Thyroid cancer (TC), the most frequent malignancy of the endocrine system, currently ranks in the United States as the 13th most common cancer diagnosis overall and the sixth most common among women
[1][2][1,2]. Among the histological types, papillary thyroid carcinoma (PTC) accounts for approximately 90% of all cases, followed by follicular thyroid carcinoma (FTC) (4%), Hürthle-cell carcinoma (2%), medullary thyroid carcinoma (2%), and anaplastic thyroid carcinoma (ATC) (1%)
[2]. In 2020, the estimated number of new cases of TC was approximately 449,000 in women and 137,000 in men globally, with most countries having an age-standardized incidence rate that is about three times higher in women (10.1 per 100,000) than in men (3.1 per 100,000)
[3]. Since the mid-1970s to 2013, the incidence of TC has markedly risen in the United States, with an annual rate increase of 3%, mainly owing to small (<2 cm) PTCs
[4]. A similar trend was also observed in other developed countries while, in contrast, mortality rates remained stable or declined in most territories
[3][5][3,5]. These epidemiological features have been largely attributed to overdiagnosis
[3]. Indeed, the changes in clinical practice guidelines recommended by the American Thyroid Association, including the reclassification of the non-invasive encapsulated follicular variant subtype of PTC (FVPTC) from a malignant to an in situ neoplasm (“non-invasive follicular thyroid neoplasm with papillary-like nuclear features”), having an extremely low risk of adverse outcomes, like tumor recurrence or spread, coincided with a decline of FVPTC incidence by 10% in recent years
[6][7][6,7]. On the other hand, a continuous increase in the incidence of larger classical PTC and other PTC variants was recorded over time, indicating that the reasons underlying TC incidence trends were multifactorial
[6]. Although more than 95% of TC cases belong to differentiated TC (PTC, FTC, and Hürthle-cell carcinoma), deriving from thyroid follicular epithelial cells and characterized by an excellent prognosis
[8][9][8,9], the etiology of TC is not fully clarified
[10]. While childhood exposure to ionizing radiation, a history of benign thyroid nodules and goiter, and a family history of proliferative thyroid disease are established risk factors for TC, the role of other modifiable factors, such as dietary patterns and microbiota composition in TC carcinogenesis, have been recently explored
[2][11][12][13][2,11,12,13]. Deficiency of iodine, considered a trace element essential for the formation of thyroid hormones, has been associated with an increased risk of TC, promoting the development of FTC and ATC, while the effect of iodine supplementation, though still controversial, may influence the ratio of PTC to FTC, suggesting that an excessive iodine intake could act as a risk factor for PTC
[11][14][11,14]. However, other nutritional factors, like selenium (Se), zinc (Zn), and flavonoids, not only play a crucial role in the thyroid gland, but, thanks to their antioxidant properties, might exert protective effects against impaired redox homeostasis, the signature of certain thyroid pathologies, including TC
[15]. Growing evidence supports the contribution of increased production of reactive oxygen species (ROS) in the pathogenesis and progression of TC
[16][17][16,17], with oxidative stress inversely correlated with tumor differentiation and directly correlated with the presence of somatic mutations and with worse TC presentation and higher TC aggressiveness
[17].
2. The Microbiota and Thyroid Axis
The microbiota, which collectively refers to microorganisms (bacteria, viruses, fungi, etc.) resident in the human body, plays a vital role in both the maintenance of normal physiology and the occurrence of clinical outcomes
[27][28][27,28]. The gut microbiota consists of almost 1200 bacterial species (at least 160 such species in each individual) and around 90% of the total human cells, whose gene count exceeds the human genome’s gene count by ~100-fold
[13][29][30][13,29,30]. The community of gut bacteria, most of which are strictly anaerobic and represent a mass of approximately 1.5–2 kg, is mainly composed (>90%) of Bacteroidetes, Firmicutes, Actinobacteria, Proteobacteria, and Verrucomicrobia, with Firmicutes and Bacteroidetes accounting for almost 90% of the population of the total gut microbiota
[19][31][32][19,31,32].
