When research on osteogenic differentiation in dental follicle cells (DFCs) began, projects focused on bone morphogenetic protein (BMP) signaling. The BMP pathway induces the transcription factor DLX3, whichh in turn induces the BMP signaling pathway via a positive feedback mechanism. However, this BMP2/DLX3 signaling pathway only seems to support the early phase of osteogenic differentiation, since simultaneous induction of BMP2 or DLX3 does not further promote differentiation. Data showed that inhibition of classical protein kinase C (PKCs) supports the mineralization of DFCs and that osteogenic differentiation is sensitive to changes in signaling pathways, such as protein kinase B (PKB), also known as AKT. Small changes in the lipidome seem to confirm the participation of AKT and PKC in osteogenic differentiation.
- protein kinase C
- dental follicle cells
- osteogenic differentiation
1. Introduction
Discovery of the BMP2/DLX3 Signaling Pathway
2. Protein Kinase C (PKC) Signaling and Biomineralization
2.1. PKC in Osteogenic Progenitor Cells
One factor appears to be PKC, which can be divided into several groups, but only classical PKCs are involved in the osteogenic differentiation of DFCs [50]. While the mechanism of the PKC signaling pathway, which is described below, is well known, little is known about its role in the osteogenic differentiation of DFCs. Interestingly, previous studies using both immortalized cell lines and somatic stem cells have demonstrated the involvement of PKC in osteogeogenic differentiation, with these studies showing that PKC can have both promoting and inhibitory effects. Miraoui et al. [51] showed that fibroblast growth factor receptor 2 (FGFR2)-induced osteoblast differentiation in murine mesenchymal C3H10T1/2 depended on PKC activation. In contrast, Nakura et al. have shown that PKC inhibits osteogenic differentiation in mouse preosteoblastic cell line MC3T3-E1, but promotes cell proliferation [52]. Similar conclusions were drawn with C2C12 cells by Lee and co-workers [53], who showed that PKC inhibits osteogenic differentiation by regulating the transcription factor MSX2, which in turn inhibits the expression of the osteogenic transcription factor RUNX2. The more recent studies with somatic stem cells also showed similar results. Li et al. [54], for example, showed that the inhibitory effect of miR-26a-5p on osteogenic differentiation of murine adipose-tissue-derived mesenchymal stem cells depends, among other things, on PKC inhibition. However, a study by Lotz et al. showed that PKC inhibition in human bone marrow-derived stem cells increased osteocalcin expression, but inhibited BMP2 expression [55]. Results from another study showed that activation of PKC, specifically, PKCβ1, resulted in repression of muscle ring finger protein-1 (MURF1)-mediated ubiquitylation of the peroxisome proliferator-activated receptor γ2 (PPARγ2) transcription factor. Stabilized PPARγ2 proteins enhanced adipogenesis and consequently reduced osteoblastogenesis from MSCs, showing that PKC activation suppresses osteogenic differentiation [56]. Similar inhibitory effects of PKC on osteogenic differentiation of PDL stem cells have recently been described. Wang et al. showed that Advanced glycation end product (AGE proteins) impaired the osteogenic potential via PKCβ2 [57].2.2. PKC Signaling in DFCs
