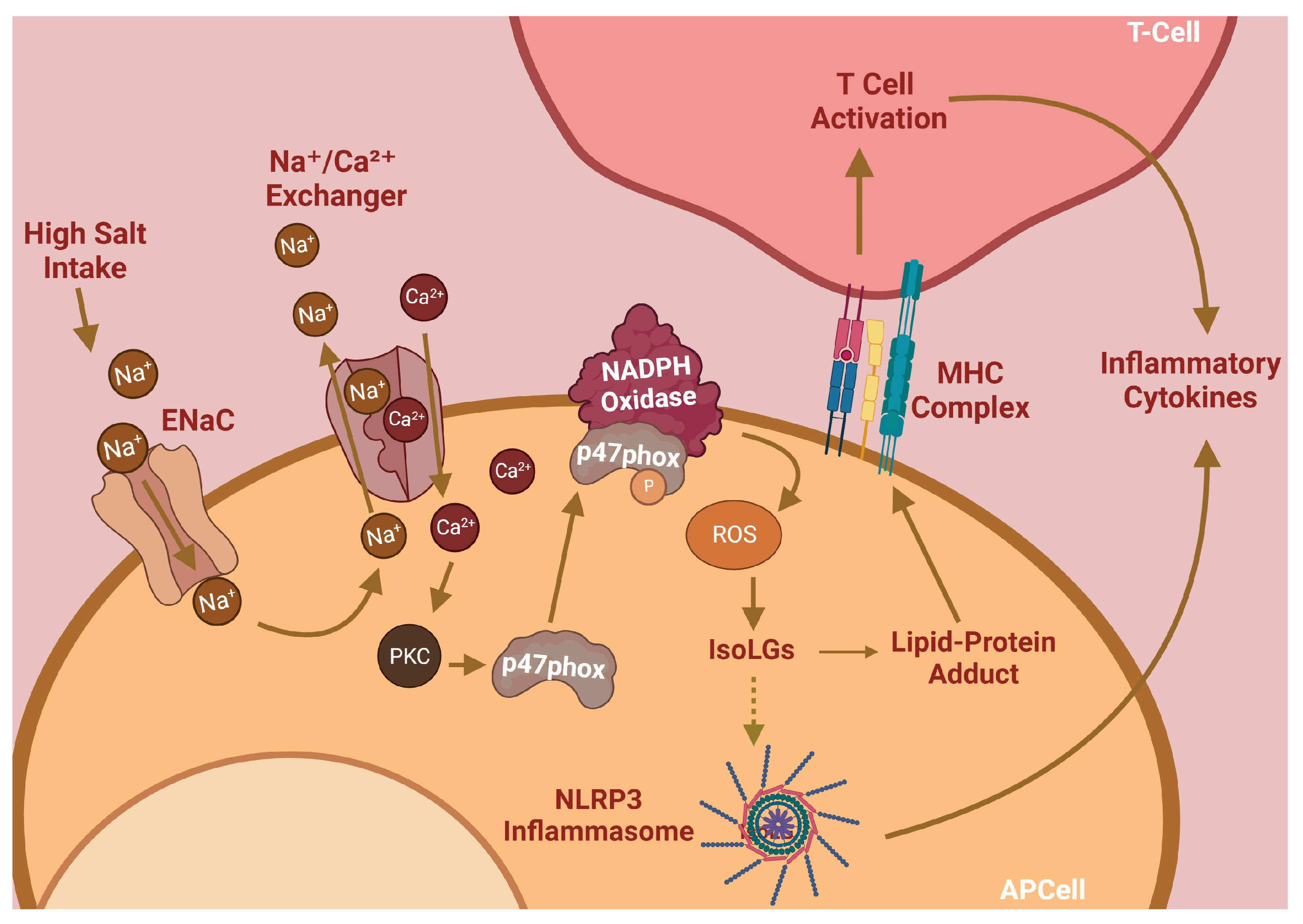
Hypertension is the leading remediable risk factor for cardiovascular morbidity and mortality in the United States. Excess dietary salt consumption, which is a catalyst of hypertension, initiates an inflammatory cascade via activation of antigen-presenting cells (APCs). This pro-inflammatory response is driven primarily by sodium ions (Na
+
) transporting into APCs by the epithelial sodium channel (ENaC) and subsequent NADPH oxidase activation, leading to high levels of oxidative stress. Flavonoids, a natural phenolic compound, have these therapeutic benefits and can potentially serve as anti-hypertensives. Flavones are a type of flavonoid that have increased anti-inflammatory effects that may allow them to act as therapeutic agents for hypertension, including diosmetin, which is able to induce significant arterial vasodilation in several different animal models.