If, on the one hand, health depends on nutritional, metabolic, and immune functions of the microbial communities that are in symbiosis with the host, on the other hand, gut microbiota dysbiosis, a condition characterized by an alteration in the composition and physiological functions of the gastrointestinal microbiota as a consequence of diseases, changes of dietary habits, stress, or antibiotic use, may increase the prevalence of type 2 diabetes, cardiovascular disease, autoimmune disease, inflammatory bowel disease, and central nervous system disorders
[18][29][33][34][18,29,33,34]. A gut-endocrine–homeostasis-thyroid axis has been shown in recent studies, which reported an altered composition of the gut microbiota in patients with Hashimoto’s disease and Graves’ disease, further suggesting that microbiota analysis could provide an alternative non-invasive diagnostic methodology for thyroid diseases
[21][23][24][34][35][21,23,24,34,35]. The intestine, in fact, is a target organ of thyroid hormones, namely, triiodothyronine (T3), whose actions mostly depend on its interaction with nuclear thyroid receptor (TR) alpha 1, the main TR isoform expressed in the intestine epithelial cells
[36][37][36,37]. On the other hand, the gut microbiota plays a key role in both the homeostasis of thyroid function and thyroid disease pathogenesis via different mechanisms (see
[32] for more details):
-
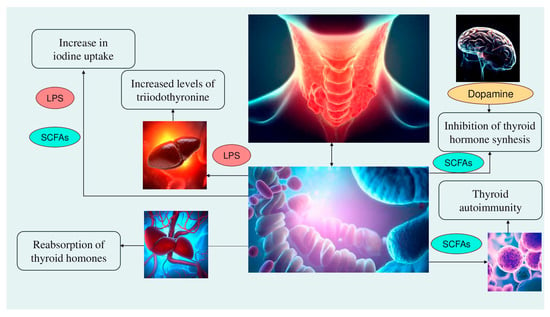
Alteration of iodine uptake, the main rate-limiting step in thyroid hormonogenesis, affecting the activity of sodium iodide symporter (NIS) through two processes: (a) The binding of the Gram-negative bacterial endotoxin lipopolysaccharide (LPS), released by the gut microbiota, to the thyroid cell toll-like receptor 4 (TLR-4). TLR-4 in turn activates the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), which subsequently promotes NIS transcription through paired box 8 (PAX8)
[38]. (b) Alternatively, enhancement of NIS expression may also occur through histone deacetylase (HDAC) inhibition by an important metabolite of the gut microbiota, butyrate
[39][40][39,40];
-
Modulation of activities of iodothyronine deiodinases, enzymes responsible for the conversion of thyroxine (T4) to its active form T3 by type 1 and type 2 deiodinases (D1, expressed mostly in the liver, kidney, thyroid, and pituitary, and D2, expressed primarily in the thyroid, central nervous system, pituitary, developing cochlea, brown adipose tissue, and skeletal muscle
[41]) or to reverse T3, its inactive form, by type 3 deiodinase—D3
[42]. This occurs through a complex thyroid–gut axis pathway involving LPS capable of inducing the decrease in D1 activity in the liver
[43] and, at the same time, activating D2 in the mediobasal hypothalamus, ultimately promoting the conversion of T4 to T3
[44];
-
Modulation of T3 and T4 bioavailability through the deconjugation of sulfoconjugated and glucuroconjugated iodothyronines by bacterial sulfate esterase or β-glucuronidase, respectively, thus inducing the reabsorption of thyroid hormones in the enterohepatic circulation. In humans, a recycling mechanism has been described for steroids hormones, biliary acids, and vitamins, while as for thyroid hormones, direct proof has been only established in animal models
[31];
-
Regulation of the SCFAs-mediated balance between T helper 17 (Th17) cells and regulatory T cells (Treg), two subtypes of CD4+ lymphocytes exerting opposite effects (release of pro-inflammatory cytokines, i.e., interleukin—IL-17 or anti-inflammatory IL-10, respectively), in autoimmune inflammatory diseases and immune tolerance
[35][45][35,45]. All these immune cells play a role in the pathogenesis of autoimmune thyroid disease (AITD), like Hashimoto disease (HD) and Graves’ disease. For instance,
Prevotella is correlated with reduced proinflammatory Th17 polarization and increased differentiation of anti-inflammatory Treg; therefore, it has been speculated that the Th17/Treg homeostasis regulation might be a potential pathogenic pathway for
Prevotella in HT patients
[32][46][32,46];
-
Involvement of the microbiota–gut–brain signaling in dopamine release, synthesis, and bioavailability. Certain species up-/downregulate the system dopamine transporter/dopamine binding efficiency, while others are positively or negatively correlated with the activity of tyrosine hydroxylase, an enzyme involved in dopamine synthesis
[47][48][47,48]. Furthermore, butyrate’s intrinsic HDAC inhibitor activity influences neurotransmitter levels
[47]. Since dopamine inhibits synthesis and secretion of the thyroid-stimulating hormone (TSH), thyroid function may be affected
[35] (
Figure 1).
