Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Huawei Zhang.
Fungi are an important and prolific source of secondary metabolites (SMs) with diverse chemical structures and a wide array of biological properties. Fungal–fungal co-culture is the major source of new SMs (1–109) and consists of two types including liquid state fermentation (LSF) and solid state fermentation (SSF). Potato dextrose broth (PDB) and rice are the most common co-culture media for fungal LSF and SSF, respectively.
- fungus
- co-culture
- secondary metabolite
- natural product
1. Liquid State Fermentation (LSF)
Although the growth interference between two co-cultured fungal strains is very complex, it is well recognized that LSF can facilitate metabolite transportation and exchange, and trigger new SMs biosynthesis [33][1]. By the end of 2022, a total of 75 new SMs (1–75) had been obtained from LSF co-culture, such as polyketides, macrolides, terpenes, etc.
1.1. Potato Dextrose Broth (PDB)
Potato dextrose broth (PDB) is the most widespread used medium for growing fungi under aerobic condition. Among these LSF-derived new SMs, 54 compounds (1–54, Figure 1) were discovered in PDB using fungal–fungal co-culture. Chemical investigation of the co-culture broth of Nigrospora oryzae and Beauveria bassiana led to isolation of five new azaphilones (1–5), in which compound 2 had an unprecedented skeleton with a bicyclic oxygen bridge and compounds 4 and 5 showed significant nitric oxide (NO) inhibitory activity at 50 µM with inhibition rates of 37% and 39%, respectively [34][2].

Figure 1.
New SMs (
1
–
54
) isolated from fungal–fungal co-cultures using PDB.
Two new aryl esters (6 and 7) and six new protoilludane-type sesquiterpenes (8–13) were produced by Armillaria sp. when co-cultured with an endophytic fungus Epicoccum sp., and compound 13 showed moderate in vitro cytotoxic activities toward human cancer cell lines (HL-60, A549, MCF-7, SMMC-7721, and SW480) with IC50 values ranging from 15.80 to 23.03 μM and weak inhibitory activity against acetylcholinesterase (AChE) [35][3]. The co-culture of N. oryzae and Irpex lacteus from seeds of Dendrobium officinale resulted in production of four new SMs (14–17) belonging to two backbones of pulvilloric acid-type azaphilone as well as a tremulane sesquiterpene (18) equipped with strong anti-AChE activity at 50 μM [36][4]. Three new zinniol analogues, pleoniols A–C (19–21), were detected in the co-culture extract of two endophytic strains Pleosporales sp. F46 and Acremonium pilosum F47 [37][5]. The co-culture of two symbiotic fungi Phoma sp. YUD17001 and Armillaria sp. derived from Gastrodia elata afforded four polyketones (22–25) and a new nitrogenous compound (26) [38][6]. Phomretones A–F (27–32) were new bicyclic polyketides purified from the co-culture of Armillaria sp. and the endophytic strain Phoma sp. YUD17001 [39][7]. The co-culture of Penicillium fuscum and P. camembertii/clavigerum afforded the production of eight new 16-membered-ring macrolides (33–40), of which compound 33 exhibited the most potent antimicrobial activity against MRSA strains as well as Bacillus anthracis, Streptococcus pyogenes, Candida albicans, and C. glabrata. The mechanism of action (MoA) study indicated that 33 did not inhibit bacterial protein synthesis nor target their ribosomes, suggesting a novel mode of action for its antibiotic activity [40][8]. A novel alkaloid named harziaphilic acid (41) was produced in the co-culture of two plant beneficial fungi Trichoderma harzianum M10 and Talaromyces pinophilus F36CF and displayed selectively inhibitory effect on the proliferation of cancer cells [41][9]. Co-cultivation of Aspergillus nidulans with Epicoccum dendrobii produced eight new SMs (42–49) as well as six known compounds. The mechanisms that trigger metabolic changes during fungal–funal interactions was determined that VeA1 regulation requires the transcription factor SclB and velvet complex members LaeA and VelB to generate aspernidines as representative formation of SM in A. nidulans [1][10]. When co-cultured with Botrytis cinerea, the biocontrol fungus Purpureocillium lilacinum was shown to manufacture a new unusual linear polypeptide leucinostatin (50) in PDB detected by matrix-assisted laser desorption ionization-time of flight mass spectrometry imaging mass spectrometry (MALDI-TOF-IMS) [42][11]. The co-culture of Phellinus orientoasiaticus and Xylodon flaviporus yielded three new sesquiterpenes (51–53) [43][12]. One new 10-membered lactone (54) was separated from the co-culture of Nigrospora sp. and Stagonosporopsis sp. and exhibited antifungal activities against P. janthinellum, Aspergillus fumigatus, Phomopsis sp. and Alternaria sp. [44][13].
