You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Tasneem Solomon-Rakiep.
Chronic hepatitis B caused by persistent infection with the hepatitis B virus (HBV) is a major public health threat in endemic regions like the World Health Organization (WHO) Africa region. Chronic infection with HBV poses a 15–25% lifetime risk of acquiring liver cirrhosis or hepatocellular carcinoma. This is inversely proportionate to the age of acquisition and in the absence of interventions, ~90% of babies born to mothers testing positive for the hepatitis B surface (HBsAg) or e (HBeAg) antigens will develop chronic infection, raising significant global public health concern.
- Africa
- birth-dose
- hepatitis B
- health systems
- vaccine
1. Current Knowledge on the Risk of HBV MTCT in the WHO Africa Region
1. Current Knowledge on the Risk of Hepatitis B Virus Mother-To-Child Transmission in the World Health Organization Africa Region
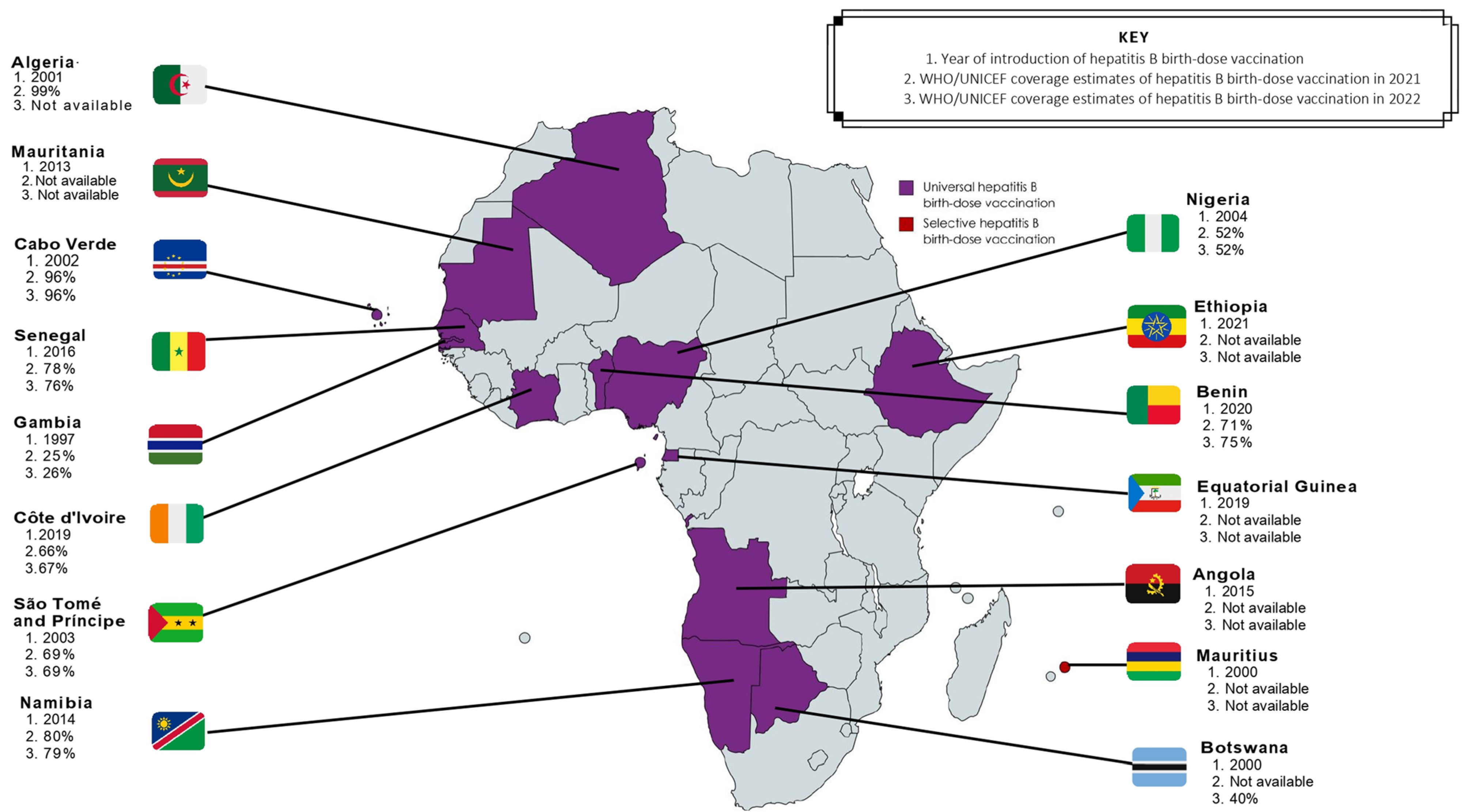
Areas of high endemicity are defined as having an HBsAg prevalence of ≥8%, moderate endemicity between 2–7% and low endemicity of <2% in the general population [2,6][1][2]. In Africa, an estimated HBsAg prevalence of 7.5% is recorded, and Africa is considered home to approximately 28% of the world’s 296 million chronic carriers [16,17][3][4]. Prior to regional adoption of routine hepatitis B vaccination, >95% of all infections occurring in infancy were associated with horizontal transmission [3][5]. In recent years, a growing risk of vertical transmission (>5%) has been observed and is cited to be influenced by the HIV epidemic, as subsequently discussed in Section 3.1.2 [3,19]. Despite this, strategies employed to control the burden of hepatitis B depend almost solely on the 1992 WHO recommendation of universal hepatitis B infant vaccination