Figure 1. The mechanisms involved in the microbiota–thyroid axis. Abbreviations: LPS: lipopolysaccharide; SCFAs: short chain fatty acids.
Therefore, although further data are still required to elucidate the specific relationships and mechanisms between the gut microbiota and the thyroid, intestinal microorganisms appear to act directly or indirectly on the gland by mainly influencing iodothyronine synthesis, conversion, and storage, as well as through immune regulation
[32].
3. The Association between Microbiota and Thyroid Cancer
The gut microbiota has been associated with the development, diagnosis, and treatment of various tumors (e.g., hepatocellular carcinoma, pancreatic, gastric, and breast cancers)
[27][49][50][27,49,50], with evidence supporting a causal role for gut dysbiosis in the development of colorectal cancer
[51][52][53][51,52,53]. Despite the multifactorial etiology of cancer, which is the result of a complex interaction between genetic alterations and environmental factors, it has been estimated that approximately 15% of malignancies worldwide are caused by infections with oncogenic pathogens
[34][54][34,54]. Besides the “oncomicrobes”, 11 microorganisms (7 viruses, 1 bacterium, and 3 parasites, e.g., Epstein–Barr virus, Hepatitis B/C virus, Human Papillomaviruses, Human Immunodeficiency Virus, and
Helicobacter pylori—Hp, labeled as Group 1 carcinogens
[55]) of the estimated ~10
30 distinct microbial species living on Earth
[56], an increasing amount of evidence supports the existence of another category of microorganisms, which is not causally related to cancer but able to promote tumor development and modulate both tumor progression and responses to numerous forms of cancer therapy
[30][57][30,57]. Although studies on the role of the microbiota in cancer are in their infancy, the technological advancement with the application of 16S ribosomal ribonucleic acid (rRNA) gene sequencing, has allowed establishing a close relationship between the activity of intestinal microorganisms, particularly their metabolites, and the protective or promoting effects against cancers
[34][49][34,49].
Overall, the research performed demonstrated a significant difference in the richness, diversity, and composition of intestinal microbial communities between TC patients and healthy controls, suggesting the predictive value of the gut microbiota in discriminating TC statuses
[20][34][20,34]. Feng et al.
[20], analyzing 30 TC patients and 35 healthy controls’ fecal samples by 16S rRNA gene sequencing, found that the TC group had a greater gut microbiota richness and diversity (α-diversity) compared to the control group, with TC patient samples enriched in the abundance of Firmicutes and Proteobacteria, and samples of controls enriched in Bacteroidetes. Furthermore, β-diversity, defined as the extent of similarity between two microbiota communities, was also significantly different between TC and control individuals, for a total of 21 different genera, 5 of them more effective in distinguishing TC patients from controls
[20]. Of note, many genera of Proteobacteria, such as
Enterobacter and
Haemophilus, were also increased in fecal samples from patients with primary liver cancer
[58]. Similar results were found by Zhang et al.
[59], who compared the gut microbiome of 20 patients with TC, 18 individuals with thyroid nodules, and 36 matched healthy controls, showing higher microbial abundance and distinct composition in individuals with thyroid disease than in the healthy control group. In particular, the gut microbiome of the TC group was characterized by the relative dominance of
Neisseria and
Streptococcus, which have also been associated with the development of gastric cancer in the absence of Hp infection
[60],
Streptococcus accounting for the largest proportion in Hp-negative gastric cancer at the family level
[61] and related to colorectal carcinogenesis, as well
[62], supporting the potential of these bacteria possibly playing a role in thyroid disease. Additionally, both studies found a lower relative abundance of certain genera, including
Butyricimonas,
Lactobacillus,
Bacteroides, and the Lachnospiraceae family
[20][59][20,59]. Overall, these microorganisms are known to participate in the production of SCFAs (acetate, propionate, butyrate, and valerate), the main metabolites produced in the colon by bacterial fermentation of dietary fibers and resistant starch, which in turn play an essential role in the modulation of gut microbiota physiology and composition by regulating immunity and suppressing or promoting inflammatory responses, as described thereinafter in the text
[63][64][65][66][63,64,65,66]. Importantly, some
Lactobacillus strains can fix inorganic selenite into selenoproteins
[67], such as glutathione peroxidase (GPx) and thioredoxin reductase (TrxR), key factors for oxidative stress control
[68], and iodothyronine deiodinases, essential enzymes for thyroid function
[69]. Furthermore, altered gut microbiota genera in the TC group were significantly associated with both serum lipid (e.g., lipoprotein A, apolipoprotein A and B) and lipid metabolite levels (e.g., linolenic acid, gamma-aminobutyric acid)
[20], confirming that dysregulation of lipid metabolism represents an important metabolic alteration in cancer, including TC
[70][71][72][70,71,72] and disease indices, namely, increased serum TSH levels
[59], recognized as positively associated with the incidence of nodular goiter and PTC
[73]. Previously, the study by Shen et al.