- diosmetin
- flavone
- flavonoid
- hypertension
- inflammation
1. Introduction
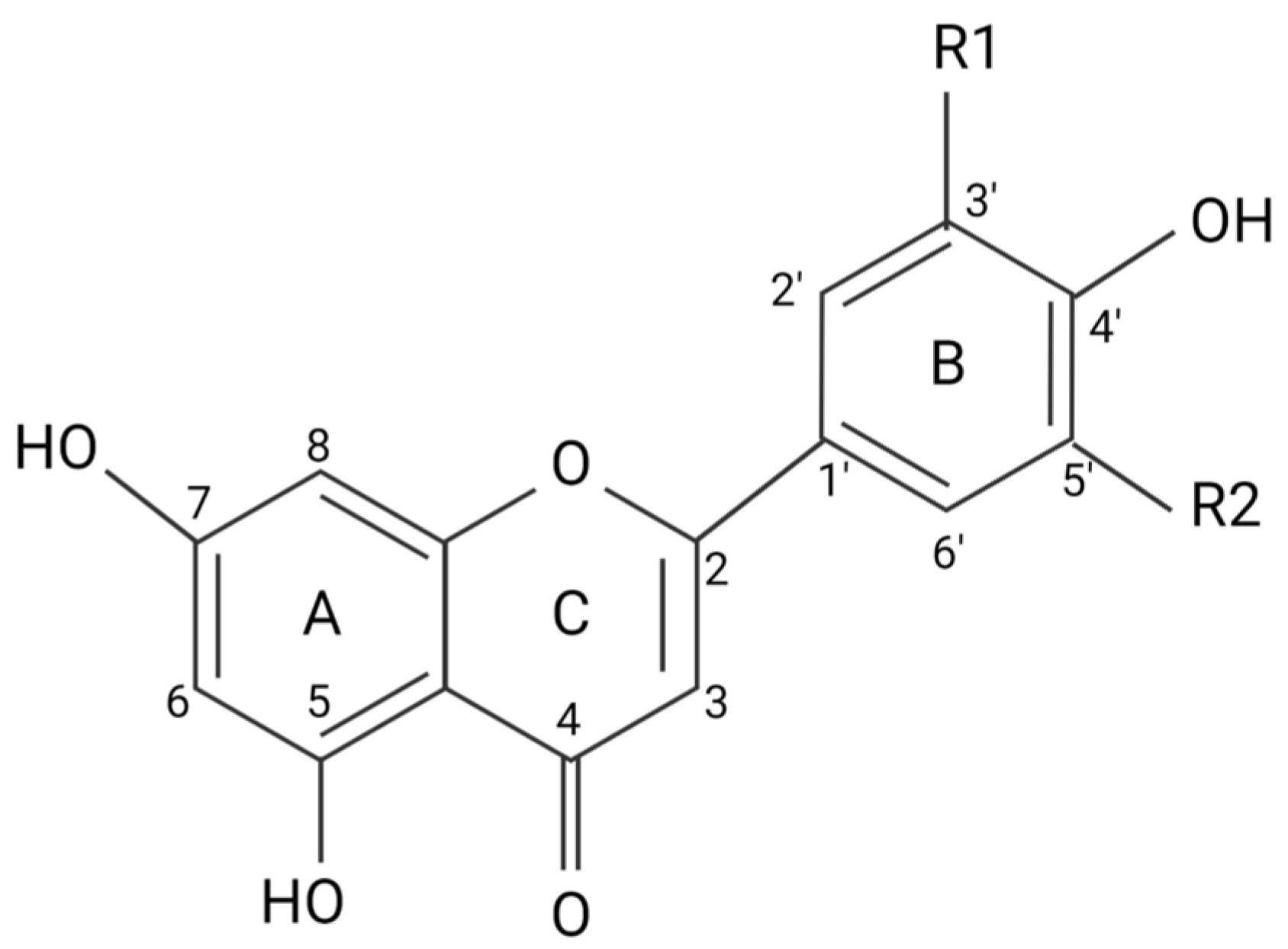
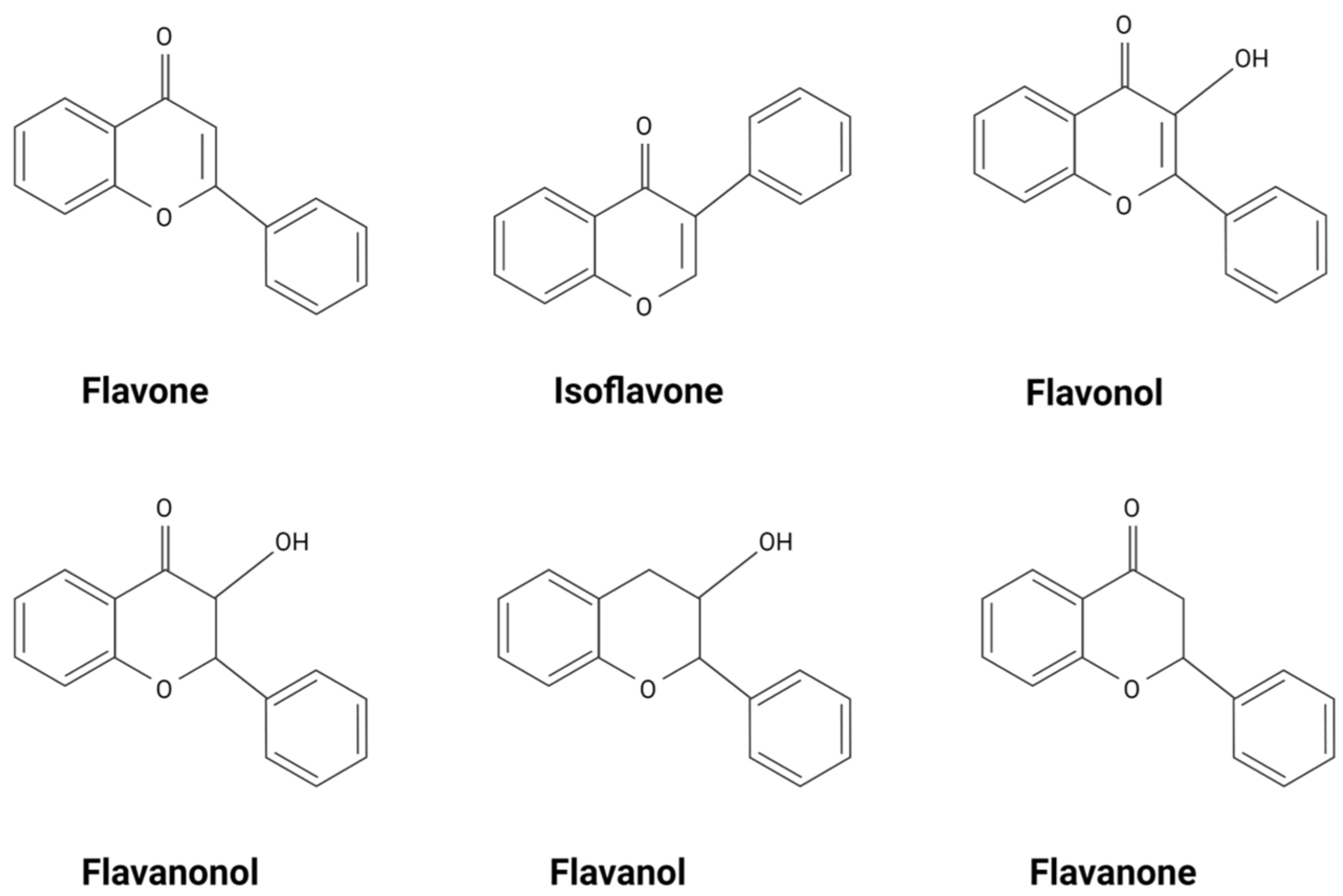
2. Flavonoids Exert Their Effects by Targeting Intracellular Proteins