1.2. Other Liquid Media
Several other liquid media were uncommonly used in fungal–fungal co-culture system and resulted in production of 21 new SMs (55–75, Figure 2). Pleurotusins A (55) and B (56) along with five known terpenoids were produced by two edible fungi Pleurotus ostreatus SY10 and P. eryngii SY302 when co-cultivated in liquid medium consisting of glucose 10 g/L, KH2PO4 1 g/L, MgSO4 0.5 g/L, peptone 2 g/L, and 1 L sterilized water [45,46][14][15]. A new N-methoxypyridone analog (57) was not produced by the monoculture of the two endophytic strains Camporesia sambuci FT1061 and Epicoccum sorghinum FT1062, but was synthesized in the co-culture, which consisted of mannitol 20 g/L, sucrose 10 g/L, monosodium glutamate 5 g/L, KH2PO4 0.5 g/L, MgSO4·7 H2O 0.3 g/L, yeast extract 3 g/L, corn steep liquor 2 mL/L, and 1 L distilled water [47][16]. Owing to the appearance of pigments in adversarial zones between Penicillium pinophilum FKI-5653 and Trichoderma harzianum FKI-5655 on PDA plate, chemical study of their co-culture resulted in the isolation of a novel diphenyl ether (58) from glucose–peptone broth [48][17]. A new chlorinated bianthrone (59) was isolated from the co-culture of two different developmental stages of a marine alga-derived Aspergillus alliaceus strain in malt liquid medium and showed weak cytotoxic activity against the HCT-116 colon cancer and SK-Mel-5 melanoma cell lines [49][18]. This is the first example of self-induced metabolomic changes resulting in the production of allianthrones. Fungal strains Chaunopycnis sp. CMB-MF028 and T. hamatum CMB-MF030 were co-isolated from the inner tissue of an intertidal rock platform mollusc; co-cultivation of these fungi in ISP2 broth resulted in transcriptional activation of a new 2-alkenyl-tetrahydropyran (60) [50][19].
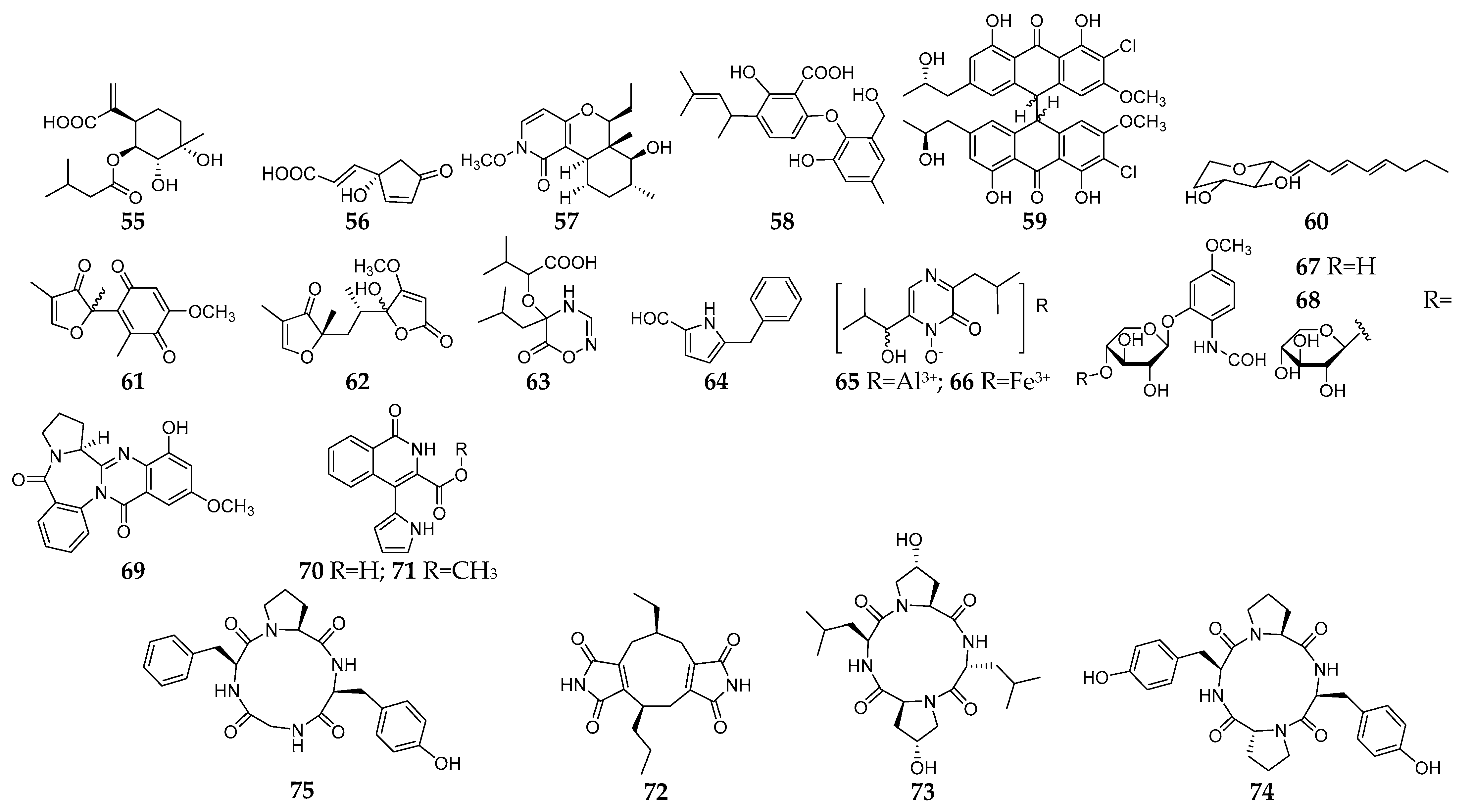
Figure 2.
New SMs (
55
–
75
) isolated from fungal–fungal co-cultures using other liquid medium.