[71] revealed that the serum of patients with distant metastatic PTC (n = 37) was characterized by an elevated concentration of gamma-aminobutyric acid (GABA), which could be implicated, along with its receptors, in the oncogenesis/metastasis of various tumors
[74][75][76][74,75,76]. These subjects also had increased levels of serum aminooxyacetic acid, a nonselective inhibitor of transaminases, including GABA transaminase
[77], thereby increasing GABA concentration, and of 4-deoxypyridoxine, a potent antagonist of vitamin B6 coenzyme (that is involved in the regulation of immune responses)
[78]. Since both are not endogenous metabolites, they could be related to diet–gut microbiota interactions, suggesting that serum metabolomics profiling could significantly discriminate PTC patients according to distant metastasis
[71]. In contrast to prior findings, a recent cross-sectional study
[79] reported a reduced richness and diversity of the gut microbiota in stool samples of TC patients (n = 60) compared to those of healthy controls (n = 60), probably because of differences in the demographics of controls, dietary habits, and tumor TNM status. In addition, although there was no significant difference in the Firmicutes/Bacteroidetes ratio (accepted to have an important influence on maintaining normal intestinal homeostasis
[80]), between the two groups, about 70% of TC patients showed a relatively higher abundance of Proteobacteria, a signature of microbial dysbiosis possibly related to obesity
[81], in accordance with Feng and co-workers
[20]. Notably, a four-genus microbial signature was able to distinguish TC patients with metastatic lymphadenopathy from those without it; however, no significant difference in gut microbiota richness or diversity was observed between the two groups
[79]. Overall, these results, albeit needing to be supported by animal models, provide relevant information on the potential role of the gut microbiome in TC pathogenesis and how it might be important to prevent and regulate intestinal dysbiosis.
Microbial Communities in Thyroid Cancer Tissues
Although the presence of bacteria in tumor tissues, traditionally considered sterile, dates back to more than 100 years ago, only thanks to a combination of imaging, sequencing, and cultivation techniques, and genetically engineered and germ-free animal models (grown in sterile conditions and completely free of intestinal bacteria
[82]), was it possible to exclude the possibility of contamination and detect the very low microbial content in tumors
[30][83][30,83]. Thus, in recent years, tumor-type specific bacteria have been observed in a variety of tumors, e.g., melanoma, colorectal, pancreatic, gastric, breast, lung, ovarian, prostate, and bladder cancers, suggesting their involvement in processes related to tumorigenesis and cancer progression
[27][28][27,28]. Nejman et al.
[84], analyzing the tumor microbiome of 1526 tumors and their adjacent normal tissues across seven cancer types, found that the intratumor bacteria are mostly intracellular and are present in both cancer and immune cells, with the phyla Proteobacteria and Firmicutes representing the majority of bacterial sequences detected in all tumor types, while the Actinobacteria phylum dominates in non-gastrointestinal tumors. The tumor microenvironment, recognized as a pivotal player in tumorigenesis, consists of both proliferating malignant cells and non-malignant components, including tumor stromal cells (stromal fibroblasts and immune cells, such as microglia, macrophages, and lymphocytes), elements of the extracellular matrix, and endothelial cells
[28][85][28,85]. If genetic/epigenetic alterations promote the process of tumor initiation and progression
[28][85][28,85], the microbiome and its metabolites, despite their low biomass, can influence the components of the tumor microenvironment by modulating the processes of inflammation, proliferation, and cell death, therefore playing a key role in shaping tumor development
[86].
Compared to other tumors, the intratumoral microbiome of TC has been poorly explored; however, it can be hypothesized that the thyroid can be colonized by microorganisms, since gastric mucosal cells and thyroid follicular cells derive from primitive gut cells during embryonic development
(Table 2). The study by Liu et al.