commencing at 4 or 6 weeks of life [5][6]. In the absence of strategies targeting the interruption of vertical transmission, the cycle of chronic infection continues to fuel the morbidity and mortality [2,15][1][7]. The immaturity of the neonatal immune system increases the risk of viral replication and is suggested as the reason for delayed clearance of HBeAg [44][8], whereas acute infections in immunocompetent adults are likely to be cleared [15][7]. In a meta-analysis of 15 articles investigating HBV infection among women in sub-Saharan Africa, a total of 14,239 women were screened for HBsAg and a further 951 for HBeAg [45][9]. Among these studies, HBeAg positivity was shown to increase the risk of vertical transmission to 38.3% compared to 4.8% in HBeAg negative women [45][9]. Therefore, assessing the increased risk of HBV MTCT, the influence of HIV co-infection, and the strategies available for effective prevention of HBV MTCT in Africa is essential.
3.2. Status of HBV MTCT Mitigation Strategies in the WHO Africa Region
2. Status of Hepatitis B Virus Mother-To-Child Transmission Mitigation Strategies in the World Health Organization Africa Region
Safe and effective strategies are available for prevention of HBV MTCT to neonates and infants from as early as the in-utero stage, as shown in Figure 1. Antenatal screening has the advantage of identifying those at risk for HBV MTCT who can then be timely linked to appropriate care such as HBV-active antiviral prophylaxis [47][10]. During antenatal visits, pregnant women should also be provided with information on HBV infection, the lifetime risk for chronic liver disease associated with HBV MTCT, and the prevention strategies available to them and their babies including hepatitis B birth-dose vaccination [23,47][10][11]. Unfortunately, for some