Sclerotiorumins A–C (61–63) together with one pyrrole derivative 1-(4-benzyl-1H-pyrrol-3-yl) ethanone (64) and two metallo-organic complexes (65 and 66) were synthesized by two marine-derived fungi A. sclerotiorum and P. citrinum in the co-culture liquid medium which consisted of glucose, soluble starch, MgSO4, KH2PO4, peptone, and sea salt [51][20]. When strains Trametes versicolor and Ganoderma applanatum co-cultured in a medium containing glucose (10 g/L), KH2PO4 (1 g/L), MgSO4 (0.5 g/L), and peptone (2 g/L), two novel formamide derivatives (67 and 68) were highly synthesized and compound 68 displayed the potential to enhance the cell viability of a human immortalized bronchial epithelial cell line [52][21]. A novel alkaloid named as aspergicin (69) was isolated from the co-cultured mycelia of two marine-derived mangrove epiphytic Aspergillus sp. in GYP medium [53][22]. Two new antibacterial 1-isoquinolone analogs (70 and 71) were produced by two mangrove endophytic fungi (strain Nos. 1924 and 3893) in a co-culture system which consisted of glucose 10 g/L, peptone 2 g/L, yeast extracts 1 g/L, crude marine salt 3.5 g/L, and water 1 L [54,55][23][24]. A new diimide derivative (72) and three new cyclic peptides (73–75) were isolated and characterized from the co-culture of two mangrove fungi Phomopsis sp. K38 and Alternaria sp. E33 in liquid medium (glucose 10 g/L, peptone 2 g/L, yeast extract 1 g/L, NaCl 30 g/L), and compound 72 had weak cytotoxic activity against Hep-2 and HepG2 cells and compounds 73–75 exhibited moderate to high antifungal activities as compared with the positive control (Ketoconazole) [55,56,57][24][25][26].
2. Solid State Fermentation (SSF)
Metabolite exchange between co-cultured strains is limited in solid state fermentation (SSF) and could reduce direct growth competition or interference between co-cultured members and facilitate co-cultured SMs biosynthesis [33][1]. By the end of 2022, 34 new SMs (76–109, Figure 3, Figure 4 and Figure 5) were obtained from fungal–fungal co-culture using SSF method. Rice and potato dextrose agar (PDA) are the most commonly used SSF media for fungal–fungal co-culture.

Figure 3.
New SMs (
76
–
95
) isolated from fungal–fungal co-cultures using rice solid medium.