First experiments with DFCs showed that the expression of classical PKCs, e.g., PKCα, is inhibited from day 7 of osteogenic differentiation, but these experiments also showed that manipulating PKC activity had little effect on ALP activity, which is an early marker of osteogenic differentiation peaking at day 7 [50]. On the other hand, inhibition of classical PKCs supports the mineralization of DFCs, and this is the case even if PKCs were inhibited only a few days, but later than 1 week, after induction of differentiation, since inhibition of PKC occurring in the first week of osteogenic differentiation had no impact on the mineralization [50]. These results suggest that PKC does not affect the early phase of differentiation. Therefore, the interaction of PKC with AKT, which had a positive influence on osteogenic differentiation in previous work [48], was investigated [50]. Interestingly, and in contradiction to this previous work, in this study, the induction of differentiation with both BMP2 and dexamethasone appears to inhibit AKT expression/activity [50]. However, after inhibition of PKC, induction of osteogenic differentiation leads to activation of AKT [50]. Thus, AKT seems to play a complex role in differentiation, which is why regulation of AKT activity during differentiation has been investigated. These experiments showed that AKT does not seem to have a direct impact on mineralization, since both the inhibitor MK2206 and the activator SC-79 inhibited mineralization in a dexamethasone-based differentiation medium [50]. If one assumes that, as shown in this publication, PKC is essential for the activation of AKT, conversely, it should be investigated whether simultaneous inhibition of PKC and activation of AKT influences mineralization. When BMP2 was used for osteogenic differentiation, a stimulatory effect on the differentiation due to AKT inhibition was observed. These studies show that AKT is involved in the mechanism of differentiation. However, the importance of AKT on further signaling pathways, such as the BMP pathway, is ambiguous and depends on the cell line. Two recent studies on DFCs during osteogenic differentiation showed contradictory results regarding the activation of the BMP signaling pathway during differentiation. While Pieles et al. hypothesized an inhibitory effect of AKT on the BMP signaling pathway, Viale-Bouroncle and colleagues showed that AKT supports the BMP signaling pathway. These conflicting results suggest that AKT does not directly affect BMP signaling or that other, as yet unknown, signaling pathways influence AKT’s impact on BMP signaling. On the other hand, however, AKT activation seems to be essential for the inactivation of GSK-3β as a factor involved in the induction of β-catenin of the WNT pathway [50]. The canonical WNT signaling pathway and, in particular, its transcription factor β-catenin, which is also induced by BMP2 in DFCs, play a role in the early phase of DFC differentiation [47,58][47][58]. While PKCs have a direct impact on non-canonical WNT signaling activation, the active form of β-catenin is induced after PKC inhibition [50]. Since there have been results in recent years on the importance of the canonical WNT signaling pathway in DFCs, which had both a promoting and an inhibitory effect on osteogenic differentiation [47[47][58][59][60],58,59,60], this signaling pathway appears to have a modulating property for differentiation similar to the kinase AKT, which is characterized by the constitution of the individual DFC cell line. However, studies suggest that classical PKCs and canonical WNT signaling have a similar inhibitory effect on osteogenic differentiation of DFCs [47,50][47][50]. It will, therefore, be important for understanding differentiation to uncover by which biological processes classical PKCs are linked to the canonical WNT signaling pathway in DFCs during osteogenic differentiation. A signaling pathway that is of importance for cell differentiation is the NFκ-B signaling pathway. This signaling pathway both inhibits and promotes the differentiation of osteogenic progenitor cells and is inhibited in dental follicle cells after induction of differentiation [61,62,63,64][61][62][63][64]. However, it appears to have an inhibitory effect on osteogenic differentiation of DFCs, as decreased