[1], including 93 sample tissues (divided into tumor, paratumor, and normal tissues) and stool samples from 25 TC patients (19 malignant cases, 6 benign cases), reported a higher α-diversity of fecal samples than that of thyroid tissues, while the total number of microorganisms in tissue samples decreased with the increasing distance from the cancerous tissue. The predominance of Proteobacteria, and, in particular, of
Pseudomonas mucidolens, was found in all three types of tissue samples, especially in patients with malignant TC, suggesting that they might participate in TC development, but not in stool samples that were instead characterized by the predominance of Firmicutes
[1]. While other
Pseudomonas species have been associated with bacteremia, nosocomial infections, and cystic fibrosis
[87][88][87,88],
Pseudomonas aeruginosa has recently been shown to enter cancer cells and induce apoptosis, without any effect on normal cells
[89]. The phylum Proteobacteria consists of facultative anaerobic bacteria, which are not dominant in the healthy intestine, where over 90% of the gut microbiota is characterized by strict anaerobes
[90]. Moreover, since the authors observed only a very partial overlap of sequences and metabolic pathways (especially in fatty acid degradation) between the thyroid and intestine, excluding a linkage between gut and thyroid microbes, the abundance of Proteobacteria in thyroid tissues could be caused by TC cells, which possibly give rise to a unique microbial community
[1]. Another research study
[12], characterizing tumor tissues and matched peritumor (approximately 3 cm adjacent to the cancer tissue) tissues from 55 TC patients at the early stage (stages I and II) who underwent thyroidectomy, reported significantly lower α-diversity and richness in tumors than in peritumor tissues, consistent with Yu et al.
[79] and with that observed for other cancers
[91][92][91,92]. Furthermore, microbial diversity and composition were significantly different between tumor and peritumor microenvironments: while
Sphingomonas, which has also been identified as the dominant genus in thymic epithelial tumors
[93], colitis-associated cancer
[94], and gastric mucosa-associated lymphoid lymphoma
[95], predominated in tumor tissue,
Comamonas, also associated with lymph node metastasis in pancreatic cancer
[96], had higher abundance in peritumor tissues. The combination of these genera could therefore serve both as a signature to distinguish tumors from peritumor tissues and as a prognostic marker in cancer progression in patients with early-stage TC
[12]. Of note, the authors also reported higher α-diversity of the thyroid microbiome from patients at the N1 stage in comparison to those at N0, but no significant differences in α-diversity and richness between female and male patients
[12]. Gnanasekar and co-authors
[97], based on data of microbial sequences obtained from the Genomic Data Commons legacy archive for a total of 563 TC patients, as well as confirming that the tumor tissue contained lower microbe abundance than the adjacent normal tissue, as in Dai et al.
[12], found heterogeneity in the composition of the carcinoma microbiome between males and females, and between PTC (classical, follicular variant, and tall cell—TCPTC) subtypes. The aggressiveness of TCPTC could depend on the dominance of
Micrococcus lutheus, which has been associated with infections in severely immunocompromised patients
[98][99][98,99], and of
Bradyrhizobium sp.
BTAi1, correlated with a lower free-survival probability in cervical cancer cases
[100]. At the same time, the microbe abundance in male samples was related to a greater number of chromosomal alterations and inversely associated with tumor suppressive pathways, explaining the worse prognosis of TCPTC in males than in female patients
[101]. Of interest,
Frankia sp. and
Anabaena sp.
K119, both enriched in normal tissue samples of all PTC subtypes, were inversely correlated with the pathologic M stage
[97]. A recent study
[82] investigated the role of the PTC tumor microbiome in cancer progression, showing that the tumor bacterial α-diversity was significantly higher in patients with advanced lesions (T3 or T4) than those with relatively mild lesions (T1 or T2). In contrast to Dai et al.
[12], the α-diversity was higher in females (who are expected to have a greater PTC incidence compared to male patients), indicating that the microbiome presents specific characteristics that vary by sex, in addition to tumor staging. The authors further observed significant differences in β-diversity, with
Pseudomonas, the most abundant genus in all groups, presenting higher relative abundance in patients with T1 and T2 PTC than in those with T3 or T4 PTC. Moreover, an interaction between intratumoral bacteria and AITD-related antibodies was found. In particular, Prevotellaceae,
Bacteroides, and
Bifidobacteria showed a negative relationship with anti-thyroperoxidase (TPO) levels, corroborating previous findings
[102] and suggesting a role of these microbial genera in the pathogenesis of AITD by molecular mimicry
[82]. These immunoregulatory effects of the tumor microbiome may in turn enhance or impair the immune response against the tumor and, consequently, affect the final outcome of PTC
[82]. Hence, the TC microbiome appears to play a crucial role not only in the tumor progression, but, by interacting with autoimmune antibodies, might also contribute to tumor invasion.