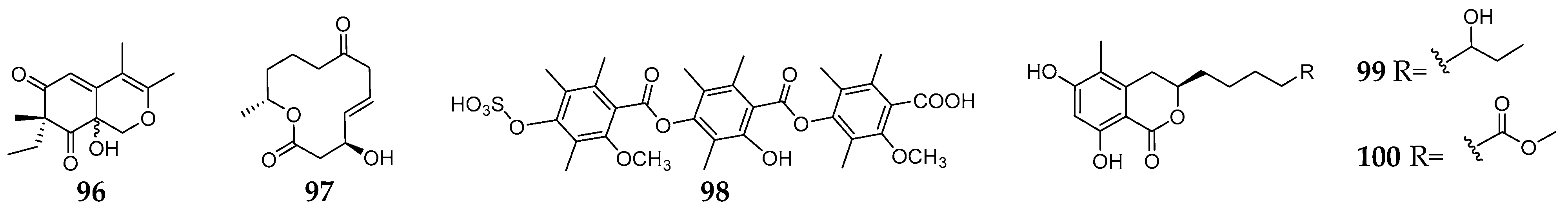
Figure 4.
New SMs (
96
–
100
) were derived from fungal–fungal co-cultures using PDA.
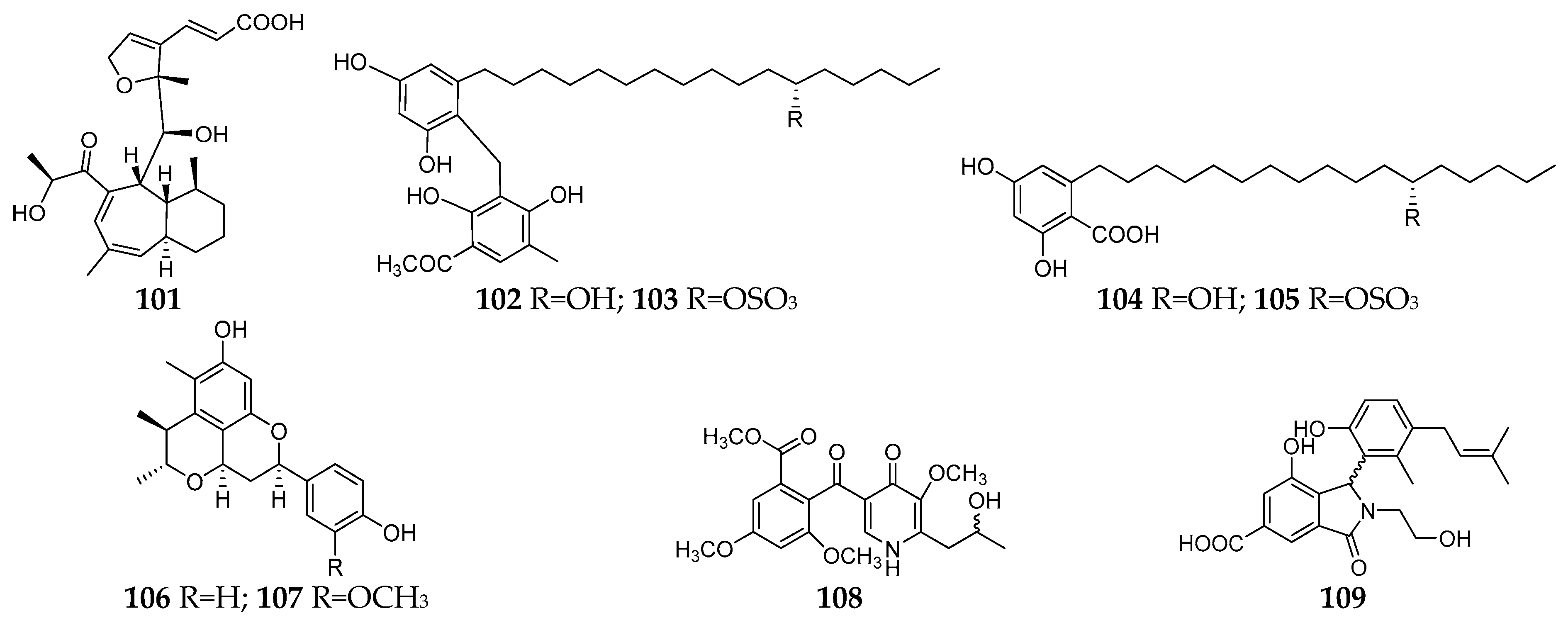
Figure 5.
New SMs (
101
–
109
) isolated from fungal–fungal co-cultures using PDA.