mineralization was shown after NFκ-B induction with NFκ-B activator PMA. Interestingly, PMA activates NFκ-B, but independently of classical PKC [65]. Novel PKCs, which do not influence osteogenic differentiation of DFCs, can directly activate the NFκ-B signaling pathway [65]. However, a previous study showed that classical PKCs caused the activation of RELB of noncanonical NF-κB signaling, but not RELA of canonical NF-κB signaling, in cancer cells [66]. So, this could be an explanation why it was not possible to induce osteogenic differentiation markers by inhibiting the canonical NFκ-B signaling pathway. Interestingly, regulation of AKT has only little influence on the expression of NFκ-B signaling pathway proteins after induction of osteogenic differentiation with dexamethasone. On the other hand, NF-κB can be induced by AKT, and both signaling pathways play important roles in cell viability [67]. WResearchers believe that the NFκ-B signaling pathway does not play a significant role in osteogenic differentiation. Previous studies on the proteome and transcriptome did not find any significant evidence that the NFκ-B signaling pathway is involved in differentiation [68]. It is possible that PMA induces PKC, but impairs cell viability or cell proliferation, as well [69], and it is therefore possible that PMA suppressed the mineralization of DFCs by impairing cell viability without activation of NFκ-B signaling, which could support cell viability [70]. This conclusion is supported by the following observation from a previous study. The inducer of the non-canonical WNT signaling pathway WNT5A, which supports cell viability during osteogenic differentiation [71], inhibited the activation and expression of classical PKCs in DFCs. The results presented here can be summarized as follows [50]. PKCs inhibit osteogenesis specifically by regulating the kinase AKT, thereby affecting the downstream activity of β-catenin and the NF-κB signaling pathway (Figure 1).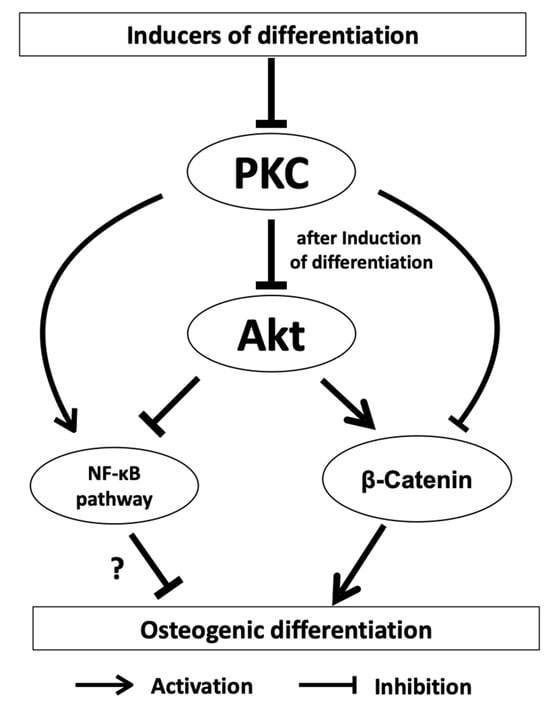
References
- Fu, Y.; Miyazaki, K.; Chiba, Y.; Funada, K.; Yuta, T.; Tian, T.; Mizuta, K.; Kawahara, J.; Zhang, L.; Martin, D.; et al. Identification of GPI-anchored protein LYPD1 as an essential factor for odontoblast differentiation in tooth development. J. Biol. Chem. 2023, 299, 104638.
- Zheng, H.; Fu, J.; Chen, Z.; Yang, G.; Yuan, G. Dlx3 Ubiquitination by Nuclear Mdm2 Is Essential for Dentinogenesis in Mice. J. Dent. Res. 2022, 101, 1064–1074.
- Zhang, F.; Yang, S.; Jiang, L.; Liu, J.; He, Y.; Sheng, X.; Chen, H.; Kang, J.; Jia, S.; Fan, W.; et al. Melatonin-mediated malic enzyme 2 orchestrates mitochondrial fusion and respiratory functions to promote odontoblastic differentiation during tooth development. J. Pineal Res. 2023, 74, e12865.
- Meng, Z.S.; Liu, J.C.; Feng, Z.P.; Guo, S.L.; Wang, M.Z.; Wang, Z.; Li, Z.; Li, H.J.; Sui, L. N-acetylcysteine regulates dental follicle stem cell osteogenesis and alveolar bone repair via ROS scavenging. Stem Cell Res. Ther. 2022, 13, 466.
- Guo, H.; Zhao, W.; Liu, A.; Wu, M.; Shuai, Y.; Li, B.; Huang, X.; Liu, X.; Yang, X.; Guo, X.; et al. SHED promote angiogenesis in stem cell-mediated dental pulp regeneration. Biochem. Biophys. Res. Commun. 2020, 529, 1158–1164.
- Morsczeck, C. Dental stem cells for tooth regeneration: How far have we come and where next? Expert. Opin. Biol. Ther. 2023, 23, 527–537.
- Hermans, F.; Hemeryck, L.; Bueds, C.; Pereiro, M.T.; Hasevoets, S.; Kobayashi, H.; Lambrechts, D.; Lambrichts, I.; Bronckaers, A.; Vankelecom, H. Organoids from mouse molar and incisor as new tools to study tooth-specific biology and development. Stem Cell Rep. 2023, 18, 1166–1181.
- Li, Z.; Yue, M.X.; Liu, Y.S.; Zhang, P.; Qing, J.; Liu, H.; Zhou, Y.S. Advances of Engineered Hydrogel Organoids within the Stem Cell Field: A Systematic Review. Gels 2022, 8, 379.