African countries, antenatal screening for HBV infection can be expensive and impractical if laboratory facilities are not situated close to antenatal clinics [23,47][10][11]. Despite this, the feasibility of antenatal screening for HBV infection has been proven in South Africa [15][7], and cost-effectiveness has been demonstrated in Namibia as part of the national HBV MTCT prevention package [3][5]. Maximum gains can be achieved if HBV antenatal screening is integrated with existing HIV and syphilis point-of-care testing infrastructure [15,55][7][12].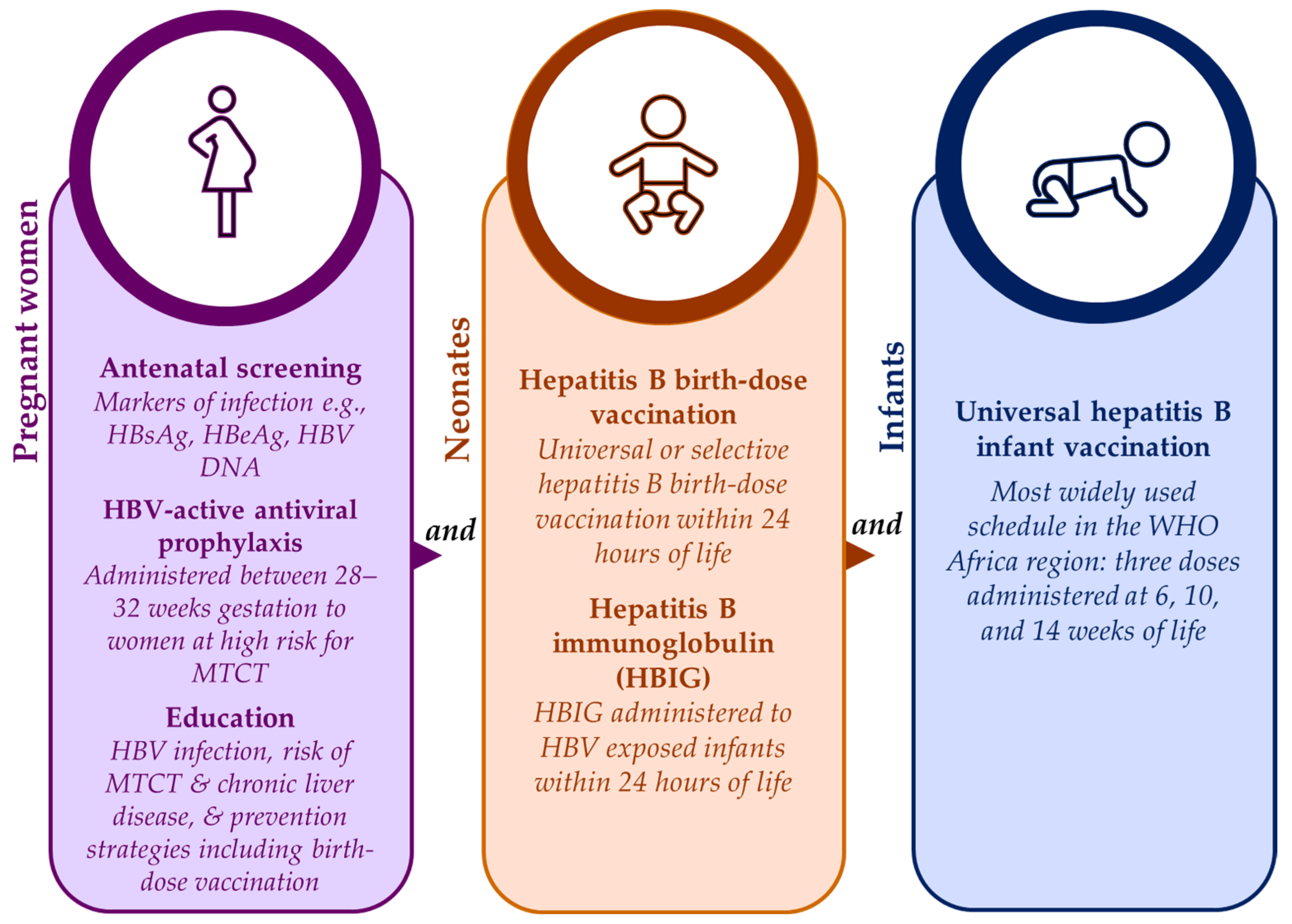
References
- Breakwell, L.; Tevi-Benissan, C.; Childs, L.; Mihigo, R.; Tohme, R. The Status of Hepatitis B Control in the African Region. Pan Afr. Med. J. 2017, 27, 17.
- Sadoh, A.; Sadoh, W. Does Nigeria Need the Birth Dose of the Hepatitis B Vaccine? Niger. J. Paediatr. 2014, 41, 104–109.
- World Health Organization. Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections, 2021. Accountability for the Global Health Sector Strategies 2016–2021: Actions for Impact; World Health Organization: Geneva, Switzerland, 2021.
- World Health Organization; Annex, W. 1. Key Data at a Glance. In Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections, 2021. Accountability for the Global Health Sector Strategies 2016–2021: Actions for Impact; World Health Organization: Geneva, Switzerland, 2021.
- Tamandjou Tchuem, C.R.; Andersson, M.I.; Wiysonge, C.S.; Mufenda, J.; Preiser, W.; Cleary, S. Prevention of Hepatitis B Mother-to-Child Transmission in Namibia: A Cost-Effectiveness Analysis. Vaccine 2021, 39, 3141–3151.
- Howell, J.; Lemoine, M.; Thursz, M. Prevention of Materno-Foetal Transmission of Hepatitis B in Sub-Saharan Africa: The Evidence, Current Practice and Future Challenges. J. Viral Hepat. 2014, 21, 381–396.