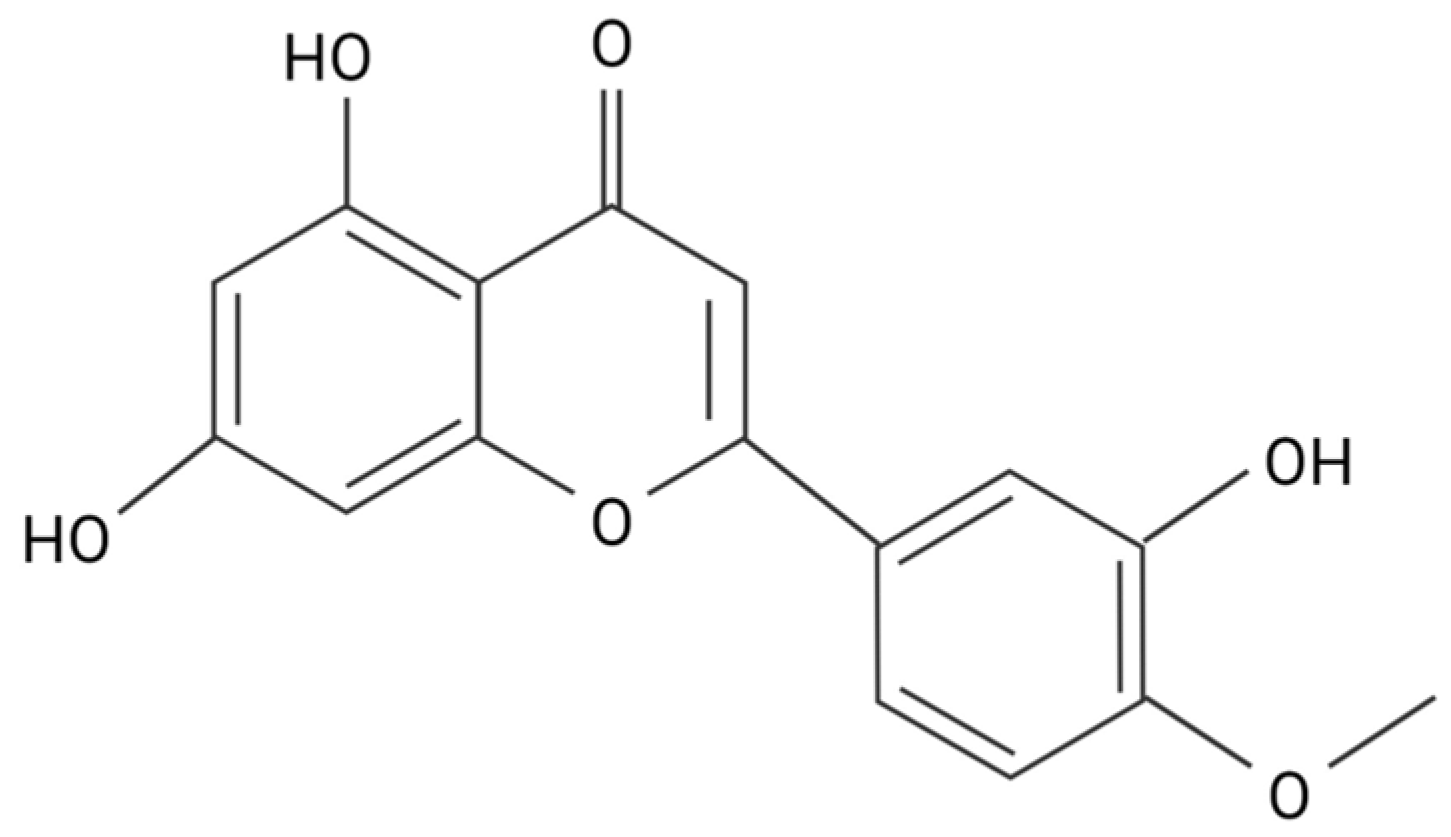
3. Flavones Target NADPH Oxidase Transcription by Inhibiting Smad3 Phosphorylation
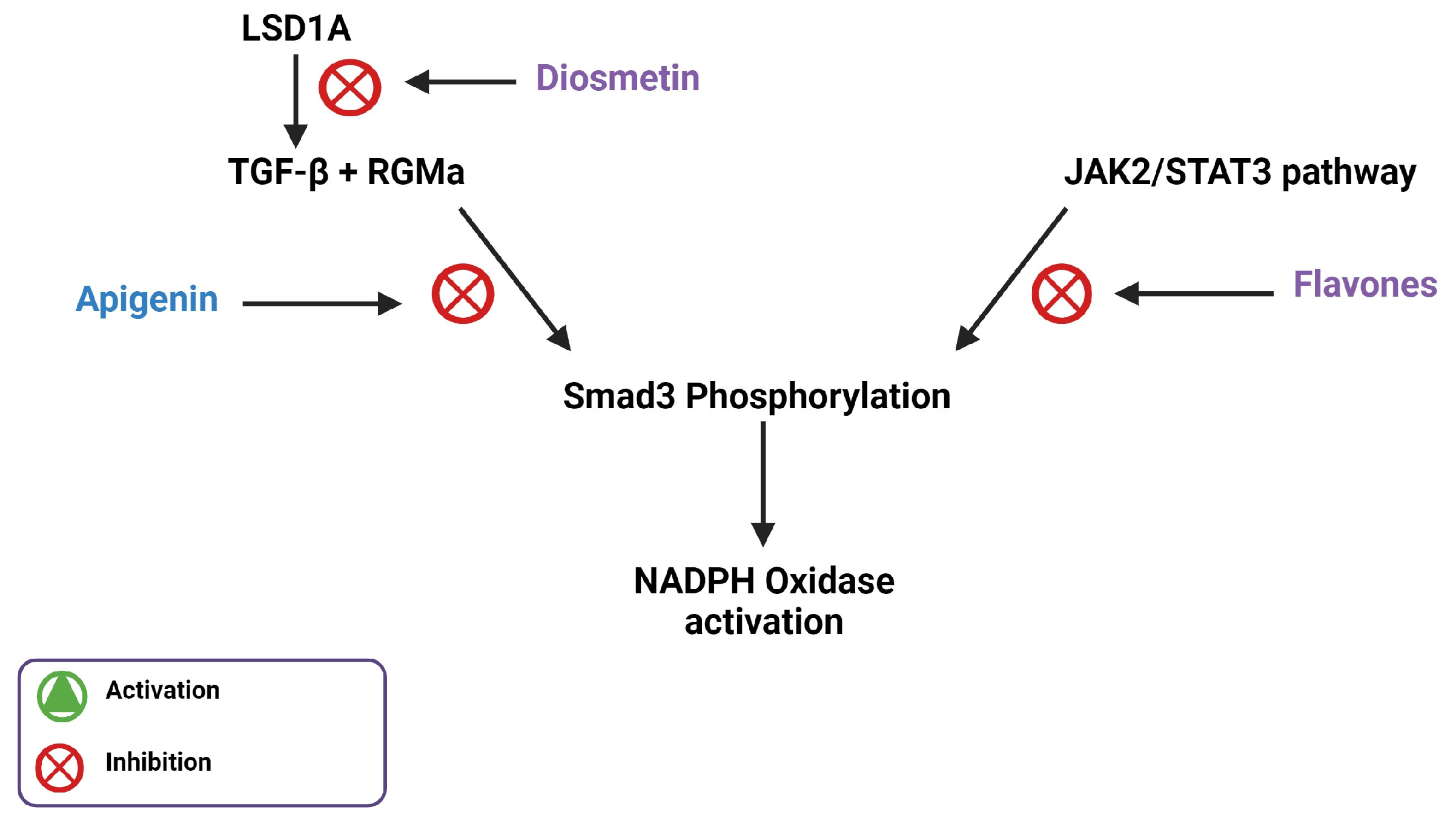
4. Flavones Affect Mitochondrial Biogenesis, Dynamics, and Energetics
References
- GBD 2013 Risk Factors Collaborators; Forouzanfar, M.H.; Alexander, L.; Anderson, H.R.; Bachman, V.F.; Biryukov, S.; Brauer, M.; Burnett, R.; Casey, D.; Coates, M.; et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 2287–2323.
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, e13–e115.
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357.
