Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Chin-Kun Wang and Version 2 by Rita Xu.
Phytochemicals are plant secondary metabolites that show health benefits for humans due to their bioactivity. There is a huge variety of phytochemicals that have already been identified, and these compounds can act as antimicrobial and neuroprotection agents. Due to their anti-microbial activity and neuroprotection, several phytochemicals might have the potency to be used as natural therapeutic agents, especially for
Helicobacter pylori
infection and neurodegenerative disease, which have become a global health concern nowadays.
- phytochemical
- neurodegenerative disease
- Helicobacter pylori
1. Introduction
Helicobacter pylori infection is one of the global health problems. More than 50% of the population in the world is affected, mostly in developing countries [1]. H. pylori attaches to the human stomach; induces a change in gastric physiology; and is highly associated with gastric ulcers, which further progress into gastric cancer [2]. H. pylori can colonize and infect gastric tissue because of virulent factors such as urease, lipopolysaccharide (LPS), vacuolating cytotoxin A (VacA), cytotoxin-associated gene A (CagA), and some others [3]. Until now, the main treatment for H. pylori infection is to use the combination of two antibiotics together with a bismuth compound and/or antacid agent such proton pump inhibitor (PPI), which is called quadruple therapy and provides an eradication rate of more than 80% [4]. The usage of antibiotics in H. pylori offers another concern of some side effects as well as antibiotic resistance problems [5]. Recent studies show that H. pylori infection contributes to the progression of neurodegenerative diseases.
Neurodegenerative diseases (NDs) are disorders that affect the central nervous system and that are mostly caused by neuronal cell death, which causes impairment of the cognitive and motoric system [6]. There are many risk factors associated with ND progression, but its pathogenesis has still been unclear until now. Several diseases are classified as NDs such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and Huntington’s disease (HD) [7]. These diseases have different characteristics, but most of them share the same hallmarks, which are neuronal cell death and neuroinflammation [8][9][8,9]. Until now, ND has been classified as an incurable disease, and medication might have a small impact on improving a patient’s condition [10]. Evidence of nutraceuticals on NDs is still deficient, in terms of whether together with normal medication, they could provide better effects on subjects with NDs.
There are several hypotheses about the possible connection between H. pylori infection and NDs. H. pylori affect the absorption of folate and vitamin B-12, which causes the elevation of homocysteine level and induces neurotoxicity. Furthermore, H. pylori cross the blood–brain barrier and induce amyloid deposition in the brain [11]. Another study showed that the outer membrane vesicles of H. pylori that were injected into mice altered astrocyte function and induced neuronal damage in the mouse brain [12]. In PD, it showed that H. pylori infection is related to the progression of the disease and increases the requirement of medication for PD [13]. This evidence might provide a clue about the connection between neurodegenerative disease and H. pylori infection.
Phytochemicals are secondary metabolites of plants, which are non-nutritive bio-active compounds synthesized for natural defenses of the plant against pests [14][15][16][17][14,15,16,17]. Phytochemicals found in fruits, vegetables, nuts, and grains provide health benefits. Many studies showed that phytochemicals from different natural sources act as antibacterial agents or neuroprotective agents [17][18][17,18]. Önem et al. showed that stalk extracts from two different cultivars of Prunus avium L. inhibited Gram-positive bacteria and reduced the biofilm formation of bacteria by up to 75% [19]. Li et al. showed that supplementation of proanthocyanidins (PAC)-rich cranberry juice (44 mg of PAC per portion) twice a day for 8 weeks significantly reduced H. pylori infection [20]. Desideri et al. reported that high intake (990 mg/day) of dietary flavonols from cocoa for 8 weeks significantly improved cognitive function in mild cognitive impairment subjects compared to those of low intake (45 mg/day) of cocoa flavonols [21]. Kent et al. pointed out that the intervention of anthocyanin-rich cherry juice for 12 weeks significantly improved verbal fluency and short-term and long-term memory in subjects with dementia [22]. Past studies showed that phytochemicals can be used as drug alternatives to treat H. pylori and neurodegenerative disease and reduce the risk of antibiotic resistance and complications due to the medication.
