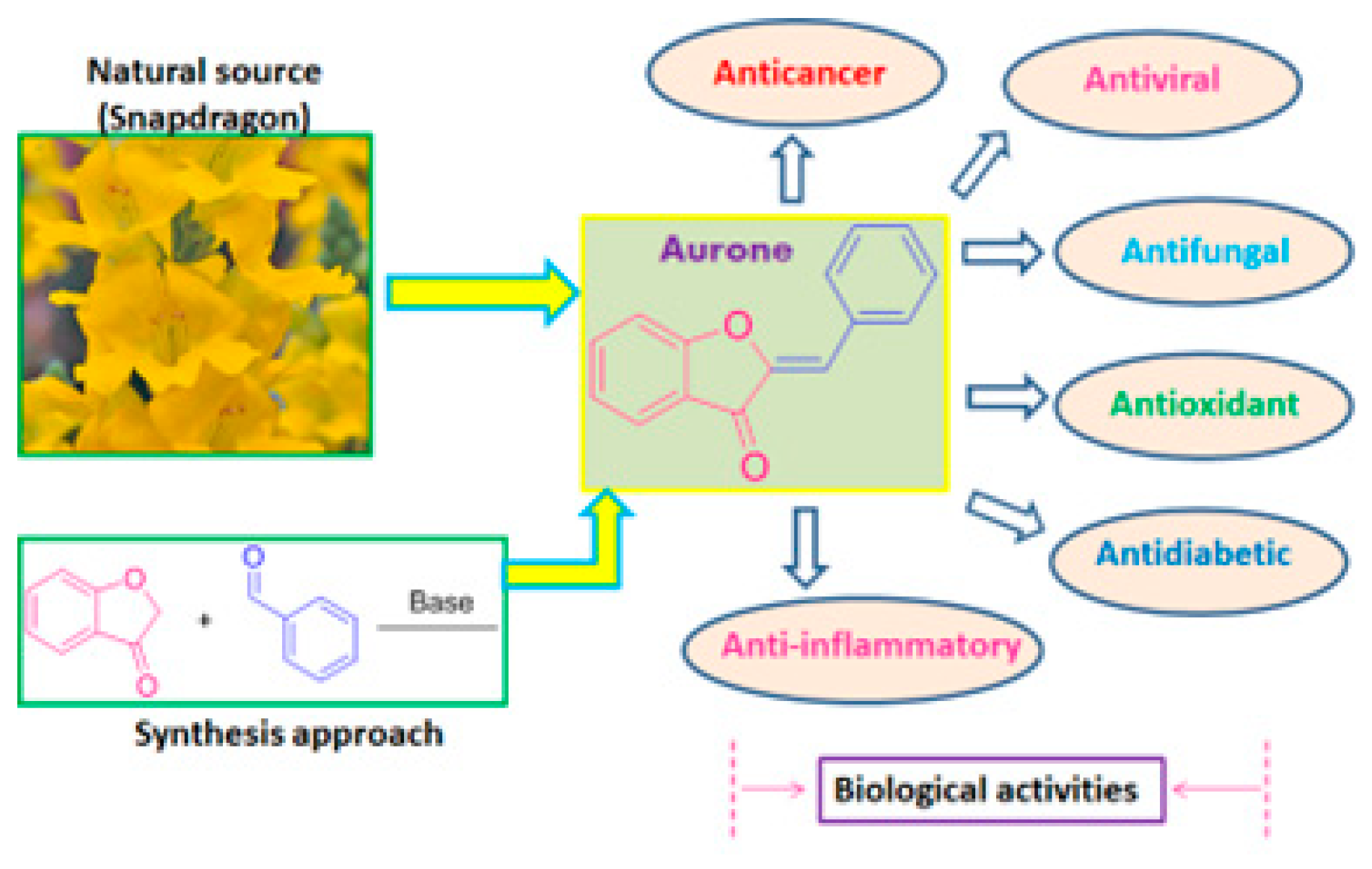
Natural products are a boundless source for the development of pharmaceutical agents against a wide range of human diseases. Accordingly, naturally occurring aurones possess various biological benefits, such as anticancer, antioxidant, antimicrobial, antidiabetic, anti-inflammatory, antiviral and neuroprotective effects. In addition, various studies have revealed that aurones are potential templates for the regulation of diabetes mellitus and its associated complications. Likewise, certain aurones and their analogues have been found to be remarkable kinase inhibitors of DARK2, PPAR-γ, PTPM1, AGE, α-amylase and α-glucosidase, which represents a promising approach for the treatment of chronic metabolic disorders such as diabetes.