2.1. Rice Solid Medium
Chlorotetralone (76) was a new antifungal aromatic polyketide produced by the endophytic N. oryzae co-cultured with B. bassiana on rice solid medium [58][27]. Six new isoprenylated chromanes (77–80) including two new isoprenylated phenol glucosides (81–82) were obtained from the co-cultured rice medium of Pestalotiopsis sp. and P. bialowiezense [59[28][29],60], and compound 77 showed significant β-glucuronidase inhibitory potency [32][30]. Two new citrinin analogs (83 and 84) were detected in the co-culture of two marine algal-derived endophytic strains A. sydowii EN-534 and P. citrinum EN-535 [61][31]. Chermebilaene A (85) together with a new orthoester meroterpenoid (86) was the first natural sesquiterpene hybridized with octadecadienoic acid from the co-culture of two marine strains P. bilaiae MA-267 and P. chermesinum EN-480, and showed potent inhibitory activity against pathogenic fungi Ceratobasidium cornigerum and Edwardsiella tarda [62][32]. A new antimicrobial terrein derivative, namely asperterrein (87), was metabolized by a marine red alga-derived endophytic fungus A. terreus EN-539 co-cultured with symbiotic fungus Paecilomyces lilacinus EN-531, which was isolated from the inner tissues of the marine red alga Laurencia okamurai [63][33]. Five new prenylated indole alkaloids (88–92) were isolated from a co-culture of marine-derived fungi A. sulphureus KMM 4640 with Isaria felina KMM 4639 on rice solid medium, and compound 89 was able to inhibit the colony formation of human prostate cancer cells 22Rv1 at non-cytotoxic concentration of 10 μM [64][34]. Chemical investigation of a co-culture of the marine-derived fungi I. felina KMM 4639 and A. carneus KMM 4638 on rice led to the discovery of three new drimane-type sesquiterpenes (93–95) [65][35].
2.2. Potato Dextrose Agar (PDA)
Until now, only three chemical studies using PDA medium for fungal–fungal co-culture have been reported. Large-scale co-cultivation of Plenodomus influorescens and Pyrenochaeta nobilis on PDA resulted in isolation of a new azaphilone (96) and a new macrolides (97) [66][36]. Trichophyton rubrum and Bionectria ochroleuca were derived from different environments and were shown to generate a long-distance interaction zone, which led to the discovery of a substituted trimer of 3,5-dimethylorsellinic acid (98) [67][37]. Its nonsulfated form and three other known analogs were found in the monoculture of B. ochroleuca. Co-cultivation of Cosmospora sp. and Magnaporthe oryzae resulted in the production of two new dihydro-isocoumarins, soudanones H-I (99–100) on PDA medium [68][38].
2.3. Other Solid Media
Several other solid media have been used for fungal–fungal co-culture followed by chemical investigation. Whelone (101) was a new polyketide isolated and identified from the co-culture of A. fischeri NRRL 181 and T. labelliformis G536 on oatmeal [69][39]. Four new alkyl aromatics, penixylarins A−D (102–105), were isolated from a co-culture of an Antarctic deep-sea-derived fungus Penicillium crustosum PRB-2 and the mangrove-derived fungus Xylaria sp. HDN13-249 on solid medium mainly consisting of soluble starch, yeast extract, sucrose, maltose, bean flour, peptone, and agar [70][40]. Biological tests indicated that compound 104 exhibited potential antituberculosis effects. Citrifelins A (106) and B (107) possessing a unique tetracyclic framework were separated from the co-culture of Penicillium citrinum and Beauveria felina in wheat bran broth medium (100 mL of naturally sourced and filtered seawater, 100 g of wheat bran, and 0.6 g of dried potato powder) and showed inhibitory activities against several human and aquatic pathogens [71][41]. Co-cultivation of strains T. pinophilus 17F4103 and Paraphaeosphaeria sp. 17F4110 on a malt extract agar medium led to production of a new γ-pyridone (108) and enhancement of the production of penicillone C and D [72][42]. A new isoindolinone alkaloid, irpexine (109), was detected in the co-culture of I. lacteus with Phaeosphaeria oryzae, which consisted of malt extract 20 g/L, peptone 5 g/L, agar 15 g/L, and 1 L deionized water. In addition, a known green pigment hypoxyxylerone was detected in the axenic culture of I. lacteus, but its production was markedly enhanced by the co-culture [73][43].
References
- Zhuang, L.; Zhang, H. Utilizing cross-species co-cultures for discovery of novel natural products. Curr. Opin. Biotechnol. 2021, 69, 252–262.
- Zhang, Z.X.; Yang, X.Q.; Zhou, Q.Y.; Wang, B.Y.; Hu, M.; Yang, Y.B.; Zhou, H.; Ding, Z.T. New azaphilones from Nigrospora oryzae co-cultured with Beauveria bassiana. Molecules 2018, 23, 1816.
