You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Varol TUNALI and Version 2 by Fanny Huang.
The shifting landscape of parasitic infections affecting the central nervous system (CNS) is challenging the established paradigms in Europe. While traditionally confined to low- and middle-income countries, these infections are now encroaching upon non-endemic regions, propelled with escalating international travel, immunosuppression trends, and climatic variations.
- central nervous system
- parasitic infections
- one health
- Europe
- travel medicine
1. Introduction
The shifting landscape of parasitic infections affecting the central nervous system (CNS) is challenging the established paradigms in Europe. While traditionally confined to low- and middle-income countries, these infections are now encroaching upon non-endemic regions, propelled with escalating international travel, immunosuppression trends, and climatic variations [1][2][1,2]. The augmentation of risk via prolonged immunosuppression and immunosuppressive medications accentuates the gravity of the situation [3]. However, the limited availability of empirical data, due to underreporting, underscores the exigency for a prompt and comprehensive comprehension of this evolving scenario [4].
CNS parasitic infections, such as neurocysticercosis, represent a growing global health concern, particularly in developing nations, where it is the leading cause of preventable epilepsy [5]. Yet, obtaining accurate global estimates of the prevalence and burden of CNS parasitic infections remains challenging due to limited population-wide data, the vast heterogeneity in infection types and locations, and a suspected underdiagnosis and underreporting in resource-limited regions [6].
2. Protozoal Infections of Central Nervous System
2.1. Amebiasis (
Entamoeba histolytica
)
Amebiasis, caused by Entamoeba histolytica, presents a global health challenge, particularly in regions with inadequate sanitation. Endemic areas in Africa, Asia, and Latin America report an estimated annual burden of around 50 million symptomatic cases, accompanied with a considerable number of asymptomatic carriers [7][10]. GeoSentinel Surveillance Network data underscore E. histolytica as the third most common pathogen among travelers returning with infectious gastrointestinal disease, accounting for 12.5% of confirmed cases and an estimated incidence of 14 cases per 1000 returning travelers [8][11]. In parts of Asia, Europe, North America, and Australia, specific populations, including gays, bisexuals, and other men who have sex with men (MSM), are identified as being at higher risk of acquiring amebiasis [9][12]. In Europe, while relatively infrequent, instances of amebiasis are often linked to travel, immigration, or localized transmission, necessitating vigilant surveillance and strategic intervention strategies [9][12]. There have been reports of brain abscesses caused by E. histolytica from Turkey and Spain in Europe [10][11][13,14] (Table 1).
Table 1. Epidemiology, mode of transmission, diagnosis, and treatment of protozoal diseases of the CNS.
| Parasites and Diseases | Countries with Reported Cases (Europe) | Mode of Transmission | Diagnosis | Treatment |
|---|---|---|---|---|
| Entamoeba histolytica Amebiasis |
Turkey, Spain [10][11][13,14] | Ingestion of cysts | Radiology, serology, molecular | Metronidazole, surgical drainage |
| Free living amoeba Naegleria fowleri Acanthamoeba spp. Balamuthia mandrillaris Sappinia |
Belgium, Czech Republic, Italy, the Netherlands, United Kingdom [12][13][14][20[,2215,23],24] | Trophozoites through nasal passage, olfactory nerve Cysts or trophozoites through eye, nasal passage, lung, or skin |
Microscopy, molecular | Symptomatic |
| Plasmodium falciparum Cerebral malaria |
United Kingdom, Switzerland [16][17][33,34], Italy, Germany, France, Denmark, Belgium [18][32] | Mosquito bite | Microscopy, molecular | Quinine and artemisinin |
| Toxoplasma gondii Toxoplasmosis |
France, Spain, Czech Republic, United Kingdom, Germany, Denmark, Serbia [19][39] | Ingestion of oocysts or tissue cysts | Radiology, serology | Pyrimethamine and sulfadiazine |
| Trypanosoma brucei African trypanosomiasis (HAT) |
France, Italy, Spain, the United Kingdom, Germany, the Netherlands, Belgium, Norway, Sweden, Switzerland, Poland [20][56] | Tsetse fly bite | Microscopy, molecular | Pentamidine, eflornithine, nifurtimox, melarsoprol, suramin |
| Trypanosoma cruzi South American trypanosomiasis (Chagas Disease (CD)) |
Spain, Portugal, Italy, France, the United Kingdom, Switzerland [21][54] | Metacyclic trypomastigotes through mucous membranes or skin abrasions | Microscopy, molecular | Nifurtimox, benznidazole |
The pathogenesis of an amoebic brain abscess intricately involves E. histolytica’s interactions with the host’s immune system and brain tissue [22][15]. Trophozoites originating from the intestines, following hematogenous spread, reach distant locations, including the brain. By adhering to endothelial cells and overcoming the blood–brain barrier, these trophozoites infiltrate brain tissue [23][16]. Subsequent immune responses elicit inflammation and tissue damage [24][17]. The culmination of these events leads to the formation of pus-filled abscesses within the brain. The resultant abscess formation underscores severe clinical manifestations, including severe headaches, fever, seizures, and neurological deficits [22][15]. A timely diagnosis is pivotal in curbing brain tissue damage and potential complications associated with this life-threatening condition.