- Morsczeck, C. Mechanisms during Osteogenic Differentiation in Human Dental Follicle Cells. Int. J. Mol. Sci. 2022, 23, 5945.
- Guo, H.; Bai, X.Y.; Wang, X.L.; Qiang, J.B.; Sha, T.; Shi, Y.; Zheng, K.J.; Yang, Z.M.; Shi, C. Development and regeneration of periodontal supporting tissues. Genesis 2022, 60, e23491.
- Nagata, M.; Ono, N.; Ono, W. Mesenchymal Progenitor Regulation of Tooth Eruption: A View from PTHrP. J. Dent. Res. 2020, 99, 10.
- Morsczeck, C. Effects of Cellular Senescence on Dental Follicle Cells. Pharmacology 2021, 106, 137–142.
- Morsczeck, C.; Moehl, C.; Gotz, W.; Heredia, A.; Schaffer, T.E.; Eckstein, N.; Sippel, C.; Hoffmann, K.H. In vitro differentiation of human dental follicle cells with dexamethasone and insulin. Cell Biol. Int. 2005, 29, 567–575.
- Kemoun, P.; Narayanan, A.S.; Brunel, G.; Salles, J.P.; Laurencin-Dalicieux, S.; Rue, J.; Farges, J.C.; Gennero, I.; Conte-Auriol, F.; Briand-Mesange, F.; et al. Human dental follicle cells acquire cementoblast features under stimulation by BMP-2/-7 and enamel matrix derivatives (EMD) in vitro. Cell Tissue Res. 2007, 329, 283–294.
- Honda, M.J.; Imaizumi, M.; Suzuki, H.; Ohshima, S.; Tsuchiya, S.; Satomura, K. Stem Cells Isolated from Human Dental Follicles Have Osteogenic Potential. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2011, 111, 700–708. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21147007 (accessed on 6 September 2023).
- Popowics, T.; Foster, B.L.; Swanson, E.C.; Fong, H.; Somerman, M.J. Defining the Roots of Cementum Formation. Cells Tissues Organs 2005, 181, 248–257. Available online: http://www.ncbi.nlm.nih.gov/pubmed/16612090 (accessed on 6 September 2023).
- Wise, G.E.; Frazier-Bowers, S.; D’Souza, R.N. Cellular, molecular, and genetic determinants of tooth eruption. Crit. Rev. Oral Biol. Med. An. Off. Publ. Am. Assoc. Oral. Biol. 2002, 13, 323–334.
- Diekwisch, T.G. The Developmental Biology of Cementum. Int. J. Dev. Biol. 2001, 45, 695–706. Available online: http://www.ncbi.nlm.nih.gov/pubmed/11669371 (accessed on 6 September 2023).
- Morsczeck, C.; Gotz, W.; Schierholz, J.; Zeilhofer, F.; Kuhn, U.; Mohl, C.; Sippel, C.; Hoffmann, K.H. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005, 24, 155–165.
- Morsczeck, C.; Frerich, B.; Driemel, O. Dental Stem Cell Patents. Recent. Pat. DNA Gene Seq. 2009, 3, 39–43. Available online: http://www.ncbi.nlm.nih.gov/pubmed/19149737 (accessed on 6 September 2023).
- Friedenstein, A.; Kuralesova, A.I. Osteogenic precursor cells of bone marrow in radiation chimeras. Transplantation 1971, 12, 99–108.
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630.
- Brunel, G.; Kémoun, P.; Laurencin-Dalicieux, S.; Rue, J.; Vaysse, F.; Roméas, A.; Arzate, H.; Conte-Auriol, F.; Farges, J.C.; Salles, J.P. Localization of STRO-1, BMP-2/-3/-7, BMP Receptors and Phosphorylated Smad-1 during the Formation of Mouse Periodontium. Tissue Cell 2007, 39, 257–266. Available online: http://www.ncbi.nlm.nih.gov/pubmed/17662325 (accessed on 6 September 2023).
- Völlner, F.; Ernst, W.; Driemel, O.; Morsczeck, C. A Two-Step Strategy for Neuronal Differentiation In Vitro of Human Dental Follicle Cells. Differ. Res. Biol. Divers. 2009, 77, 433–441. Available online: http://www.ncbi.nlm.nih.gov/pubmed/19394129 (accessed on 6 September 2023).