- Li, H.-T.; Tang, L.-H.; Liu, T.; Yang, R.-N.; Yang, Y.-B.; Zhou, H.; Ding, Z.-T. Protoilludane-type sesquiterpenoids from Armillaria sp. by co-culture with the endophytic fungus Epicoccum sp. associated with Gastrodia elata. Bioorg. Chem. 2020, 95, 103503.
- Zhou, Q.-Y.; Yang, X.-Q.; Zhang, Z.-X.; Wang, B.-Y.; Hu, M.; Yang, Y.-B.; Zhou, H.; Ding, Z.-T. New azaphilones and tremulane sesquiterpene from endophytic Nigrospora oryzae cocultured with Irpex lacteus. Fitoterapia 2018, 130, 26–30.
- Wang, Z.-R.; Yang, H.-X.; Peng, X.-P.; Li, G.; Lou, H.-X. Induced production of zinniol analogues by co-cultivation of two endophytic fungi in the same ecological niche. Phytochem. Lett. 2020, 35, 206–210.
- Li, H.T.; Zhou, H.; Duan, R.T.; Li, H.Y.; Tang, L.H.; Yang, X.Q.; Yang, Y.B.; Ding, Z.T. Inducing secondary metabolite production by co-culture of the endophytic fungus Phoma sp. and the symbiotic fungus Armillaria sp. J. Nat. Prod. 2019, 82, 1009–1013.
- Li, H.-T.; Liu, T.; Yang, R.; Xie, F.; Yang, Z.; Yang, Y.; Zhou, H.; Ding, Z.-T. Phomretones A-F, C-12 polyketides from the co-cultivation of Phoma sp. YUD17001 and Armillaria sp. RSC. Adv. 2020, 10, 18384–18389.
- Stierle, A.A.; Stierle, D.B.; Decato, D.; Priestley, N.D.; Alverson, J.B.; Hoody, J.; McGrath, K.; Klepacki, D. The berkeleylactones, antibiotic macrolides from fungal coculture. J. Nat. Prod. 2017, 80, 1150–1160.
- Vinale, F.; Nicoletti, R.; Borrelli, F.; Mangoni, A.; Parisi, O.A.; Marra, R.; Lombardi, N.; Lacatena, F.; Grauso, L.; Finizio, S.; et al. Co-culture of plant beneficial microbes as source of bioactive metabolites. Sci. Rep. 2017, 7, 14330.
- Wang, G.; Ran, H.; Fan, J.; Keller, N.P.; Liu, Z.; Wu, F.; Yin, W.B. Fungal-fungal cocultivation leads to widespread secondary metabolite alteration requiring the partial loss-of-function VeA1 protein. Sci. Adv. 2022, 8, eabo6094.
- Liu, R.; Khan, R.A.A.; Yue, Q.; Jiao, Y.; Yang, Y.; Li, Y.; Xie, B. Discovery of a new antifungal lipopeptaibol from Purpureocillium lilacinum using MALDI-TOF-IMS. Biochem. Biophys. Res. Commun. 2020, 527, 689–695.
- Pham, H.T.; Doan, T.P.; Kim, H.W.; Kim, T.W.; Park, S.Y.; Kim, H.; Lee, M.; Kim, K.H.; Oh, W.K.; Lim, Y.W.; et al. Cyclohumulanoid sesquiterpenes induced by the noncompetitive coculture of Phellinus orientoasiaticus and Xylodon flaviporus. J. Nat. Prod. 2022, 85, 511–518.
- Chen, J.X.; Xia, D.D.; Yang, X.Q.; Yang, Y.B.; Ding, Z.T. The antifeedant and antifungal cryptic metabolites isolated from tobacco endophytes induced by host medium and coculture. Fitoterapia 2022, 163, 105335.
- Ge, X.; Wang, Y.; Sun, C.; Zhang, Z.; Song, L.; Tan, L.; Li, D.; Yang, S.; Yu, G. Secondary metabolites produced by coculture of Pleurotus ostreatus SY10 and Pleurotus eryngii SY302. Chem. Biodivers. 2022, 19, e202100832.
- Zhang, B.; Li, Y.; Zhang, F.; Linhardt, R.J.; Zeng, G.; Zhang, A. Extraction, structure and bioactivities of the polysaccharides from Pleurotus eryngii: A review. Int. J. Biol. Macromol. 2020, 150, 1342–1347.
- Li, C.; Sarotti, A.M.; Yang, B.; Turkson, J.; Cao, S. A new N-methoxypyridone from the co-cultivation of hawaiian endophytic fungi Camporesia sambuci FT1061 and Epicoccum sorghinum FT1062. Molecules 2017, 22, 1166.