- aurones
- chalcone
1. Introduction
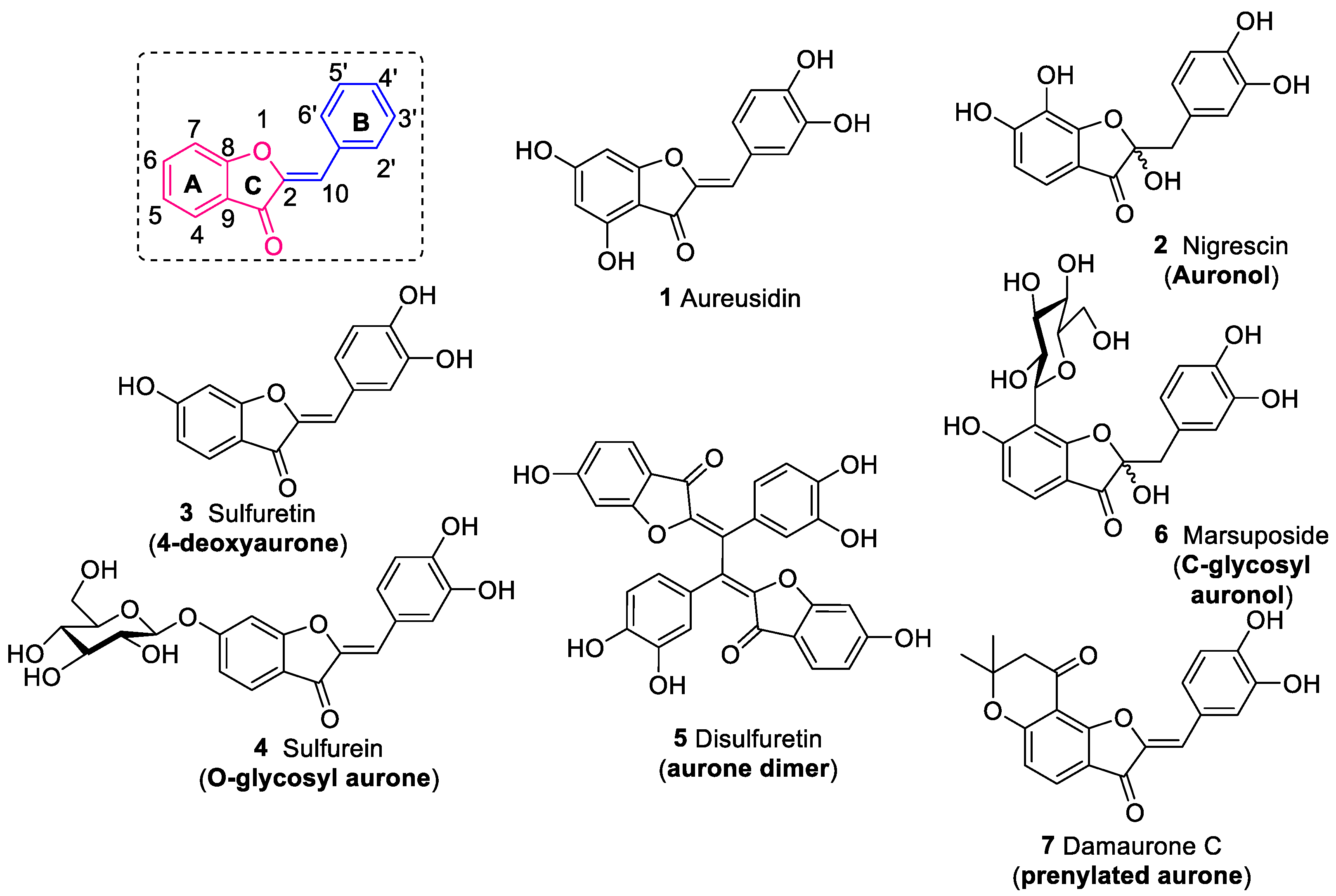
2. Occurrence and Distribution of Aurones
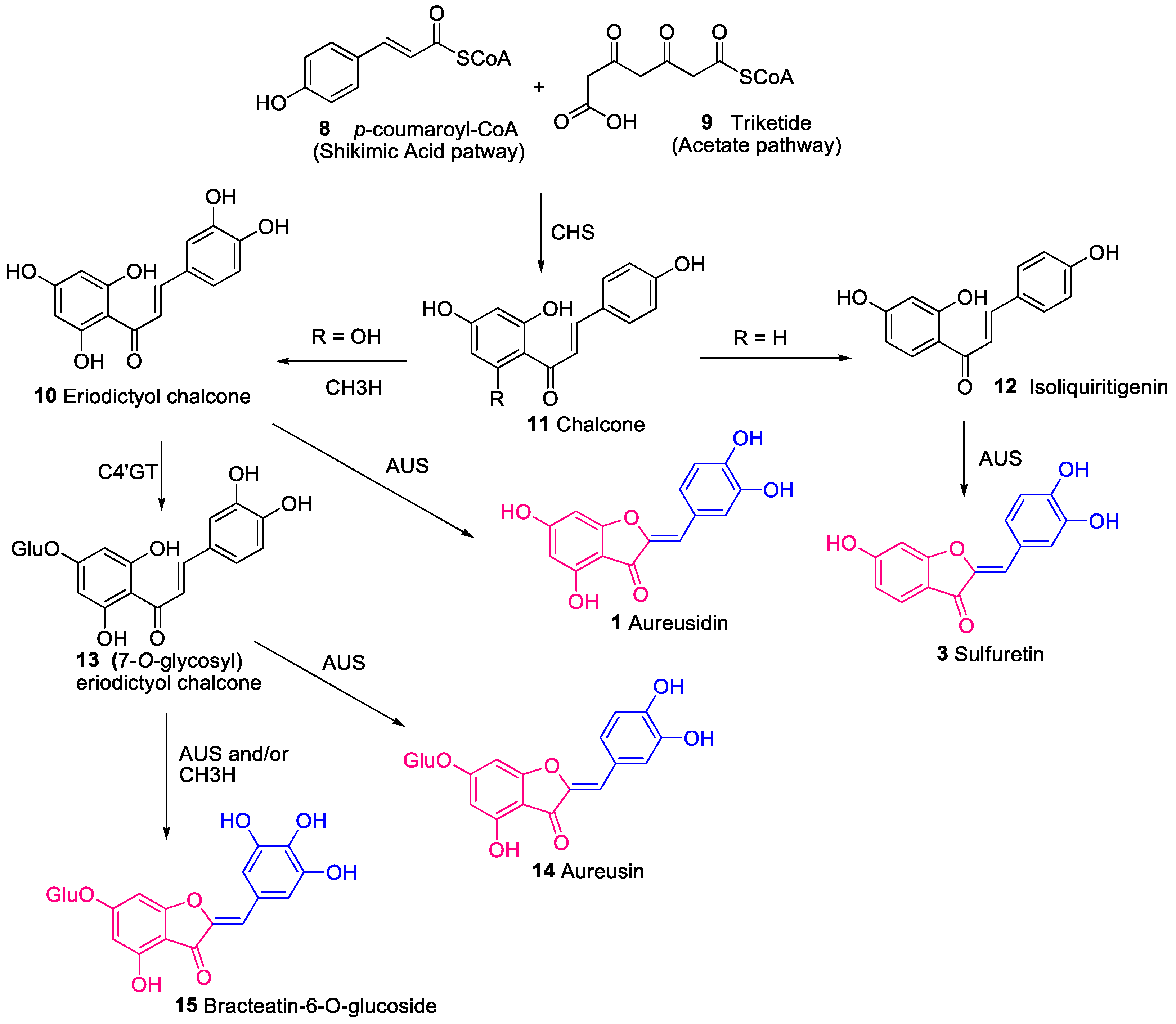
3. Biosynthesis of Aurones
References
- Punjot, P.; Bishnoi, R.; Kant, R.; Sharma, S.K. Factors associated with peripheral neuropathy among patients with type 2 diabetes mellitus: A cross-sectional study. J. Cardio-Diabetes Metab. Disord. 2021, 1, 25–30.
- Schwandt, P. On the occasion of the world diabetes day: Diabetes mellitus–a globally increasing health problem. Int. J. Prev. Med. 2012, 3, 747–748.
- Dahlén, A.D.; Dashi, G.; Maslov, I.; Attwood, M.M.; Jonsson, J.; Trukhan, V.; Schiöth, H.B. Trends in antidiabetic drug discovery: FDA approved drugs, new drugs in clinical trials and global sales. Front. Pharmacol. 2022, 12, 807548.