- Huang, Y.L.; Liu, L.; Liu, Q.; Huo, F.J.; Hu, X.Y.; Guo, S.J.; Tian, W.D. Dental follicle cells-derived small extracellular vesicles inhibit pathogenicity of Porphyromonas gingivalis. Oral. Diseases 2022, 29, 2297–2309.
- Zhang, J.; Lan, T.; Han, X.; Xu, Y.; Liao, L.; Xie, L.; Yang, B.; Tian, W.; Guo, W. Improvement of ECM-based bioroot regeneration via N-acetylcysteine-induced antioxidative effects. Stem Cell Res. Ther. 2021, 12, 202.
- Yang, B.; Yang, X.T.; Luo, X.Y.; Chen, G.; Chen, J.L.; Huo, F.J.; Zhu, Z.L.; Tian, Y.; Guo, W.H.; Tian, W.D. DFCs/TDM based artificial bio-root to obtain long-term functional root regeneration in non-human primate. Chem. Eng. J. 2023, 451, 138738.
- Raju, R.; Oshima, M.; Inoue, M.; Morita, T.; Huijiao, Y.; Waskitho, A.; Baba, O.; Inoue, M.; Matsuka, Y. Three-dimensional periodontal tissue regeneration using a bone-ligament complex cell sheet. Sci. Rep. 2020, 10, 1656.
- Tu, R.; Tang, X.A.; Xu, R.; Ping, Z.; Yu, Z.; Xie, T. Gap junction-transported cAMP from the niche controls stem cell progeny differentiation. Proc. Natl. Acad. Sci. USA 2023, 120, e2304168120.
- Kopecny, L.R.; Lee, B.W.H.; Coroneo, M.T. A systematic review on the effects of ROCK inhibitors on proliferation and/or differentiation in human somatic stem cells: A hypothesis that ROCK inhibitors support corneal endothelial healing via acting on the limbal stem cell niche. Ocul. Surf. 2023, 27, 16–29.
- Della Sala, F.; di Gennaro, M.; Lista, G.; Messina, F.; Valente, T.; Borzacchiello, A. Effect of Composition of Lung Biomimetic Niche on the Mesenchymal Stem Cell Differentiation toward Alveolar Type II Pneumocytes. Macromol. Biosci. 2023, 23, e2300035.
- Lee, C.; Hong, S.N.; Kim, E.R.; Chang, D.K.; Kim, Y.H. Depletion of Intestinal Stem Cell Niche Factors Contributes to the Alteration of Epithelial Differentiation in SAMP1/YitFcsJ Mice With Crohn Disease-Like Ileitis. Inflamm. Bowel Dis. 2021, 27, 667–676.
- Chua, J.S.; Muruganandam, G.; Sung, D.; Saijoh, Y.; Balagurunathan, K. Applications of Xylosides in the Manipulation of Stem Cell Niche to Regulate Human Neural Stem Cell Differentiation and Neurite Outgrowth. Methods Mol. Biol. 2022, 2303, 779–788.
- Clemot, M.; Senos Demarco, R.; Jones, D.L. Lipid Mediated Regulation of Adult Stem Cell Behavior. Front. Cell Dev. Biol. 2020, 8, 115.
- Saugspier, M.; Felthaus, O.; Viale-Bouroncle, S.; Driemel, O.; Reichert, T.E.; Schmalz, G.; Morsczeck, C. The Differentiation and Gene Expression Profile of Human Dental Follicle cells. Stem Cells Dev. 2010, 19, 707–717. Available online: http://www.ncbi.nlm.nih.gov/pubmed/20491563 (accessed on 6 September 2023).
- Silvério, K.G.; Davidson, K.C.; James, R.G.; Adams, A.M.; Foster, B.L.; Nociti, F.H., Jr.; Somerman, M.J.; Moon, R.T. Wnt/β-Catenin Pathway Regulates Bone Morphogenetic Protein (BMP2)-Mediated Differentiation of Dental Follicle Cells. J. Periodontal Res. 2012, 47, 309–319. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22150562 (accessed on 6 September 2023).