- Nonaka, K.; Abe, T.; Iwatsuki, M.; Mori, M.; Yamamoto, T.; Shiomi, K.; Omura, S.; Masuma, R. Enhancement of metabolites productivity of Penicillium pinophilum FKI-5653, by co-culture with Trichoderma harzianum FKI-5655. J. Antibiot. 2011, 64, 769–774.
- Mandelare, P.E.; Adpressa, D.A.; Kaweesa, E.N.; Zakharov, L.N.; Loesgen, S. Coculture of two developmental stages of a marine-derived Aspergillus alliaceus results in the production of the cytotoxic bianthrone allianthrone A. J. Nat. Prod. 2018, 81, 1014–1022.
- Shang, Z.; Salim, A.A.; Capon, R.J. Chaunopyran A: Co-cultivation of marine mollusk-derived fungi activates a rare class of 2-alkenyl-tetrahydropyran. J. Nat. Prod. 2017, 80, 1167–1172.
- Bao, J.; Wang, J.; Zhang, X.Y.; Nong, X.H.; Qi, S.H. New furanone derivatives and alkaloids from the co-culture of marine-derived fungi Aspergillus sclerotiorum and Penicillium citrinum. Chem. Biodivers. 2017, 14, e1600327.
- Yao, L.; Zhu, L.P.; Xu, X.Y.; Tan, L.L.; Sadilek, M.; Fan, H.; Hu, B.; Shen, X.T.; Yang, J.; Qiao, B.; et al. Discovery of novel xylosides in co-culture of basidiomycetes Trametes versicolor and Ganoderma applanatum by integrated metabolomics and bioinformatics. Sci. Rep. 2016, 6, 33237.
- Zhu, F.; Li, J.-S.; Xie, W.-C.; Shi, J.-J.; Xu, F.; Song, Z.-F.; Liu, Y.-L. Structure revision of aspergicin by the crystal structure of aspergicine, a co-occurring isomer produced by co-culture of two mangrove epiphytic fungi. Nat. Prod. Res. 2017, 31, 2268–2272.
- Zhu, F.; Lin, Y.C. Marinamide, a novel alkaloid and its methyl ester produced by the application of mixed fermentation technique to two mangrove endophytic fungi from the South China Sea. Chinese Sci. Bull. 2006, 51, 1426–1430.
- Li, C.; Wang, J.; Luo, C.; Ding, W.; Cox, D.G. A new cyclopeptide with antifungal activity from the co-culture broth of two marine mangrove fungi. Nat. Prod. Res. 2014, 28, 616–621.
- Li, C.Y.; Ding, W.J.; Shao, C.L.; She, Z.G.; Lin, Y.C. A new diimide derivative from the co-culture broth of two mangrove fungi (strain no. E33 and K38). J. Asian Nat. Prod. Res. 2010, 12, 809–813.
- Huang, S.; Ding, W.; Li, C.; Cox, D.G. Two new cyclopeptides from the co-culture broth of two marine mangrove fungi and their antifungal activity. Pharmacogn. Mag. 2014, 10, 410–414.
- Zhang, Z.-X.; Yin, H.-Y.; Yang, Y.-B.; Wang, D.-L.; Zhao, T.-D.; Wang, C.-F.; Yang, X.-Q.; Ding, Z.-T. A new chlorinated tetralone from co-culture of insect-pathogenic Beauveria bassiana and phytopathogenic Nigrospora oryzae. Chem. Nat. Compd. 2021, 57, 297–299.
- Yang, B.; Tong, Q.; Lin, S.; Guo, J.; Zhang, J.; Liu, J.; Wang, J.; Zhu, H.; Hu, Z.; Zhang, Y. Cytotoxic butenolides and diphenyl ethers from the endophytic fungus Pestalotiopsis sp. Phytochem. Lett. 2019, 29, 186–189.
- Zhang, Q.; Yang, B.; Li, F.; Liu, M.; Lin, S.; Wang, J.; Xue, Y.; Zhu, H.; Sun, W.; Hu, Z.; et al. Mycophenolic acid derivatives with immunosuppressive activity from the coral-derived fungus Penicillium bialowiezense. Mar. Drugs 2018, 16, 230.
- Li, F.; Yan, S.; Huang, Z.; Gao, W.; Zhang, S.; Mo, S.; Lin, S.; Wang, J.; Hu, Z.; Zhang, Y. Inducing new bioactive metabolites production from coculture of Pestalotiopsis sp. and Penicillium bialowiezense. Bioorg. Chem. 2021, 110, 104826.