2. H. pylori
H. pylori is a Gram-negative spiral bacterium that is found in the human stomach and is associated with gastric ulcer and advanced gastric cancer [2][23][24][2,23,24]. The infection of H. pylori shows no symptoms in most cases, but it depends on the immune response of the individual and the severity of the syndrome. Most symptoms of H. pylori infection are correlated with the gastric ulcer and inflammation in the gastric tissue [25]. H. pylori is considered a special bacterium due to the virulence factors (Figure 1) that help to colonize in the human stomach, such as VacA, CagA, urease, LPS, and different kinds of adhesins [3].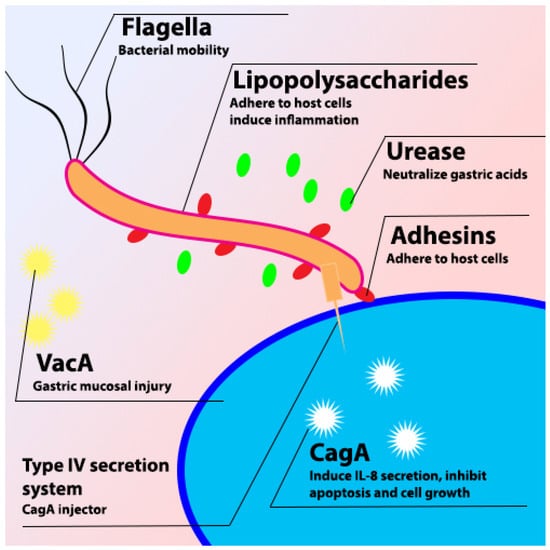
Figure 1. Schematic diagram of H. pylori virulence factor.
2.1. VacA
VacA is one of the virulence factors possessed by H. pylori. VacA is the major toxic 88 kDa protein that is secreted from H. pylori through type V auto transport secretion system (T5SS), which binds to the host cell and causes vacuolation of the cell [26]. VacA plays an important role in the colonization of H. pylori in the gastric mucosa, stimulating the autophagy pathway in cells and disrupting lysosomal trafficking that causes the accumulation of dysfunctional autophagosomes and the formation of large intracellular vacuoles to promote the intracellular survival of H. pylori [27]. Furthermore, it induces different responses in infected cells such as vacuole formation, cytochrome c release, and forming channels in the mitochondria [28]. It also induces cell apoptosis because of increasing cytochrome c release from mitochondria. Cytochrome c combines with Apaf-1 and caspase-9 to stimulate the production of caspase-3 and caspase-7, resulting in cell apoptosis [29][30][29,30]. VacA can disrupt the tight junction to alter the tissue structure and increase the adhesion of H. pylori to epithelial cells [26][31][32][26,31,32].2.2. CagA
CagA is a 120 to 145 kDa protein that can be injected into the host cell by using a type IV secretion system (T4SS) after the adhesion of H. pylori to the host cell [33]. H. pylori is divided into two different strains based on the presence of CagA: CagA-positive and CagA-negative strains. The cagA-positive strain is more virulent than the CagA-negative strain and is associated with higher gastric inflammation [34]. The effects of CagA on the host cell are independent of the phosphorylation process. The most noticeable is to disrupt the cell’s tight junction and induce cell morphology changes [35]. Non-phosphorylated CagA also can activate serum response elements further affect the cell cycle and induce inflammatory response [36].2.3. Urease
Urease is a 550 kDa molecule consisting of UreA and UreB subunits. Urease plays a crucial role in the survival of H. pylori in the human stomach. H. pylori produces urease in acidic conditions, which breaks down urea and releases ammonia to neutralize the acidic condition in the human stomach [37]. pH increases in the stomach alter the protective mucous layer and also dysregulate the gastric epithelial cell tight junction [38].2.4. Pathophysiology of H. pylori Infection