Diagnosing amoebic brain abscesses involves a multifaceted approach, including clinical evaluation, imaging techniques, and laboratory confirmation [23][16]. Identification of E. histolytica trophozoites in brain tissue samples, obtained via biopsy or surgical drainage, confirms the diagnosis [9][12]. Efficient management entails a combined pharmacological approach and surgical intervention if needed. Metronidazole, effective against E. histolytica, is coupled with other antibiotics to address potential bacterial infections [23][16]. Surgical drainage alleviates pressure and removes infected tissue, warranting neurosurgical consultation and close monitoring.
2.2. Free-Living Amebiasis
Free-living amoebae (FLA), including Naegleria fowleri, Acanthamoeba spp., Balamuthia mandrillaris, and Sappinia, cause severe CNS diseases and infections in humans and animals [25][18]. FLA are found in water and soil worldwide, with infections often linked to water exposure or contact lenses. Since its establishment in 1962, the CDC has conducted passive surveillance for Primary Amebic Meningoencephalitis (PAM) in the United States, with 145 reported cases largely originating from southern states, while globally, Naegleria fowleri, the amoeba responsible for PAM, has been detected on every continent except Antarctica, with recent estimates of 235 to 260 reported cases worldwide [26][19]. In Europe, PAM cases have been primarily associated with locations like natural warm springs in the City of Bath, United Kingdom, and artificially heated areas such as power stations and baths in Belgium and the Czech Republic [12][20].
FLA’s complex pathogenesis involves trophozoites and cyst stages, cytopathic effects, invasion, and immune responses [27][21]. The most severe FLA infections involve the CNS. Granulomatous amoebic encephalitis (GAE) and primary amoebic meningoencephalitis (PAM) are the main categories [25][18]. GAE is characterized by subacute to chronic neurological symptoms, including headache, visual disturbances, neurological deficits, and coma, with a high mortality rate. PAM, caused by N. fowleri, progresses rapidly with flu-like symptoms, followed by severe neurological signs and death within days [25][18]. Differential diagnoses vary based on the site of infection and risk factors. Although rare, cases of GAE, PAM, and keratitis associated with the FLA have been reported in Europe [13][14][15][22,23,24].
The diagnosis of FLA infections relies on microscopy, histopathology, and molecular tests. Treatment effectiveness is highest when administered early in the course of the disease. Combination antimicrobial therapies are often used, with miltefosine showing promise in treatment [28][25]. In Europe, FLA infections are less common than in some other regions, and there is a focus on awareness, prevention, and a prompt diagnosis. Surveillance, awareness campaigns, and proper contact lens hygiene education are important in managing these rare but serious infections.