- Yang, S.Q.; Li, X.M.; Li, X.; Li, H.L.; Meng, L.H.; Wang, B.G. New citrinin analogues produced by coculture of the marine algal-derived endophytic fungal strains Aspergillus sydowii EN-534 and Penicillium citrinum EN-535. Phytochem. Lett. 2018, 25, 191–195.
- Meng, L.H.; Li, X.M.; Li, H.L.; Wang, B.G. Chermebilaenes A and B, new bioactive meroterpenoids from co-cultures of marine-derived isolates of Penicillium bilaiae MA-267 and Penicillium chermesinum EN-480. Mar. Drugs 2020, 18, 339.
- Li, H.L.; Li, X.M.; Yang, S.Q.; Cao, J.; Li, Y.H.; Wang, B.G. Induced terreins production from marine red algal-derived endophytic fungus Aspergillus terreus EN-539 co-cultured with symbiotic fungus Paecilomyces lilacinus EN-531. J. Antibiot. 2020, 73, 108–111.
- Afiyatullov, S.S.; Zhuravleva, O.I.; Antonov, A.S.; Berdyshev, D.V.; Pivkin, M.V.; Denisenko, V.A.; Popov, R.S.; Gerasimenko, A.V.; von Amsberg, G.; Dyshlovoy, S.A.; et al. Prenylated indole alkaloids from co-culture of marine-derived fungi Aspergillus sulphureus and Isaria felina. J. Antibiot. 2018, 71, 846–853.
- Zhuravleva, O.I.; Belousova, E.B.; Oleinikova, G.K.; Antonov, A.S.; Khudyakova, Y.V.; Rasin, A.B.; Popov, R.S.; Menchinskaya, E.S.; Trinh, P.T.H.; Yurchenko, A.N.; et al. Cytotoxic drimane-type sesquiterpenes from co-culture of the marine-derived fungi Aspergillus carneus KMM 4638 and Beauveria felina (=Isaria felina) KMM 4639. Mar. Drugs 2022, 20, 584.
- Oppong-Danquah, E.; Budnicka, P.; Blumel, M.; Tasdemir, D. Design of fungal co-cultivation based on comparative metabolomics and bioactivity for discovery of marine fungal agrochemicals. Mar. Drugs 2020, 18, 73.
- Bertrand, S.; Schumpp, O.; Bohni, N.; Monod, M.; Gindro, K.; Wolfender, J.L. De novo production of metabolites by fungal co-culture of Trichophyton rubrum and Bionectria ochroleuca. J. Nat. Prod. 2013, 76, 1157–1165.
- Oppong-Danquah, E.; Blumel, M.; Scarpato, S.; Mangoni, A.; Tasdemir, D. Induction of isochromanones by co-cultivation of the marine fungus Cosmospora sp. and the phytopathogen Magnaporthe oryzae. Int. J. Mol. Sci. 2022, 23, 782.
- Knowles, S.L.; Raja, H.A.; Isawi, I.H.; Flores-Bocanegra, L.; Reggio, P.H.; Pearce, C.J.; Burdette, J.E.; Rokas, A.; Oberlies, N.H. Wheldone: Characterization of a unique scaffold from the coculture of Aspergillus fischeri and Xylaria flabelliformis. Org. Lett. 2020, 22, 1878–1882.
- Yu, G.; Sun, Z.; Peng, J.; Zhu, M.; Che, Q.; Zhang, G.; Zhu, T.; Gu, Q.; Li, D. Secondary metabolites produced by combined culture of Penicillium crustosum and a Xylaria sp. J. Nat. Prod. 2019, 82, 2013–2017.
- Meng, L.H.; Liu, Y.; Li, X.M.; Xu, G.M.; Ji, N.Y.; Wang, B.G. Citrifelins A and B, citrinin adducts with a tetracyclic framework from cocultures of marine-derived isolates of Penicillium citrinum and Beauveria felina. J. Nat. Prod. 2015, 78, 2301–2305.
- Murakami, S.; Hayashi, N.; Inomata, T.; Kato, H.; Hitora, Y.; Tsukamoto, S. Induction of secondary metabolite production by fungal co-culture of Talaromyces pinophilus and Paraphaeosphaeria sp. J. Nat. Med. 2020, 74, 545–549.
- Sadahiro, Y.; Kato, H.; Williams, R.M.; Tsukamoto, S. Irpexine, an isoindolinone alkaloid produced by coculture of endophytic fungi, Irpex lacteus and Phaeosphaeria oryzae. J. Nat. Prod. 2020, 83, 1368–1373.
More
