Imaging within the realm of biomedical applications can be categorized into two domains based on object size: macroscopic and microscopic imaging. The substantive importance of macroscopic imaging has been demonstrated prominently in medical practices, encompassing X-ray imaging, positron emission tomography (PET), magnetic resonance imaging (MRI), and ultrasonic echo imaging. Although these modalities offer undeniable utility, they are not devoid of limitations. Even with recent progress in X-ray detection, the ionizing radiation inherent to X-ray imaging engenders challenges related to repeated exposure. Similarly, the utilization of PET and MRI is impeded by the considerable scale of the necessary apparatus, thereby hindering seamless bedside deployment. The domain of ultrasound imaging presents difficulty involving a tradeoff between spatial resolution and penetration depth in animal bodies. An additional contender for noninvasive macroscopic structural imaging of animal bodies has emerged: optical imaging.
1. Two-Dimensional Transillumination Imaging
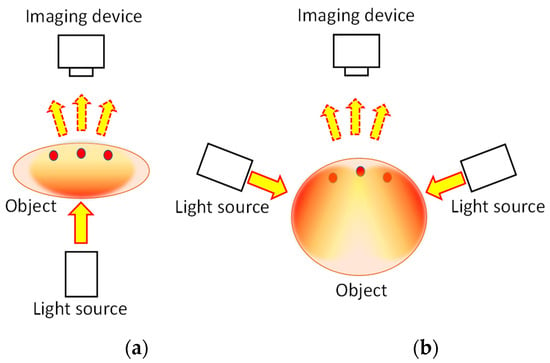
Figure 1 presents the fundamental principle of transillumination imaging
[1][2][3][4][5][6][7][21,22,23,24,25,26,49].
RWe
searchers illuminate the animal body from one side and capture the resulting image from the opposite side (
Figure 1a). This technique provides a simple and safe tool to investigate the internal structure of an animal body noninvasively. However, for NIR light, an animal body is regarded as a scattering-dominant turbid medium containing an inhomogeneous, light-absorbing internal structure. The scattering coefficient (
μs) of human body tissue is typically of approximately 10 mm
−1 in
near-infrared (NIR
) wavelengths
[8][9][10][11][12][13][15,16,17,18,19,20]. This fact implies that the non-scattered component of light decays rapidly within a few millimeters in body tissue. However, when the body is less than a few centimeters thick, the internal light-absorbing structure can be visualized through transillumination imaging
[7][49]. For instance, in human and animal bodies,
rwe
searchers c can observe a subcutaneous blood vessel network because of the marked differences in the absorption coefficients (
μa) of hemoglobin and the surrounding tissues
[8][9][10][11][12][13][15,16,17,18,19,20].
Figure 1.
Principle of transillumination imaging: (
a
) transmission mode and (
b
) backscattering mode.
A transillumination image represents the spatial distribution of light that reaches an imaging device through collimation optics, such as a human eye or a camera. In the transmitted light emerging from an animal body, the image-composing light is not the non-scattered component but the component that results from repeated scattering in the forward direction. When
rwe
searchers illuminate an animal body with a collimated light beam,
researchers cwe can observe a portion of the light in the same direction as the incident beam even through a few centimeters of thickness. Animal body tissue is known to have an anisotropy factor (g) close to 1
[8][9][10][11][12][13][15,16,17,18,19,20], which indicates that the scattering pattern of body tissue is highly oriented towards the forward direction. Consequently, some of the light can emerge through the body tissue in the same direction as the incident light after undergoing repeated forward scattering.
RWe
searchers refer to this component as near-axis scattered light (NASL)
[14][50]. It shares a similar concept to the snake photon
[15][16][17][51,52,53] or quasi-ballistic light
[18][19][20][21][54,55,56,57]. From experimentation,
rwe
searchers h have confirmed that the NASL intensity remains detectable even after passing through 1 cm thick animal tissue
[14][50]. Because NASL emerges from the animal body in the same direction as the incident light beam, the internal structure can be visualized by employing collimated optics using a regular camera lens with a small aperture.