2.3. Cerebral Malaria
Cerebral malaria is a severe complication of Plasmodium falciparum infection and is characterized by the sequestration of infected red blood cells (iRBCs) in the cerebral microvasculature, leading to endothelial activation, inflammation, and disruption of the blood–brain barrier [29][26]. The pathogenesis of cerebral malaria involves a complex interplay between parasite-related factors, host immune responses, and endothelial dysfunction. One of the key mechanisms in the development of cerebral malaria is the sequestration of iRBCs in the cerebral microvasculature. This sequestration leads to the activation of endothelial cells, triggering the release of pro-inflammatory cytokines, chemokines, and adhesion molecules [30][27]. This inflammatory response contributes to the recruitment and activation of immune cells, including monocytes, T cells, and platelets. These cells release additional inflammatory mediators, amplifying the immune response and exacerbating endothelial dysfunction [31][28]. Endothelial dysfunction and disruption of the blood–brain barrier further contribute to the pathogenesis of cerebral malaria. Impaired endothelial function results in increased vascular permeability, allowing the leakage of fluid and proteins into the brain parenchyma, leading to cerebral edema and increased intracranial pressure [32][29].
According to a Eurosurveillance report, malaria stood out as the predominant arthropod-borne ailment among travelers from Africa, with 34,235 reported cases (with an incidence rate of 28.8 per 100,000 travelers) between 2015 and 2019, exhibiting a steady rise in the number of cases during this period except for a decline in 2016, while the case fatality ratio consistently remained below 1% [33][30]. According to a meta-analysis, 13.2% of the imported malaria cases in Europe were allocated as severe malaria, with a 6.3% prevalence of deaths among severe malaria patients. Asia, on the other hand, had the highest proportion of deaths from severe imported malaria, followed by Europe [34][31]. Between 2006 and 2014, a multicenter study conducted by The European Network for Tropical Medicine and Travel Health (TropNet) analyzed 185 patients with severe malaria treated in 12 European countries, reporting that 46 (25%) of these patients presented with cerebral malaria, which was found to be associated with older age [18][32].
A retrospective study conducted at the Hospital for Tropical Diseases in London, United Kingdom, included 124 patients with severe falciparum malaria admitted to the ICU, revealing that cerebral malaria was the most prevalent condition, and both cerebral malaria and acute kidney injury were observed earlier (median day 1) compared to acute respiratory distress syndrome (median day 3) [16][33]. According to a recent report from Switzerland, malaria cases in Switzerland primarily originate from West Africa, especially among travelers, indicating the need for focused travel medicine efforts in that region, while potential future waves of migrants from Afghanistan and climate change could contribute to the establishment of P. vivax malaria transmission in Switzerland, and post-pandemic travel trends may increase malaria cases [17][34].
The debated post-malaria neurological syndrome (PMNS), which was defined in 1997 by Nguyen et al., is another manifestation of P. falciparum in the CNS. This syndrome is characterized by the emergence of encephalitic signs in patients after a symptom-free period (with a median time of 96 h) following a malaria cure, and, notably, these patients have negative blood smears for P. falciparum and negative results in investigations for other potential causes [35]. Despite its association with mefloquine treatment by Nguyen et al., several reports from Europe challenge the notion of an association or causative effect between mefloquine and the condition, instead highlighting numerous cases treated with quinine and artemisinin [36][37][36,37].
2.4. Toxoplasmosis
Toxoplasma gondii is a parasite that causes zoonotic infections in humans and infects a wide range of animals. While it usually only results in mild illness in healthy individuals, toxoplasmosis can be a common opportunistic infection with high mortality in immunocompromised individuals, often due to reactivation of infection in the CNS [38]. During the acute phase of infection, interferon-dependent immune responses control rapid parasite expansion and mitigate acute disease symptoms. However, after dissemination, the parasite differentiates into semi-dormant cysts within muscle cells and neurons, where they persist for life in the infected host [38] (Figure 1). T. gondii uses various strategies to breach the blood–brain barrier and enter the CNS. These mechanisms include potential Trojan Horse-like entry via infected monocytes and efficient transmigration of extracellular tachyzoites, which may be the primary mode of parasite entry, ultimately leading to the formation of tissue cysts in the CNS. The mechanisms of reactivation are not fully understood but it is thought that it may be due to a decline in cell-mediated immunity. Recent studies suggest that cellular stress is a key factor not only in prompting development of bradyzoites but also in maintaining the encysted form. In immunocompromised patients, reactivation of Toxoplasma infection is typically seen after the CD4+ T-cell count drops below 100–200 cells/mm3 [38].