- Viale-Bouroncle, S.; Gosau, M.; Morsczeck, C. Laminin regulates the osteogenic differentiation of dental follicle cells via integrin-α2/-β1 and the activation of the FAK/ERK signaling pathway. Cell Tissue Res. 2014, 357, 345–354.
- Viale-Bouroncle, S.; Gosau, M.; Morsczeck, C. Collagen I induces the expression of alkaline phosphatase and osteopontin via independent activations of FAK and ERK signalling pathways. Arch. Oral. Biol. 2014, 59, 1249–1255.
- Aonuma, H.; Ogura, N.; Takahashi, K.; Fujimoto, Y.; Iwai, S.; Hashimoto, H.; Ito, K.; Kamino, Y.; Kondoh, T. Characteristics and osteogenic differentiation of stem/progenitor cells in the human dental follicle analyzed by gene expression profiling. Cell Tissue Res. 2012, 350, 317–331.
- Morsczeck, C.; Petersen, J.; Völlner, F.; Driemel, O.; Reichert, T.; Beck, H.C. Proteomic Analysis of Osteogenic Differentiation of Dental Follicle Precursor Cells. Electrophoresis 2009, 30, 1175–1184. Available online: http://www.ncbi.nlm.nih.gov/pubmed/19288589 (accessed on 6 September 2023).
- Viale-Bouroncle, S.; Felthaus, O.; Schmalz, G.; Brockhoff, G.; Reichert, T.E.; Morsczeck, C. The Transcription Factor DLX3 Regulates the Osteogenic Differentiation of Human Dental Follicle Precursor Cells. Stem Cells Dev. 2012, 21, 1936–1947. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22107079 (accessed on 6 September 2023).
- Morsczeck, C.; Reichert, T.E. The dexamethasone induced osteogenic differentiation of dental follicle cells. Histol. Histopathol. 2017, 32, 1223–1229.
- Morsczeck, C.; Schmalz, G.; Reichert, T.E.; Völlner, F.; Saugspier, M.; Viale-Bouroncle, S.; Driemel, O. Gene Expression Profiles of Dental Follicle Cells before and after Osteogenic Differentiation In Vitro. Clin. Oral. Investig. 2009, 13, 383–391. Available online: http://www.ncbi.nlm.nih.gov/pubmed/19252934 (accessed on 6 September 2023).
- Choi, S.J.; Song, I.S.; Feng, J.Q.; Gao, T.; Haruyama, N.; Gautam, P.; Robey, P.G.; Hart, T.C. Mutant DLX 3 disrupts odontoblast polarization and dentin formation. Dev. Biol. 2010, 344, 682–692.
- Hassan, M.Q.; Javed, A.; Morasso, M.I.; Karlin, J.; Montecino, M.; van Wijnen, A.J.; Stein, G.S.; Stein, J.L.; Lian, J.B. Dlx3 Transcriptional Regulation of Osteoblast Differentiation: Temporal Recruitment of Msx2, Dlx3, and Dlx5 Homeodomain Proteins to Chromatin of the Osteocalcin Gene. Mol. Cell Biol. 2004, 24, 9248–9261. Available online: http://www.ncbi.nlm.nih.gov/pubmed/15456894 (accessed on 6 September 2023).
- Hassan, M.Q.; Tare, R.S.; Lee, S.H.; Mandeville, M.; Morasso, M.I.; Javed, A.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; Lian, J.B. BMP2 commitment to the osteogenic lineage involves activation of Runx2 by DLX3 and a homeodomain transcriptional network. J. Biol. Chem. 2006, 281, 40515–40526.
- Viale-Bouroncle, S.; Klingelhöffer, C.; Ettl, T.; Reichert, T.E.; Morsczeck, C. A protein kinase A (PKA)/β-catenin pathway sustains the BMP2/DLX3-induced osteogenic differentiation in dental follicle cells (DFCs). Cell Signal. 2015, 27, 598–605.
- Viale-Bouroncle, S.; Klingelhöffer, C.; Ettl, T.; Morsczeck, C. The AKT signaling pathway sustains the osteogenic differentiation in human dental follicle cells. Mol. Cell. Biochem. 2015, 406, 199–204.