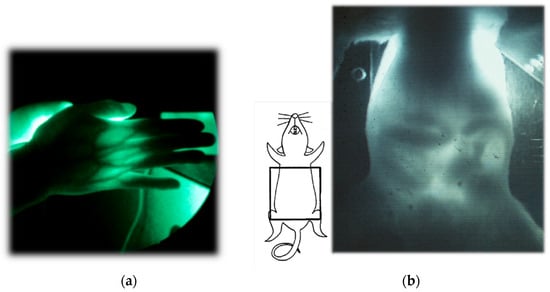
Figure 2 presents examples of transillumination images of a human and a mouse
[7][49]. Details of the experiments are reported elsewhere
[22][58]. It is noteworthy that this technique can provide real-time video images with simple instrumentation in safe settings, enabling repeated or continuous bedside monitoring. In the video image of the mouse abdomen, the peristaltic movement of its digestive tract during regular food consumption can be visualized, obviating the need for any unnatural contrast medium required for X-ray fluoroscopy.
Figure 2.
Examples of NIR transillumination images: (
a
) human hand and (
b) mouse abdomen. Modified from Ref. [7]. ) mouse abdomen. Modified from Ref. [49].
In cases where the object body is too thick to allow for light transmission in the incident beam direction, indirect light illumination can be used.
Figure 1b presents this illumination technique. Given the highly diffusive nature of general body tissue, characterized by a reduced scattering coefficient
μs′= ~1 mm
−1 in NIR wavelengths, the illuminated light from the side propagates almost uniformly after several millimeters of propagation. Consequently, the illuminated light is dispersed uniformly within the body, serving as back-illumination for imaging subsurface absorbing structures. This illumination technique has found application in finger-vein personal authentication
[23][24][59,60]. Additionally, its application in the daily management of arterio-venous fistulas in dialysis treatment has been reported
[25][61].
2. Functional Imaging
One important merit of NIR transillumination imaging is its capability for the noninvasive visualization of physiological changes occurring within animal bodies from an external perspective. Along with the recent progress in optoelectronic devices
[26][27][62,63],
rwe
searchers c can leverage the extensive utilization of spectroscopy in the biomedical realm. As explained above, a transillumination image represents the spatial distribution of NASL observed through collimation optics. The intensity is a consequence of attenuation through animal body tissue, which is a scattering-dominant turbid medium. If
researchers we disregard rigor, then the received intensity can be expressed using the following equation.
In this equation, Iin, Iout, μs, μa, dt, and S, respectively, denote the incident light intensity, output light intensity (i.e., the transillumination image), scattering coefficient, absorption coefficient, path length in body tissue, and increment factor that represents scattering from off-axis directions. The parameters (x, y) signify the spatial distribution in the plane perpendicular to the optical axis in the z-direction of the Cartesian coordinate system. If rwesearchers ignore the terms μs and S, then this equation reduces to the conventional Beer–Lambert law. Because rwesearchers c cannot neglect these terms with animal body tissue, the Beer–Lambert law is typically deemed inapplicable.
However, by obtaining transillumination images under two different conditions, wherein only the absorption condition varies while the other conditions are kept constant, absorption changes in a linear manifestation of the Beer–Lambert law can be visualized as
where designations 1 and 2, respectively, denote conditions before and after the change. Equation (2) elucidates that by calculating the logarithm of intensity ratios for two images, pre-change and post-change, the resulting image delineates the spatial distribution of absorption changes while mitigating the scattering effect. Because
μa is proportional to the molar absorption coefficient
ε and the absorber concentration
C, the observed absorption changes typically reflect alterations in either
ε or
C. By capturing transillumination images
I1 and
I2 before and after physiological changes within animal bodies, transillumination imaging can capture the internal functional changes manifesting as variations in
ε or
C.
One such functional change within animal bodies is the alteration in hemoglobin oxygenation.