- Elijovich, F.; Laffer, C.L.; Sahinoz, M.; Pitzer, A.; Ferguson, J.F.; Kirabo, A. The Gut Microbiome, Inflammation, and Salt-Sensitive Hypertension. Curr. Hypertens. Rep. 2020, 22, 79.
- Patrick, D.M.; Van Beusecum, J.P.; Kirabo, A. The role of inflammation in hypertension: Novel concepts. Curr. Opin. Physiol. 2021, 19, 92–98.
- Barbaro, N.R.; Foss, J.D.; Kryshtal, D.O.; Tsyba, N.; Kumaresan, S.; Xiao, L.; Mernaugh, R.L.; Itani, H.A.; Loperena, R.; Chen, W.; et al. Dendritic Cell Amiloride-Sensitive Channels Mediate Sodium-Induced Inflammation and Hypertension. Cell Rep. 2017, 21, 1009–1020.
- Pitzer, A.; Elijovich, F.; Laffer, C.L.; Ertuglu, L.A.; Sahinoz, M.; Saleem, M.; Krishnan, J.; Dola, T.; Aden, L.A.; Sheng, Q.; et al. DC ENaC-Dependent Inflammasome Activation Contributes to Salt-Sensitive Hypertension. Circ. Res. 2022, 131, 328–344.
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47.
- Spiegel, M.; Andruniów, T.; Sroka, Z. Flavones’ and Flavonols’ Antiradical Structure–Activity Relationship—A Quantum Chemical Study. Antioxidants 2020, 9, 461.
- Premathilaka, R.; Rashidinejad, A.; Golding, M.; Singh, J. Oral delivery of hydrophobic flavonoids and their in-corporation into functional foods: Opportunities and challenges. Food Hydrocolloid 2022, 128, 107567.
- Ahmad, T.; Javed, A.; Khan, T.; Althobaiti, Y.S.; Ullah, A.; Almutairi, F.M.; Shah, A.J. Investigation into the Antihypertensive Effects of Diosmetin and Its Underlying Vascular Mechanisms Using Rat Model. Pharmaceuticals 2022, 15, 951.
- Zuiter, A. Proanthocyanidin: Chemistry and Biology: From Phenolic Compounds to Proanthocyanidins. Chem. Mol. Sci. Chem. Eng. 2014, 1, 29.
- Wang, T.-Y.; Li, Q.; Bi, K.-S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23.
- Areias, F.M.; Rego, A.C.; Oliveira, C.R.; Seabra, R.M. Antioxidant effect of flavonoids after ascorbate/Fe2+-induced oxidative stress in cultured retinal cells. Biochem. Pharmacol. 2001, 62, 111–118.
- Bondi, C.D.; Manickam, N.; Lee, D.Y.; Block, K.; Gorin, Y.; Abboud, H.E.; Barnes, J.L. NAD(P)H Oxidase Mediates TGF-beta1–Induced Activation of Kidney Myofibroblasts. J. Am. Soc. Nephrol. 2010, 21, 93–102.
- Saleem, M.; Aden, L.; Pitzer, A.; Ishimwe, J.; Ertuglu, L.; Laffer, C.; Wanjalla, C.; Pakala, S.; Kastner, P.; Park, J.; et al. Dendritic cell-specific SMAD3, downstream of JAK2, contributes to inflammation and salt-sensitivity of blood pressure in humans and mice. Physiology 2023, 38, 5734894.
- Qiu, X.; Zhu, J.; Sun, Y.; Fan, K.; Yang, D.-R.; Li, G.; Yang, G.; Chang, C. TR4 nuclear receptor increases prostate cancer invasion via decreasing the miR-373-3p expression to alter TGFbetaR2/p-Smad3 signals. Oncotarget 2015, 6, 15397–15409.
- Li, L.H.; Lu, B.; Wu, H.K.; Zhang, H.; Yao, F.F. Apigenin inhibits TGF-beta1-induced proliferation and migration of airway smooth muscle cells. Int. J. Clin. Exp. Pathol. 2015, 8, 12557–12563.
- Ning, R.; Chen, G.; Fang, R.; Zhang, Y.; Zhao, W.; Qian, F. Diosmetin inhibits cell proliferation and promotes apoptosis through STAT3/c-Myc signaling pathway in human osteosarcoma cells. Biol. Res. 2021, 54, 40.