Currently, it is reported that 30% of the world’s population has antibodies against T. gondii and the estimated pooled prevalence of T. gondii infection in people with HIV infection is 35.8% overall, with specific rates of 30.1% in Western and Central Europe [19][39]. Toxoplasma encephalitis and Toxoplasma retinitis, present in approximately 30% of this risk group, were considered AIDS-defining opportunistic infections before the introduction of highly active antiretroviral therapy (HAART). Even today, toxoplasmosis remains the leading cause of neurological disease in HIV-positive patients, often leading to severe pathology or fatal outcomes [39][40][40,41]. Patients with CNS toxoplasmosis commonly experience one or more CNS mass lesions, resulting in symptoms such as headache, confusion, lethargy, changes in consciousness, convulsions, paralysis, emotional dysregulations, poor coordination, muscle weakness, seizures, and alterations in alertness [19][39].
In addition, several studies have suggested a potential association between toxoplasmosis and mental health disorders, particularly schizophrenia and suicidal behavior. Torrey et al. found a higher prevalence of T. gondii infection among individuals with schizophrenia, indicating a possible link between the parasite and schizophrenia symptoms [41][42]. Additionally, Sutterland et al. conducted a systematic review that provided evidence of an association between toxoplasmosis and suicide attempts [42][43].
CNS toxoplasmosis affects immunocompromised individuals and requires a swift diagnosis and treatment to prevent severe neurological complications. The diagnosis involves neuroimaging, revealing characteristic ring-enhancing lesions on MRI, alongside serological tests and a CSF analysis for antibodies and DNA detection [43][44] (Figure 1).
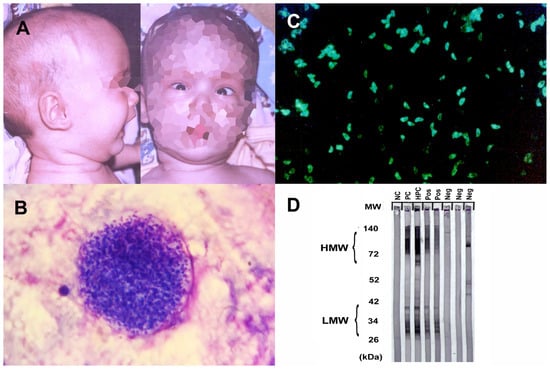
Figure 1. Physical examination and serological findings of some parasitic CNS infections. (A) Hydrocephalus and sunset eye sign due to congenital toxoplasmosis. (B) Toxoplasma gondii latent tissue cysts in the brain, which, enclosing hundreds of bradyzoites, may remain throughout the life and reactivation of these bradyzoites may lead to fatal toxoplasmic encephalitis. (C) Toxoplasma tachyzoites with bright diffuse or peripheral fluorescence in indirect immunofluorescence assay (IFA) analysis is considered as positive for anti-Toxoplasma antibodies. (D) Western blot testing for toxocariasis. The first line (NC) to the left is a negative control, the second and third lines (PC, HPC) are positive and high-positive controls, respectively, and the rest are patient samples. Bands of lower molecular weight (LMW) are specific for anti-Toxocara antibodies and considered positive for Toxocara serology. HMW, high molecular weight; MW, molecular weight; kDa, kilodalton; Pos, positive; Neg, negative).
Treatment combines antimicrobial therapy and immune restoration. Pyrimethamine and sulfadiazine form the core of antimicrobial treatment, supplemented with leucovorin to mitigate side effects. Corticosteroids might be used to control inflammation. Maintaining lower doses of antimicrobial drugs prevents relapse. Immune function restoration, often through antiretroviral therapy for HIV/AIDS patients, addresses the underlying immune deficiency. Regular monitoring is essential, and early intervention coupled with immune management generally leads to a favorable prognosis [44][45].