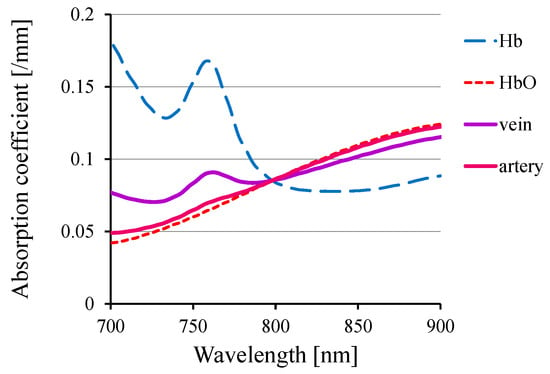
Figure 3 portrays the absorption spectra of hemoglobin and typical arterial and venous blood, showcasing distinct wavelength-dependent variations in the NIR range. Using these variations, one can visualize the distributions of venous and arterial blood separately.
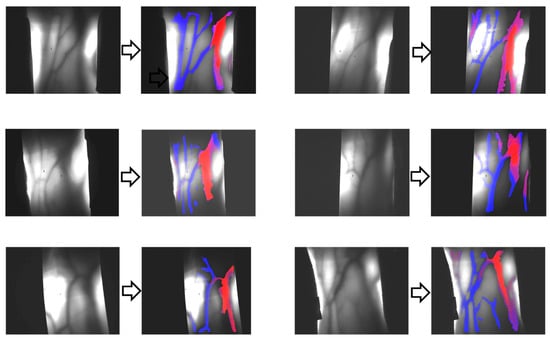
Figure 4 presents the results of venous–arterial differentiation in transillumination images obtained from different human wrists
[28][64].
Figure 3.
Absorption spectra of hemoglobin and blood.
Figure 4.
Differentiation of veins (blue color) and arteries (red color) in transillumination images of human adult wrist areas.
In the isosbestic wavelength, oxyhemoglobin and deoxyhemoglobin exhibit equal absorption. Therefore, by using this specific wavelength in the NIR range (typically of approximately 800 nm for human and rodent blood), one can independently visualize alterations in blood volume irrespective of its oxygenation status.
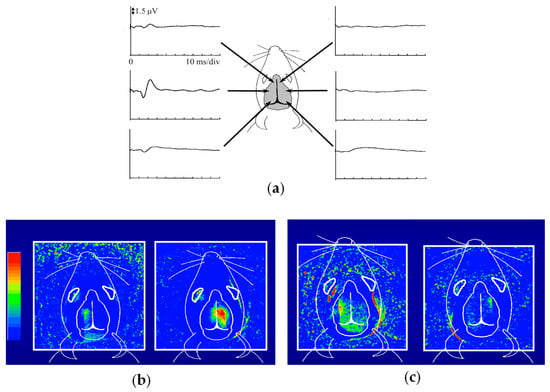
Figure 5 presents the outcomes of functional imaging in a rat brain
[29][65]. Electro-stimulation was applied to one of the rat’s forelimbs or whisker roots, with the intention of visualizing the corresponding local changes in its brain. Before conducting optical imaging,
rwe
searchers identified the cerebral cortex responsible for somatosensory perception.
Figure 5a shows the electroencephalograms (EEGs) observed via metal electrodes in direct contact with the mouse head during right forelimb stimulation.
Figure 5b presents the functional imaging results, obtained from the transillumination images taken before and after forelimb stimulation. The localized increase in light attenuation, brought about by an augmented blood volume, agreed well with the EEG-identified regions. Stimulation of the right forelimb yielded an activated region in the left cerebral hemisphere. Upon switching the stimulation from the right to the left forelimb, the activated area transitioned symmetrically across the longitudinal cerebral fissure that separates the right and left cerebrum hemispheres. As depicted in
Figure 5c, stimulation of the right/left whisker root caused different activated patterns compared to the forelimb stimulation, but remained within the respective left and right hemispheres. These positions and the localized dispersion in the somatosensory area closely correlated with measurements obtained through microelectrodes inserted into the brain
[30][31][66,67]. It is noteworthy that these observations were noninvasive, requiring no incision or skull opening. The sole intervention involved shaving the hair on the rat’s head to enhance the clarity of the transillumination images.
Figure 5. NIR functional transillumination imaging of rat brain: (a) EEG identification of activation area for somatosensory stimulation of right forelimb, (b) distribution of blood volume change in right/left forearm stimulation, and (c) right/left whisker root stimulation.