- Klingelhöffer, C.; Reck, A.; Ettl, T.; Morsczeck, C. The Parathyroid Hormone-Related Protein is Secreted during the Osteogenic Differentiation of Human Dental Follicle Cells and Inhibits the Alkaline Phosphatase Activity and the Expression of DLX3. Tissue Cell 2016, 48, 334–339. Available online: http://www.sciencedirect.com/science/article/pii/S0040816616300593 (accessed on 6 September 2023).
- Pieles, O.; Reichert, T.E.; Morsczeck, C. Classical isoforms of protein kinase C (PKC) and Akt regulate the osteogenic differentiation of human dental follicle cells via both beta-catenin and NF-kappa B. Stem Cell Res. Ther. 2021, 12, 242.
- Miraoui, H.; Oudina, K.; Petite, H.; Tanimoto, Y.; Moriyama, K.; Marie, P.J. Fibroblast growth factor receptor 2 promotes osteogenic differentiation in mesenchymal cells via ERK1/2 and protein kinase C signaling. J. Biol. Chem. 2009, 284, 4897–4904.
- Nakura, A.; Higuchi, C.; Yoshida, K.; Yoshikawa, H. PKCalpha suppresses osteoblastic differentiation. Bone 2011, 48, 476–484.
- Jeong, H.M.; Jin, Y.H.; Choi, Y.H.; Yum, J.; Choi, J.K.; Yeo, C.Y.; Lee, K.Y. PKC signaling inhibits osteogenic differentiation through the regulation of Msx2 function. Bba-Mol. Cell Res. 2012, 1823, 1225–1232.
- Li, S.S.; Hu, C.; Li, J.W.; Liu, L.; Jing, W.; Tang, W.; Tian, W.D.; Long, J. Effect of miR-26a-5p on the Wnt/Ca2+ Pathway and Osteogenic Differentiation of Mouse Adipose-Derived Mesenchymal Stem Cells. Calcif. Tissue Int. 2016, 99, 174–186.
- Lotz, E.M.; Berger, M.B.; Boyan, B.D.; Schwartz, Z. Regulation of mesenchymal stem cell differentiation on microstructured titanium surfaces by semaphorin 3A. Bone 2020, 134, 115260.
- Liu, Z.; Liu, H.; He, J.; Lin, P.; Tong, Q.; Yang, J. Myeloma cells shift osteoblastogenesis to adipogenesis by inhibiting the ubiquitin ligase MURF1 in mesenchymal stem cells. Sci. Signal 2020, 13, eaay8203.
- Wang, Z.; Wang, X.; Zhang, L.; Wang, B.; Xu, B.; Zhang, J. GLP-1 inhibits PKCbeta2 phosphorylation to improve the osteogenic differentiation potential of hPDLSCs in the AGE microenvironment. J. Diabetes Complicat. 2020, 34, 107495.
- Nemoto, E.; Sakisaka, Y.; Tsuchiya, M.; Tamura, M.; Nakamura, T.; Kanaya, S.; Shimonishi, M.; Shimauchi, H. Wnt3a signaling induces murine dental follicle cells to differentiate into cementoblastic/osteoblastic cells via an osterix-dependent pathway. J. Periodontal Res. 2015, 51, 164–174.
- Nemoto, E.; Koshikawa, Y.; Kanaya, S.; Tsuchiya, M.; Tamura, M.; Somerman, M.J.; Shimauchi, H. Wnt Signaling Inhibits Cementoblast Differentiation and Promotes Proliferation. Bone 2009, 44, 805–812. Available online: http://www.ncbi.nlm.nih.gov/pubmed/19442631 (accessed on 6 September 2023).
- Sakisaka, Y.; Tsuchiya, M.; Nakamura, T.; Tamura, M.; Shimauchi, H.; Nemoto, E. Wnt5a attenuates Wnt3a-induced alkaline phosphatase expression in dental follicle cells. Exp. Cell Res. 2015, 336, 85–93.