2.5. Trypanosomiasis
The pathophysiology of trypanosomiasis in the CNS differs depending on the type and stage of the infection. In Human African trypanosomiasis (HAT), caused by Trypanosoma brucei species, the parasites undergo a complex life cycle in the tsetse fly vector, where they multiply and develop into epimastigotes in the salivary glands. These forms are injected into the human bloodstream during a tsetse fly bite, where they differentiate into trypomastigotes and rapidly divide with binary fission [45][46]. The parasites evade the host immune system by changing their variant surface glycoproteins (VSGs), which are exposed on their plasma membrane and elicit a strong antibody response. The parasites disseminate through the bloodstream and lymphatics, reaching various organs and tissues, including the skin, spleen, liver, heart, kidneys, and eyes. Eventually, the parasites cross the blood–brain barrier into the CNS, causing the meningo-encephalitic or second stage [46][47]. The mechanisms with which the parasites invade the CNS are not fully understood but may involve direct transcytosis across endothelial cells, paracellular migration through tight junctions, or Trojan Horse mechanisms involving infected immune cells [47][48]. Once in the CNS, the parasites induce a progressive neuroinflammation that involves the activation of microglia and astrocytes, the production of pro-inflammatory cytokines and chemokines, the recruitment of peripheral immune cells, and the disruption of the blood–brain barrier integrity. These changes underlie the altered behavior in the late or secondary disease stages, prevalent in the chronic Gambian form, characterized by hypersomnia leading, and if untreated or if treatment is followed by reactive changes, to coma and death [46][47]. Reported from more than 20 countries in Africa, in 2015, a total of 2804 cases of HAT were reported to WHO, with the majority (90%) caused by T. brucei gambiense, marking a significant 90% reduction since 1999 [48][49].
In American trypanosomiasis (Chagas disease, CD), caused by Trypanosoma cruzi, the infection can be divided into three stages: acute, intermediate, and chronic. The parasites are transmitted by blood-sucking bugs of subfamily Triatominae that defecate on the skin after biting and depositing metacyclic trypomastigotes on it [49][50]. The parasites enter through mucous membranes or skin abrasions and invade various cell types, including muscle cells, macrophages, and neurons. The parasites differentiate into amastigotes and multiply intracellularly until they lyse the host cell and release trypomastigotes that can infect new cells or enter the bloodstream [49][50]. In the acute stage, which lasts for 4 to 8 weeks after infection, the parasite produces direct destructive and inflammatory changes in various organs and tissues, including the CNS. The CNS involvement can manifest as meningoencephalitis or chagomas (granulomatous lesions) that can be life-threatening, but which normally resolve spontaneously or with treatment [46][47]. The intermediate stage is a prolonged asymptomatic period that can last for years or decades, during which most parasites are suppressed by the host immune system and only low levels of parasitemia are detected. However, some parasites may persist in tissues such as cardiac muscle or neurons and cause chronic damage [50][51]. Characterized by alterations in progressive peripheral neuroimmunopathology, the chronic stage involves autoimmune destruction of various nerve components, particularly the autonomic innervation of the heart and gut. This results in cardiomyopathy or mega syndromes (such as esophagus or colon dilation), affecting approximately 30% of individuals with chronic infections [51][52]. CD remains endemic in 21 countries of continental Latin America, and globally, its prevalence decreased by 11.3%, declining from an estimated 7,292,889 cases in 1990 to 6,469,283 cases in 2019 [52][53].
CD and HAT have surfaced in Europe primarily due to migration from endemic regions. Countries like Spain, Portugal, Italy, France, the United Kingdom, and Switzerland, which host substantial migrant populations from CD-endemic areas, have encountered the impact of CD [21][54]. Spain, in particular, has documented CD cases among diverse patient groups, including those with HIV infection and rheumatologic disorders, transplant recipients, and cancer patients [53][55]. Europe has also experienced imported HAT cases, with historical data dating back to 1904–1963 and recent reports in countries like France, Italy, Spain, the United Kingdom, Germany, the Netherlands, Belgium, Norway, Sweden, Switzerland, and Poland [20][56].