- Rammohan, A.; Bhaskar, B.V.; Venkateswarlu, N.; Gu, W.; Zyryanov, G.V. Design, synthesis, docking and biological evaluation of chalcones as promising antidiabetic agents. Bioorg. Chem. 2020, 95, 103527.
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275.
- Zeng, Z.; Huang, S.Y.; Sun, T. Pharmacogenomic studies of current antidiabetic agents and potential new drug targets for precision medicine of diabetes. Diabetes Ther. 2020, 11, 2521–2538.
- Chandrashekhar, M.; Nayak, V.L.; Ramakrishna, S.; Mallavadhani, U.V. Novel triazole hybrids of myrrhanone C, a natural polypodane triterpene: Synthesis, cytotoxic activity and cell based studies. Eur. J. Med. Chem. 2016, 114, 293–307.
- Madasu, C.; Gudem, S.; Sistla, R.; Uppuluri, V.M. Synthesis and anti-inflammatory activity of some novel pyrimidine hybrids of myrrhanone A, a bicyclic triterpene of Commiphora mukul gum resin. Monatsh. Chem. 2017, 148, 2183–2193.
- Rossiter, S.E.; Fletcher, M.H.; Wuest, W.M. Natural products as platforms to overcome antibiotic resistance. Chem. Rev. 2017, 117, 12415–12474.
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216.
- Ayoka, T.O.; Ezema, B.O.; Eze, C.N.; Nnadi, C.O. Antioxidants for the Prevention and Treatment of Non-communicable Diseases. J. Explor. Res. Pharmacol. 2022, 7, 178–188.
- Rammohan, A.; Zyryanov, G.V.; Bhagath, Y.B.; Manjula, K. Antioxidants: Structure-activity of plant polyphenolics. Vitam. Horm. 2023, 121, 395–411.
- Grosso, G. Dietary antioxidants and prevention of non-communicable diseases. Antioxidants 2018, 7, 94.
- Bhaskar, B.V.; Rammohan, A.; Babu, T.M.; Zheng, G.Y.; Chen, W.; Rajendra, W.; Zyryanov, G.V.; Gu, W. Molecular insight into isoform specific inhibition of PI3K-α and PKC-η with dietary agents through an ensemble pharmacophore and docking studies. Sci. Rep. 2021, 11, 12150.