- Ren, Q.; Wang, B.; Guo, F.; Huang, R.; Tan, Z.; Ma, L.; Fu, P. Natural Flavonoid Pectolinarigenin Alleviated Hyperuricemic Nephropathy via Suppressing TGFbeta/SMAD3 and JAK2/STAT3 Signaling Pathways. Front. Pharmacol. 2021, 12, 792139.
- Zhang, R.; Wu, Y.; Xie, F.; Zhong, Y.; Wang, Y.; Xu, M.; Feng, J.; Charish, J.; Monnier, P.P.; Qin, X. RGMa mediates reactive astrogliosis and glial scar formation through TGFbeta1/Smad2/3 signaling after stroke. Cell Death Differ. 2018, 25, 1503–1516.
- Arango, D.; Morohashi, K.; Yilmaz, A.; Kuramochi, K.; Parihar, A.; Brahimaj, B.; Grotewold, E.; Doseff, A.I. Molecular basis for the action of a dietary flavonoid revealed by the comprehensive identification of apigenin human targets. Proc. Natl. Acad. Sci. USA 2013, 110, E2153–E2162.
- Gao, H.-L.; Yu, X.-J.; Hu, H.-B.; Yang, Q.-W.; Liu, K.-L.; Chen, Y.-M.; Zhang, Y.; Zhang, D.-D.; Tian, H.; Zhu, G.-Q.; et al. Apigenin Improves Hypertension and Cardiac Hypertrophy Through Modulating NADPH Oxidase-Dependent ROS Generation and Cytokines in Hypothalamic Paraventricular Nucleus. Cardiovasc. Toxicol. 2021, 21, 721–736.
- Sun, Y.; Zhou, Q.-M.; Lu, Y.-Y.; Zhang, H.; Chen, Q.-L.; Zhao, M.; Su, S.-B. Resveratrol Inhibits the Migration and Metastasis of MDA-MB-231 Human Breast Cancer by Reversing TGF-beta1-Induced Epithelial-Mesenchymal Transition. Molecules 2019, 24, 1131.
- Ashrafizadeh, M.; Najafi, M.; Orouei, S.; Zabolian, A.; Saleki, H.; Azami, N.; Sharifi, N.; Hushmandi, K.; Zarrabi, A.; Ahn, K.S. Resveratrol Modulates Transforming Growth Factor-Beta (TGF-beta) Signaling Pathway for Disease Therapy: A New Insight into Its Pharmacological Activities. Biomedicines 2020, 8, 261.
- Gilson, M.K.; Liu, T.; Baitaluk, M.; Nicola, G.; Hwang, L.; Chong, J. BindingDB in 2015: A public database for medicinal chemistry, computational chemistry and systems pharmacology. Nucleic Acids Res. 2016, 44, D1045–D1053.
- Lin, T.; Ponn, A.; Hu, X.; Law, B.K.; Lu, J. Requirement of the histone demethylase LSD1 in Snai1-mediated transcriptional repression during epithelial-mesenchymal transition. Oncogene 2010, 29, 4896–4904.
- McDonald, O.G.; Wu, H.; Timp, W.; Doi, A.; Feinberg, A.P. Genome-scale epigenetic reprogramming during epithelial-to-mesenchymal transition. Nat. Struct. Mol. Biol. 2011, 18, 867–874.
- Kim, D.; Kim, K.I.; Baek, S.H. Roles of lysine-specific demethylase 1 (LSD1) in homeostasis and diseases. J. Biomed. Sci. 2021, 28, 41.
- Muthusamy, B.P.; Budi, E.H.; Katsuno, Y.; Lee, M.K.; Smith, S.M.; Mirza, A.M.; Akhurst, R.J.; Derynck, R. ShcA Protects against Epithelial–Mesenchymal Transition through Compartmentalized Inhibition of TGF-beta-Induced Smad Activation. PLoS Biol. 2015, 13, e1002325.
- Dikalov, S.I.; Dikalova, A.E. Crosstalk Between Mitochondrial Hyperacetylation and Oxidative Stress in Vascular Dysfunction and Hypertension. Antioxid. Redox Signal. 2019, 31, 710–721.