H. pylori infection is associated with chronic gastritis, gastric ulcers, and gastric cancer [1]. Development of gastric problems due to H. pylori infection is mostly caused by alteration of the gastric physiology and microenvironment, which induces an immune response from the human body [39]. This immune response is due to the activity of the H. pylori virulence factors such as CagA, VacA, and urease, and the response might be different depending on the age [40][41][40,41]. Immune response due to H. pylori infection is mediated by Toll-like receptors (TLRs) and microRNA, which can promote or suppress the immune response [42]. After reaching the stomach, H. pylori move to the mucous layer to evade the acid condition with the help of urease and attach to epithelial cells with the help of different kinds of adhesins such as BabA, SabA, AlpA/B, HopZ, and OipA [43]. After binding to the host cell, H. pylori inject different kinds of toxins such as CagA and VacA, depending on the strain, being able to induce inflammatory responses and upregulation of pro-inflammatory cytokines secretion [1].2.5. Diagnosis and Treatment
There are various methods to identify and diagnose H. pylori. The invasive tests are based on gastric biopsy and peripheral samples to check the infection of H. pylori. On the other hand, the non-invasive method is to use the Urea Breath Test (UBT) C13 or C14 [1]. UBT is one of the most popular methods to diagnose H. pylori infection due to its high sensitivity and is considered the gold standard of the non-invasive method [1]. UBT is based on the reaction of C13-labeled urea and bacterial urease secreted from H. pylori, which produce ammonia (NH3) and C13-labeled carbon dioxide in the breath. The concentration of the C13 isotope is determined by using gas chromatography and considered positive if the Delta Over Baseline (DOB) value is ≥4‰ [44][45][46][44,45,46]. Treatment of H. pylori infection is usually conducted by using antibiotics and combination with PPI and/or with bismuth. Monotherapy (single antibiotic) was used in the past, but the efficacy was poor. The addition of PPI is used as dual therapy in some countries. Overuse of antibiotics induces the mutation and resistance of H. pylori and produces some side effects such as dizziness, vomiting, and allergy [47][48][47,48].3. Connection between H. pylori Infection and Neurodegenerative Diseases
There are several risk factors correlated with NDs, especially AD, such as age, traumatic head injury, depression, cardiovascular and cerebrovascular disease, and smoking. Recent studies showed that AD is also associated with H. pylori infection [49][50][91,92]. H. pylori is known to infect and cause several health problems in the human gastrointestinal (GI) tract such as gastric ulcers, gastritis, and gastric cancer [51][93]. In rare cases, a manifestation of extra gastric disease due to H. pylori infection might occur with several possible mechanism (Figure 2) and need to be taken into consideration. The extra gastric manifestation due to H. pylori infection, especially neurological problems, might occur through alteration of the gut–brain axis (GBA) [52][94]. The GBA is a bidirectional communication between the central nervous system (CNS) and enteric nervous system that integrates and links the gut and intestinal function with the central nervous system [53][54][55][95,96,97]. GBA modulates the GI function by regulating the GI immune system, mucosal change, and intestinal microbiome in response to stress and emotional and environmental influences [52][56][94,98].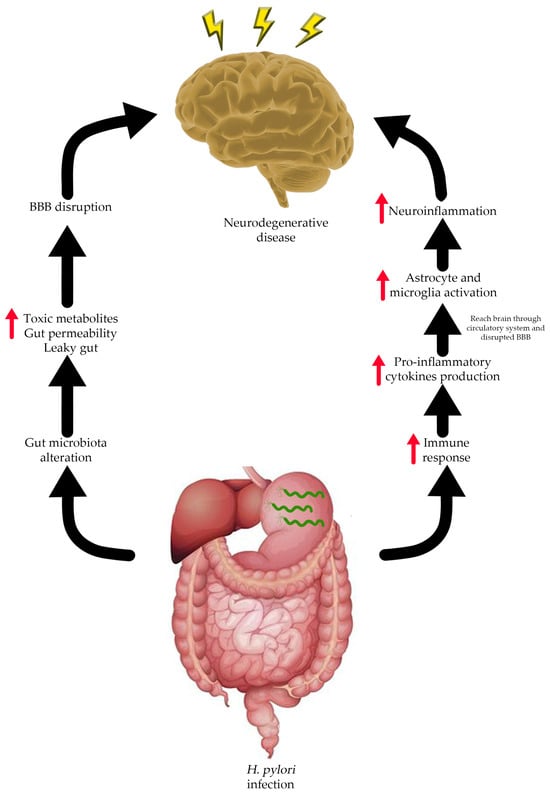
Figure 2. Possible relationship between H. pylori infection and neurodegenerative disease.
