Nitride wide-bandgap semiconductors possess a wide tunable energy bandgap and abundant coordination anionic groups. This suggests their potential to display nonlinear optical (NLO) properties in the ultraviolet (UV) wavelength spectrum.
1. Introduction
Nitrides are significant wide-bandgap semiconductor materials with a variety of optoelectronic applications in different fields of electronic devices, such as high-voltage devices, solar-blind photodetectors, and waveguide devices
[1]. Their excellent physical–chemical properties and tunable energy bandgap (
Eg) over a wide range make them ideal for these applications. Typical wide-bandgap nitride semiconductors include the third-generation semiconductor GaN
[2], ultraviolet (UV) light-emitting material AlN
[3] or its solid-solution structure (Ga,Al)N
[4], graphene-like layered material h-BN
[5], and ceramic material Si
3N
4 [6]. The wide-bandgap
Eg rendered the aforementioned materials naturally suitable for use in optoelectronic applications and the study of light–matter interaction in the UV band (100~400 nm). The UV band is typically divided into three segments: UVA (320~400 nm long-wave UV), UVB (280~320 nm mid-wave UV), and UVC (100~280 nm short-wave UV). UVC, in turn, is divided into 200~280 nm solar-blind UV and 100~200 nm deep-UV light
[7].
The UV laser is a cutting-edge high-tech solution for UV optoelectronic applications that has added significant value in many areas, including spectroscopy, medicine, and photolithography
[8][9][10][11][8,9,10,11]. One main technical route in UV laser production involves converting traditional micrometer lasers, such as the Nd: YAG 1064 nm laser
[12], into UV lasers using the cascade harmonic generation effect of nonlinear optical (NLO) crystals. This typically involves generating 355 nm for the third harmonic generation, 266 nm for the fourth harmonic generation, and 177 nm for sixth harmonic generation based on the fundamental wavelength of 1064 nm
[13]. NLO crystals serve as the irreplaceable functional material basis for laser generation, especially for the two crucial solar-blind UV and deep-UV bands. However, the need for NLO performance has increased as second-harmonic-generation (SHG) wavelengths shift to short-wave UV light, posing a challenge in crystal selection
[14].
2. Nitride Wide-Bandgap Semiconductors for UV Nonlinear Optics
Note that nitrogen (N) is only less electronegative than fluorine (F) and oxygen (O), similar to chlorine (Cl). Therefore, the relatively weak coupling strength of covalent nitride bonds may lead to a small gap developing between the highest occupied molecular orbitals (HOMO) and the lowest unoccupied molecular orbitals (LUMO). In other words, the energy bandgaps (Eg) of nitrides are typically slightly lower than those of fluorides or oxides. However, nitrides can also be bonded by the continued oxidation of F or O, resulting in the potential creation of fluoronitrides or oxynitrides. These fluoronitride or oxynitride compounds can further increase the energy bandgap Eg, making them appropriate for wider optical transparency at shorter UV wavelengths. It should be emphasized that the NLO effects and birefringence are frequently linked with covalent anionic motifs.
2.1. GaN and AlN with Tetrahedral [GaN
4
] or [AlN
4
] Motif
Group IIIA nitrides are vital semiconductor materials and they have become the most valuable semiconductor alloy system for short-wavelength light-emitting diodes (LEDs) and laser development, with various applications. The synthesis of binary and ternary nitrides of group IIIA has been critical in the growth of gas phase chemical vapor deposition (CVD) techniques
[15][38].
GaN is a third-generation semiconductor with a wide bandgap
Eg ~3.5 eV
[16][30]. However, its tetrahedral [GaN
4] framework structure (see
Figure 1a) results in relatively low structural anisotropy and a small birefringence Δ
n of only 0.017. Therefore, it cannot meet the requirements for conventional birefringent phase matching in the UV region. AlN, which has the same structure, faces a similar problem. Despite its large bandgap
Eg of 6.2 eV
[16][30], the value of birefringence Δ
n is still small, being only ~0.023, and the material does not have sufficient ability for birefringent phase matching in the UV region. Although it is feasible to develop GaAlN solid solutions with a tunable bandgap
Eg, birefringent properties are linked to the local structural anisotropy with minimal improvement. Consequently, such solid solutions remain largely incapable of producing a coherent phase-matching SHG output, especially in the UVC band. This scenario can be observed in α-Si
3N
4 with the tetrahedral [SiN
4] motif (see
Figure 1b).