- Hozhabri, N.S.T.; Benson, M.D.; Vu, M.D.; Patel, R.H.; Martinez, R.M.; Nakhaie, F.N.; Kim, H.K.W.; Varanasi, V.G. Decreasing NF-kappa B Expression Enhances Odontoblastic Differentiation and Collagen Expression in Dental Pulp Stem Cells Exposed to Inflammatory Cytokines. PLoS ONE 2015, 10, e0127494.
- Chang, J.; Liu, F.; Lee, M.; Wu, B.; Ting, K.; Zara, J.N.; Soo, C.; Al Hezaimi, K.; Zou, W.; Chen, X.; et al. NF-kappaB inhibits osteogenic differentiation of mesenchymal stem cells by promoting beta-catenin degradation. Proc. Natl. Acad. Sci. USA 2013, 110, 9469–9474.
- Li, N.; Li, Z.; Wang, Y.; Chen, Y.; Ge, X.; Lu, J.; Bian, M.; Wu, J.; Yu, J. CTP-CM enhances osteogenic differentiation of hPDLSCs via NF-kappaB pathway. Oral. Dis. 2021, 27, 577–588.
- Huang, Q.; He, W.; Weng, Y.; Wang, Y.; Liu, Y.; Xiang, Y.; Li, X.; Jiang, P.; Jin, Y.; Luo, J.; et al. Berberine inhibits osteogenic differentiation of aortic valve interstitial cells by interfering Smad1/5/8 and NF-kappaB pathways. Vascul Pharmacol. 2022, 144, 106986.
- Holden, N.S.; Squires, P.E.; Kaur, M.; Bland, R.; Jones, C.E.; Newton, R. Phorbol ester-stimulated NF-kappaB-dependent transcription: Roles for isoforms of novel protein kinase C. Cell Signal 2008, 20, 1338–1348.
- Leonard, B.; McCann, J.L.; Starrett, G.J.; Kosyakovsky, L.; Luengas, E.M.; Molan, A.M.; Burns, M.B.; McDougle, R.M.; Parker, P.J.; Brown, W.L.; et al. The PKC/NF-kappa B Signaling Pathway Induces APOBEC3B Expression in Multiple Human Cancers. Cancer Res. 2015, 75, 4538–4547.
- Niero, E.L.; Rocha-Sales, B.; Lauand, C.; Cortez, B.A.; de Souza, M.M.; Rezende-Teixeira, P.; Urabayashi, M.S.; Martens, A.A.; Neves, J.H.; Machado-Santelli, G.M. The multiple facets of drug resistance: One history, different approaches. J. Exp. Clin. Cancer Res. 2014, 33, 37.
- Morsczeck, C.; Schmalz, G. Transcriptomes and Proteomes of DENTAL follicle Cells. J. Dent. Res. 2010, 89, 445–456. Available online: http://www.ncbi.nlm.nih.gov/pubmed/20348482 (accessed on 6 September 2023).
- Kaul, N.; Forman, H.J. Activation of NF kappa B by the respiratory burst of macrophages. Free Radical Bio Med. 1996, 21, 401–405.
- Busuttil, V.; Bottero, V.; Frelin, C.; Imbert, V.; Ricci, J.E.; Auberger, P.; Peyron, J.F. Blocking NF-kappaB activation in Jurkat leukemic T cells converts the survival agent and tumor promoter PMA into an apoptotic effector. Oncogene 2002, 21, 3213–3224.
- Morsczeck, C.; Reck, A.; Reichert, T.E. WNT5A supports viability of senescent human dental follicle cells. Mol. Cell Biochem. 2018, 455, 21–28.
- Shirai, Y.; Saito, N. Activation mechanisms of protein kinase C: Maturation, catalytic activation, and targeting. J. Biochem. 2002, 132, 663–668.
- Nishizuka, Y. The Role of Protein Kinase-C in Cell-Surface Signal Transduction and Tumor Promotion. Nature 1984, 308, 693–698.
- Pieles, O.; Hoering, M.; Adel, S.; Reichert, T.E.; Liebisch, G.; Morsczeck, C. Energy Metabolism and Lipidome Are Highly Regulated during Osteogenic Differentiation of Dental Follicle Cells. Stem Cells Int. 2022, 2022, 3674931.