- Chotun, N.; Preiser, W.; van Rensburg, C.J.; Fernandez, P.; Theron, G.B.; Glebe, D.; Andersson, M.I. Point-of-Care Screening for Hepatitis B Virus Infection in Pregnant Women at an Antenatal Clinic: A South African Experience. PLoS ONE 2017, 12, e0181267.
- Shimakawa, Y.; Bottomley, C.; Njie, R.; Mendy, M. The Association between Maternal Hepatitis B e Antigen Status, as a Proxy for Perinatal Transmission, and the Risk of Hepatitis B e Antigenaemia in Gambian Children. BMC Public Health 2014, 14, 532.
- Keane, E.; Funk, A.L.; Shimakawa, Y. Systematic Review with Meta-Analysis: The Risk of Mother-to-Child Transmission of Hepatitis B Virus Infection in Sub-Saharan Africa. Aliment. Pharmacol. Ther. 2016, 44, 1005–1017.
- Tamandjou Tchuem, C.R.; Maponga, T.G.; Chotun, N.; Preiser, W.; Andersson, M.I. Is Hepatitis B Birth Dose Vaccine Needed in Africa? Pan Afr. Med. J. 2017, 27, 18.
- Diale, Q.; Pattinson, R.; Chokoe, R.; Masenyetse, L.; Mayaphi, S. Antenatal Screening for Hepatitis B Virus in HIV-Infected and Uninfected Pregnant Women in the Tshwane District of South Africa. S. Afr. Med. J. 2016, 106, 97–100.
- World Health Organization Prevention of Mother-to-Child Transmission of Hepatitis B Virus: Guidelines on Antiviral Prophylaxis in Pregnancy; World Health Organization: Geneva, Switzerland, 2020; ISBN 9789240002708.
- Chotun, N.; Nel, E.; Cotton, M.F.; Preiser, W.; Andersson, M.I. Hepatitis B Virus Infection in HIV-Exposed Infants in the Western Cape, South Africa. Vaccine 2015, 33, 4618–4622.
- Anderson, S.; Harper, L.M.; Dionne-Odom, J.; Halle-Ekane, G.; Tita, A.T.N.; Dionne-Odom, J.; Halle-Ekane, G. A Decision Analytic Model for Prevention of Hepatitis B Virus Infection in Sub-Saharan Africa Using Birth-Dose Vaccination. Int. J. Gynecol. Obstet. 2018, 141, 126–132.
- Maponga, T.G.; Matteau Matsha, R.; Morin, S.; Scheibe, A.; Swan, T.; Andrieux-Meyer, I.; Spearman, C.W.; Klein, M.B.; Rockstroh, J.K. Highlights from the 3rd International HIV/Viral Hepatitis Co-Infection Meeting-HIV/Viral Hepatitis: Improving Diagnosis, Antiviral Therapy and Access. Hepatol. Med. Policy 2017, 2, 8.
- Terrault, N.A.; Bzowej, N.H.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Murad, M.H. AASLD Guidelines for Treatment of Chronic Hepatitis B. Hepatology 2016, 63, 261–283.
- Lampertico, P.; Agarwal, K.; Berg, T.; Buti, M.; Janssen, H.L.A.; Papatheodoridis, G.; Zoulim, F.; Tacke, F. EASL 2017 Clinical Practice Guidelines on the Management of Hepatitis B Virus Infection. J. Hepatol. 2017, 67, 370–398.
- Jooste, P.; van Zyl, A.; Adland, E.; Daniels, S.; Hattingh, L.; Brits, A.; Wareing, S.; Goedhals, D.; Jeffery, K.; Andersson, M.; et al. Screening, Characterisation and Prevention of Hepatitis B Virus (HBV) Co-Infection in HIV-Positive Children in South Africa. J. Clin. Virol. 2016, 85, 71–74.
- Ekra, D.; Herbinger, K.-H.; Konate, S.; Leblond, A.; Fretz, C.; Cilote, V.; Douai, C.; Da Silva, A.; Gessner, B.D.; Chauvin, P. A Non-Randomized Vaccine Effectiveness Trial of Accelerated Infant Hepatitis B Immunization Schedules with a First Dose at Birth or Age 6 Weeks in Côte d’Ivoire. Vaccine 2008, 26, 2753–2761.
- Dionne-Odom, J.; Njei, B.; Tita, A. Elimination of Vertical Transmission of Hepatitis B in Africa: A Review of Available Tools and New Opportunities. Clin. Ther. 2018, 40, 1255–1267.