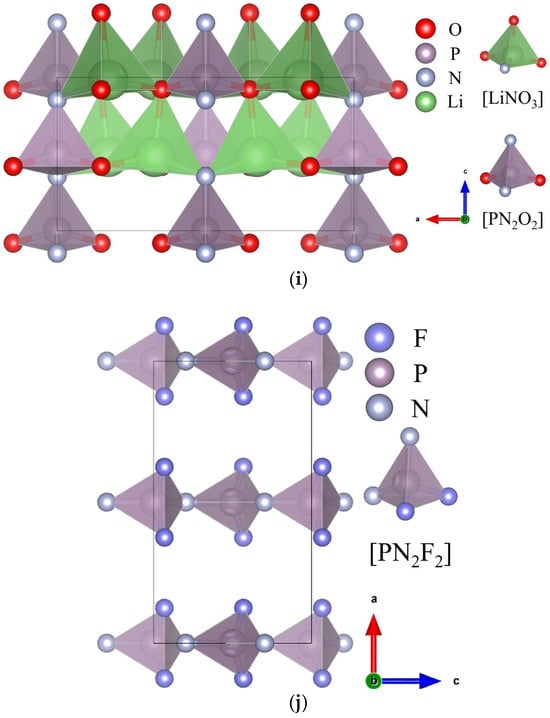
Figure 1. Crystal and motif structures of GaN or AlN (a), Si3N4 (b), α-KCN (c), β-KCN (d), AsC3N3 (e), CNI (f), NaCNO (g), Si2N2O (h), Li2PNO2 (i), and PNF2 (j). In the legends, a, b, and c indicate the orientation of the crystal axis.
2.2. KCN and AsC
3
N
3
with Chained [CN] Motif
An effective scheme to increase the birefringence Δ
n is to increase the structural anisotropy of the anionic motif. The chain-like [CN] motif is a potential nitride birefringent anionic motif. In a typical KCN compound
[17][24], the CN triple bond determines the bandgap
Eg required to reach deep-UV light (
Eg ~7.1 eV for α-KCN and 7.6 eV for β-KCN). The difference in K coordination slightly raises the bandgap
Eg from α-KCN with four coordinations (see
Figure 1c) to β-KCN with six coordinations (see
Figure 1d). Despite the staggered arrangement of isolated [CN] motifs, the resulting SHG effects (
d15 = 0.76 pm/V for α-KCN and
d11 = 0.59 pm/V for β-KCN) still exceed those of KDP (
d36 = 0.39 pm/V). Most importantly, their birefringence Δ
n are both sufficiently large, standing at ~0.117 and 0.121, respectively, for α-KCN and β-KCN. Consequently, the α-KCN and β-KCN crystals can achieve phase-matching SHG output wavelengths of 198 nm and 180 nm, which successfully expand into the deep-UV region. Furthermore, when the pyramid [AsC
3] motifs with lone-paired electrons (see
Figure 1e) are combined with the chained [CN] motifs, the bandgap
Eg in the resulting AsC
3N
3 [17][24] structure is determined by the As-C bond, reducing it to
Eg ~6.0 eV.
2.3. CNI and NaCNO with Chained [CNI] or [CNO] Motif
The birefringence and SHG effects can be significantly increased through the parallel alignment of the chained [CN] polar motifs. The coordination of isolated [CN] motifs with iodine (I) forms longer chain-like [CNI] dipole molecules (see
Figure 1f), which belong to this type of parallelly aligned structure
[17][24]. Consequently, these polar molecule crystals exhibit optimal isotropic alignment with a birefringence Δ
n of 0.7 and an SHG effect of 16 pm/V. Unfortunately, CNI has a bandgap
Eg of ~5.3 eV, much smaller than those of KCN, limiting its phase-matching SHG output to only 233 nm, which is insufficient for the deep-UV region, but still sufficient for the solar-blind UV region. Similarly, the [CN] motif in NaCNO coordinates with oxygen (O) to form a [CNO] polar anionic chain (see
Figure 1g) with a large birefringence Δ
n of 0.3, allowing the phase-matching SHG output to reach 239 nm
[17][24]. This performance is also achievable for the solar-blind UV band and superior to the UV NLO performance of the classical urea NLO crystal. However, the intrinsic dipole moment of the [CNO] motif is canceled by C and O at both ends, resulting in an SHG effect of only 0.66 pm/V, which is still larger than that of KDP.