- Sui, G.; Li, T.; Zhang, B.; Wang, R.; Hao, H.; Zhou, W. Recent advances on synthesis and biological activities of aurones. Bioorg. Med. Chem. 2021, 29, 115895.
- Alsayari, A.; Muhsinah, A.B.; Hassan, M.Z.; Ahsan, M.J.; Alshehri, J.A.; Begum, N. Aurone: A biologically attractive scaffold as anticancer agent. Eur. J. Med. Chem. 2019, 166, 417–431.
- Mander, L.; Liu, H.W. Comprehensive Natural Products II: Chemistry and Biology; Elsevier: Amsterdam, The Netherlands, 2010; Volume 1.
- Harborne, J.B. Comparative biochemistry of flavonoids—I: Distribution of chalcone and aurone pigments in plants. Phytochemistry 1966, 5, 111–115.
- Mazziotti, I.; Petrarolo, G.; La Motta, C. Aurones: A golden resource for active compounds. Molecules 2021, 27, 2.
- Boucherle, B.; Peuchmaur, M.; Boumendjel, A.; Haudecoeur, R. Occurrences, biosynthesis and properties of aurones as high-end evolutionary products. Phytochemistry 2017, 142, 92–111.
- Miosic, S.; Knop, K.; Hölscher, D.; Greiner, J.; Gosch, C.; Thill, J.; Kai, M.; Shrestha, B.K.; Schneider, B.; Crecelius, A.C.; et al. 4-Deoxyaurone formation in Bidens ferulifolia (Jacq.) DC. PLoS ONE 2013, 8, e61766.
- Wang, Y.; Liang, H.; Zhang, Q.; Cheng, W.; Yi, S. Phytochemical and chemotaxonomic study on Ficus tsiangii Merr. ex Corner. Biochem. Syst. Ecol. 2014, 57, 210–215.
- Lazinski, L.M.; Royal, G.; Robin, M.; Maresca, M.; Haudecoeur, R. Bioactive Aurones, Indanones, and Other Hemiindigoid Scaffolds: Medicinal Chemistry and Photopharmacology Perspectives. J. Med. Chem. 2022, 65, 12594–12625.
- Nakayama, T.; Yonekura-Sakakibara, K.; Sato, T.; Kikuchi, S.; Fukui, Y.; Fukuchi-Mizutani, M.; Ueda, T.; Nakao, M.; Tanaka, Y.; Kusumi, T.; et al. Aureusidin synthase: A polyphenol oxidase homolog responsible for flower coloration. Science 2000, 290, 1163–1166.
- Nakayama, T.; Sato, T.; Fukui, Y.; Yonekura-Sakakibara, K.; Hayashi, H.; Tanaka, Y.; Kusumi, T.; Nishino, T. Specificity analysis and mechanism of aurone synthesis catalyzed by aureusidin synthase, a polyphenol oxidase homolog responsible for flower coloration. FEBS Lett. 2001, 499, 107–111.
- Hoshino, A.; Mizuno, T.; Shimizu, K.; Mori, S.; Fukada-Tanaka, S.; Furukawa, K.; Ishiguro, K.; Tanaka, Y.; Iida, S. Generation of yellow flowers of the Japanese morning glory by engineering its flavonoid biosynthetic pathway toward aurones. Plant Cell Physiol. 2019, 60, 1871–1879.
- Markham, K.R.; Andersen, O.M. (Eds.) Flavonoids: Chemistry, Biochemistry, and Applications; CRC Press: Boca Raton, FL, USA, 2006.
- Rammohan, A.; Reddy, J.S.; Sravya, G.; Rao, C.N.; Zyryanov, G.V. Chalcone synthesis, properties and medicinal applications: A review. Environ. Chem. Lett. 2020, 18, 433–458.
