, B. The Role of Polyphenols in Overcoming Cancer Drug Resistance: A Comprehensive Review. Cell Mol. Biol. Lett. 2022, 27, 1.
- Huang, X.M.; Yang, Z.J.; Xie, Q.; Zhang, Z.K.; Zhang, H.; Ma, J.Y. Natural Products for Treating Colorectal Cancer: A Mechanistic Review. Biomed Pharm. 2019, 117, 109142.
- Afrin, S.; Giampieri, F.; Cianciosi, D.; Alvarez-Suarez, J.M.; Bullon, B.; Amici, A.; Quiles, J.L.; Forbes-Hernández, T.Y.; Battino, M. Strawberry Tree Honey in Combination with 5-Fluorouracil Enhances Chemosensitivity in Human Colon Adenocarcinoma Cells. Food Chem. Toxicol. 2021, 156, 112484.
- Carter, L.G.; D’Orazio, J.A.; Pearson, K.J. Resveratrol and Cancer: Focus on in Vivo Evidence. Endocr. Relat. Cancer 2014, 21, R209–R225.
- Guo, Y.; Sun, Q.; Wu, F.-G.; Dai, Y.; Chen, X.; Guo, Y.; Sun, Q.; Wu, F.; Dai, Y.; Chen Yong Loo, X. Polyphenol-Containing Nanoparticles: Synthesis, Properties, and Therapeutic Delivery. Adv. Mater. 2021, 33, 2007356.
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124.
- Hassanein, N.M.A.; Hassan, E.S.G.; Hegab, A.M.; Elahl, H.M.S. Chemopreventive Effect of Sulindac in Combination with Epigallocatechin Gallate or Kaempferol against 1,2-Dimethyl Hydrazine-Induced Preneoplastic Lesions in Rats: A Comparative Study. J. Biochem. Mol. Toxicol. 2018, 32, e22198.
- Chavva, S.R.; Deshmukh, S.K.; Kanchanapally, R.; Tyagi, N.; Coym, J.W.; Singh, A.P.; Singh, S. Epigallocatechin Gallate-Gold Nanoparticles Exhibit Superior Antitumor Activity Compared to Conventional Gold Nanoparticles: Potential Synergistic Interactions. Nanomaterials 2019, 9, 396.
- Li, Z.; Jiang, H.; Xu, C.; Gu, L. A Review: Using Nanoparticles to Enhance Absorption and Bioavailability of Phenolic Phytochemicals. Food Hydrocoll 2015, 43, 153–164.
- De Cássia Da Silveira, E.; Sá, R.; Andrade, L.N.; de Sousa, D.P. A Review on Anti-Inflammatory Activity of Monoterpenes. Molecules 2013, 18, 1227–1254.
- Cho, M.; So, I.; Chun, J.N.; Jeon, J.H. The Antitumor Effects of Geraniol: Modulation of Cancer Hallmark Pathways (Review). Int. J. Oncol. 2016, 48, 1772–1782.
- Wang, Z.; Li, M.Y.; Zhang, Z.H.; Zuo, H.X.; Wang, J.Y.; Xing, Y.; Ri, M.H.; Jin, H.L.; Jin, C.H.; Xu, G.H.; et al. Panaxadiol Inhibits Programmed Cell Death-Ligand 1 Expression and Tumour Proliferation via Hypoxia-Inducible Factor (HIF)-1α and STAT3 in Human Colon Cancer Cells. Pharmacol. Res. 2020, 155, 104727.