2.4. α-Si
3.4. α-Si
3
N
4
and Si
2
N
2
O with Tetrahedral [SiN
4
] or [SiN
3
O] Motif
Another effective way to increase the structural anisotropy of a tetrahedron is by modifying N with O, which alters the difference in bond lengths of regular tetrahedra. For example, transitioning from α-Si
3N
4 to Si
2N
2O, as shown in
Figure 1h, elevates the local anisotropy of the tetrahedral [SiN
3O] structure, raises the birefringence Δ
n of Si
2N
2O to 0.08 (exp. ~0.11), and produces a phase-matching SHG output of 285 nm
[18][26]. Regrettably, the structural anisotropy of Si
2N
2O is still not sufficiently extended, which, together with the insufficiently large bandgap
Eg ~5.0 eV, results in a phase-matching SHG output that does not reach the UVC region. Additionally, the SHG effect (
e.g.,
d24 = 1.0 pm/V) is almost doubled compared to α-Si
3N
4.
2.5. Li
2
PNO
2
and PNF
2
with Poly-Chained [PN
2
O
2
] or [PN
2
F
2
] Motif
Similar to [SiN
4], the [PN
4] motif exhibits a small degree of structural anisotropy; nevertheless, the bandgap
Eg of the P-N bond is generally larger than that of the Si-N bond. By applying the strategy to P-N structures, it may be feasible to extend the UV NLO capacity into the solar-blind UV or even the deep-UV regions. The phosphazene system has been demonstrated to undergo reasonable expansion in this regard
[19][25]. In this case, Li
2PNO
2 alters the [PN] with O, forming a tetrahedral [PN
2O
2] poly-chained arrangement (see
Figure 1i). As a result, the birefringence Δ
n increases to 0.067, enabling phase-matching SHG output at 264 nm in the solar-blind UV region. If the [PN] is further modified to become [PN
2F
2] and arranged in a chain-parallel van der Waals (vdW) polymer structure (see
Figure 1j), the resulting birefringence Δ
n of the PNF
2 structure increases to 0.16. This yields a phase-matching SHG limit of 142 nm, which is shorter than KBBF’s 161 nm limit. It is important to emphasize that this meets the requirements for the application of a 150 nm atomic clock
[20][40].
3. Conclusions
In conclusion, according to a first-principles analysis of structure–property correlations, nitride semiconductors with wide tunable energy bandgaps and abundant coordination motifs are predicted to possess potential UV NLO properties. These properties include wide UV transparency, large SHG effects, sufficient birefringence, and short phase-matching SHG output wavelengths. Regarding nitride NLO materials, a research group has previously made notable contributions to the field
[17][18][19][24,25,26]. Since then, the exploration of many nitride NLO materials has flourished. As a short summary, benefits of nitride UV NLO material used include a of UV transmittance equivalent to that of oxides; superior NLO effects; increased anisotropy of the one-dimensional structure, resulting in superior phase-matching capacity; structural diversity in coordination with oxygen or halogen elements; the application of synthetic growth conditions to oxygen-free catalogs of ternary compounds, leading to the development of semiconducting properties; and material stability. Preliminary research indicates that wide-bandgap nitride materials exhibit distinct NLO characteristics across the UV to deep-UV range. Significantly, there is enhanced efficiency in the deep-UV region. This compensates for oxides’ lack of deep-UV phase-matching capacity and improves overall NLO harmonic output efficiency in the deep-UV region. These advantages, although previously overlooked, make these materials potential alternatives to oxides and ensure they have a noteworthy impact on the study of inorganic NLO materials.