- Valdameri, G.; Herrerias, T.; Carnieri, E.G.S.; Cadena, S.M.S.C.; Martinez, G.R.; Rocha, M.E.M. Importance of the core structure of flavones in promoting inhibition of the mitochondrial respiratory chain. Chem. Interact. 2010, 188, 52–58.
- Dai, F.; Li, Q.; Wang, Y.; Ge, C.; Feng, C.; Xie, S.; He, H.; Xu, X.; Wang, C. Design, Synthesis, and Biological Evaluation of Mitochondria-Targeted Flavone–Naphthalimide–Polyamine Conjugates with Antimetastatic Activity. J. Med. Chem. 2017, 60, 2071–2083.
- Zheng, R.-F.; Du, Y.-W.; Zeng, C.; Wang, H.-F.; Xing, J.-G.; Xu, M. Total flavones of Dracocephalum moldavica L. protect astrocytes against H(2)O(2)-induced apoptosis through a mitochondria-dependent pathway. BMC Complement. Med. Ther. 2020, 20, 78.
- Serino, E.; Chahardoli, A.; Badolati, N.; Sirignano, C.; Jalilian, F.; Mojarrab, M.; Farhangi, Z.; Rigano, D.; Stornaiuolo, M.; Shokoohinia, Y.; et al. Salvigenin, a Trimethoxylated Flavone from Achillea wilhelmsii C. Koch, Exerts Combined Lipid-Lowering and Mitochondrial Stimulatory Effects. Antioxidants 2021, 10, 1042.
- Tsutsumi, R.; Yoshida, T.; Nii, Y.; Okahisa, N.; Iwata, S.; Tsukayama, M.; Hashimoto, R.; Taniguchi, Y.; Sakaue, H.; Hosaka, T.; et al. Sudachitin, a polymethoxylated flavone, improves glucose and lipid metabolism by increasing mitochondrial biogenesis in skeletal muscle. Nutr. Metab. 2014, 11, 32.
- Xiong, L.-G.; Chen, Y.-J.; Tong, J.-W.; Gong, Y.-S.; Huang, J.-A.; Liu, Z.-H. Epigallocatechin-3-gallate promotes healthy lifespan through mitohormesis during early-to-mid adulthood in Caenorhabditis elegans. Redox Biol. 2018, 14, 305–315.
- Chen, Q.; Ruan, D.; Shi, J.; Du, D.; Bian, C. The multifaceted roles of natural products in mitochondrial dysfunction. Front. Pharmacol. 2023, 14, 1093038.
- Wang, C.C.; Ho, Y.H.; Hung, C.F.; Kuo, J.R.; Wang, S.J. Xanthohumol, an active constituent from hope, affords protection against kainic acid-induced excitotoxicity in rats. Neurochem. Int. 2020, 133, 104629.
- Li, Y.; Dang, Q.; Li, Z.; Han, C.; Yang, Y.; Li, M.; Li, P. Restoration of Mitochondrial Function Is Essential in the Endothelium-Dependent Vasodilation Induced by Acacetin in Hypertensive Rats. Int. J. Mol. Sci. 2022, 23, 11350.
- Li, H.-L.; Wei, Y.-Y.; Li, X.-H.; Zhang, S.-S.; Zhang, R.-T.; Li, J.-H.; Ma, B.-W.; Shao, S.-B.; Lv, Z.-W.; Ruan, H.; et al. Diosmetin has therapeutic efficacy in colitis regulating gut microbiota, inflammation, and oxidative stress via the circ-Sirt1/Sirt1 axis. Acta Pharmacol. Sin. 2022, 43, 919–932.
- Wang, C.; Li, S.; Ren, H.; Sheng, Y.; Wang, T.; Li, M.; Zhou, Q.; He, H.; Liu, C. Anti-Proliferation and Pro-Apoptotic Effects of Diosmetin via Modulating Cell Cycle Arrest and Mitochondria-Mediated Intrinsic Apoptotic Pathway in MDA-MB-231 Cells. Med. Sci. Monit. 2019, 25, 4639–4647.
- Mo, G.L.; He, Y.; Zhang, X.Q.; Lei, X.; Luo, Q. Diosmetin exerts cardioprotective effect on myocardial ischaemia injury in neonatal rats by decreasing oxidative stress and myocardial apoptosis. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1713–1722.