- Hipgrave, D.B.; Maynard, J.E.; Biggs, B.-A. Improving Birth Dose Coverage of Hepatitis B Vaccine. Bull. World Health Organ. 2006, 84, 65–71.
- Dadari, I.K.; Zgibor, J.C. How the Use of Vaccines Outside the Cold Chain or in Controlled Temperature Chain Contributes to Improving Immunization Coverage in Low- and Middle-Income Countries (LMICs): A Scoping Review of the Literature. J. Glob. Health 2021, 11, 4004.
- Thompson, P.; Morgan, C.E.; Ngimbi, P.; Mwandagalirwa, K.; Ravelomanana, N.L.R.; Tabala, M.; Fathy, M.; Kawende, B.; Muwonga, J.; Misingi, P.; et al. Arresting Vertical Transmission of Hepatitis B Virus (AVERT-HBV) in Pregnant Women and Their Neonates in the Democratic Republic of the Congo: A Feasibility Study. Lancet Glob. Health 2021, 9, e1600–e1609.
- United Nations Children’s Fund Immunization Coverage Estimates Data Visualization. Available online: https://data.unicef.org/resources/immunization-coverage-estimates-data-visualization/ (accessed on 15 August 2023).
- World Health Organization. Global Health Sector Strategy on Viral Hepatitis 2016–2021. Towards Ending Viral Hepatitis; World Health Organization: Geneva, Switzerland, 2016; ISBN 9789241503501.
- Kabore, J. Hepatitis B Birth Dose (HepB BD) Vaccination in the WHO African Region. In Proceedings of the Building a Community of Practice to Assist hepB Birth Dose Introduction in African Countries, Online, 17–18 March 2021; Coalition for Global Hepatitis Elimination: Decatur, GA, USA, 2021.
- Njuguna, H. HepB-BD and Infant HepB3 Coverage Status in Africa. In Proceedings of the Hepatitis B Birth Dose in the African Region: Bridging Ccience and Advocacy to Eliminate Mother-to-Child Transmission of HBV, Online, 22 August 2022.
- Bassoum, O.; Kimura, M.; Dia, A.T.; Lemoine, M.; Shimakawa, Y. Coverage and Timeliness of Birth Dose Vaccination in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. Vaccines 2020, 8, 301.
- World Health Organization. Hepatitis B Vaccination Coverage. Available online: https://immunizationdata.who.int/pages/coverage/HEPB.html (accessed on 15 August 2023).
- World Health Organization. Global Hepatitis Report 2017; World Health Organization: Geneva, Switzerland, 2017.
- Sanou, A.M.; Ilboudo, A.K.; Meda, Z.C.; Togozia, A.; Coulibaly, A.; Sagna Tani, A.C.; Dramane, K.; Tarnagda, Z. Hepatitis B Vaccination in Burkina Faso: Prevalence of HBsAg Carriage and Immune Response in Children in the Western Region. J. Infect. Dev. Ctries. 2018, 12, 1002–1008.
- Apiung, T.; Ndanu, T.A.; Mingle, J.A.A.; Sagoe, K.W.C. Hepatitis B Virus Surface Antigen and Antibody Markers in Children at a Major Paediatric Hospital after the Pentavalent DTP-HBV-Hib Vaccination. Ghana Med. J. 2017, 51, 13–19.
- Metodi, J.; Aboud, S.; Mpembeni, R.; Munubhi, E. Immunity to Hepatitis B Vaccine in Tanzanian Under-5 Children. Ann. Trop. Paediatr. 2010, 30, 129–136.
- Breakwell, L.; Marke, D.; Kaiser, R.; Tejada-Strop, A.; Pauly, M.D.; Jabbi, S.; Yambasu, S.; Kabore, H.J.; Stewart, B.; Sesay, T.; et al. Assessing the Impact of the Routine Childhood Hepatitis B Immunization Program and the Need for Hepatitis B Vaccine Birth Dose in Sierra Leone, 2018. Vaccine 2022, 40, 2741–2748.
- Andersson, M.I.; Maponga, T.G.; Ijaz, S.; Barnes, J.; Theron, G.B.; Meredith, S.A.; Preiser, W.; Tedder, R.S. The Epidemiology of Hepatitis B Virus Infection in HIV-Infected and HIV-Uninfected Pregnant Women in the Western Cape, South Africa. Vaccine 2013, 31, 5579–5584.
More
