Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Muhammad Ali Butt and Version 2 by Catherine Yang.
Smart contact lenses (SCLs) have found applications in the continuous monitoring of ocular parameters, encompassing both physical characteristics, such as pressure and temperature, as well as chemical markers like glucose levels, protein content, and pH. These physiological indicators are intricately linked to human well-being, with noteworthy implications for health.
- smart contact lenses
- non-invasive
- continuous health monitoring
- intraocular pressure
1. Materials
Modern contact lenses can be made from a variety of materials, including silicone [1][50], 2-methacryloyloxyethyl phosphorylcholine (MPC) [2][51], poly (methyl methacrylate) [3][52], poly (ethylene terephthalate) [2][51], poly (2-hydroxyethyl methacrylate) [4][53], and poly (ethylene terephthalate) [5][54]. For biomedical applications, SCLs made of silicone or poly(2-hydroxyethyl methacrylate) PHEMA hydrogels are preferred [6][55]. Designing and manufacturing SCLs for IOP monitoring presents a range of intricate challenges [7][56]. The specific needs of the human eye necessitate careful consideration of factors, such as flexibility, the clarity of the viewing window, hydrophilicity, and oxygen permeability [8][9][57,58]. Notably, pHEMA hydrogel—a prevalent material in commercial contact lenses—offers advantageous traits, like water permeability and compatibility with ocular tissue. This ensures the prolonged safe and comfortable usage of SCLs. However, a notable obstacle arises in the fusion of hydrogel-based lenses with electronic circuits, particularly rigid chips, and bulky batteries. This hurdle is attributable to the alterations in structure caused by the hydrogel’s tendency to swell. Ulu et al. created an antibacterial contact lens for the treatment of ocular infections using PHEMA hydrogel that was loaded with the antibacterial chemical boric acid [10][59]. A hydrogel based on PHEMA was created by Ashtiani et al. as a therapeutic contact lens for the administration of small-molecule medications [11][60]. Chitosan was used to alter the hydrogel in their investigation. Additionally, the therapeutic contact lens demonstrated antimicrobial efficacy and consistent ascorbic acid release. Most widely used contact lens materials are presented in Table 1.
| Material | Molecular Formula | Procs/Cons | References |
|---|---|---|---|
| PMMA | (C5H8O2)n | Outstanding optical properties, low oxygen permeability, high rigidity and toughness. | [13][61] |
| PET | (C10H8O4)n | Low glass transition temperature, low rigidity, low surface energy, hydrophobic, excellent chemical resistance, and thermal stability. | [14][62] |
| PHEMA | (C6H10O3)n | Tunable mechanical properties, relatively high water content, and good chemical and thermal stability. | [6][55] |
| PDMS | (C2H6OSi)n | Flexibility and high oxygen permeability. | [15][63] |
| 2-methacryloyloxyethyl phosphorylcholine (MPC) | C11H22NO6P | Low protein adsorption, good surface wettability, high oxygen permeability, and mechanical weakness. | [16][64] |
| Chitosan | (C6H11NO4)n | Bioadhesive, biocompatible, biodegradable. | [17][65] |
To minimize deposition-related expenses, researchers have delved into the utilization of graphene. Graphene is renowned for its outstanding electrical, chemical, and mechanical attributes, as well as its optical transparency. Furthermore, the tightly knit hexagonal lattice structure of graphene effectively inhibits water infiltration, suggesting its potential application in preventing eye dehydration. Additionally, graphene exhibits exceptional electromagnetic wave shielding capabilities, with partial absorption of electromagnetic waves when incorporated into contact lenses [18][66]. A monolayer of graphene, previously cultivated on copper foil, is transferred onto the contact lens using a solution-based method and subsequently patterned using lithography [19][67]. However, it is worth noting that the lithography procedure can lead to increased manufacturing costs. To address this concern, Tang et al. employed more cost-effective techniques, specifically drop-casting and direct laser interference printing (DLIP), to deposit single-layer graphene onto contact lenses. In drop-casting, a droplet of graphene solution is placed on the contact lens and then vaporized. DLIP, on the other hand, involves positioning the graphene-coated lens between two mirrors, where a laser beam induces interference patterns on the graphene film, resulting in the removal of graphene from areas with high-laser intensity [19][67].
The wireless SCLs, utilizing a flexible inductor–capacitor–resistor (LCR) sensor devoid of chips and batteries, holds immense promise for monitoring physiological signals. To facilitate the adoption of LCR contact lenses for clinical intraocular pressure monitoring, it is imperative to employ reliable and comfortable contact lens materials while achieving exceptional sensitivity. A novel approach to creating hydrogel-based SCLs for wireless IOP monitoring involves the conformal stacking technique, effectively addressing hydrogel swelling and seamless integration of pyramid-micro-structured dielectric elastomers [20][37]. The system successfully monitors IOP in an in vitro porcine eye, thanks to the high sensitivity of the spherical pyramid-micro-structured capacitive pressure sensor and the hydrogel substrate. Furthermore, an impedance-matching tunable reader integrated into glasses enhances signal amplitude and extends the reading distance, thus improving the portability of signal measurement equipment. These innovations signify the substantial potential of the wireless contact lens system for clinical IOP monitoring, heralding a promising future for advanced daily ocular health management [20][37].
2. Electrical Components
The ideal design criteria for SCLs should allow for simple measurement and real-time display of ocular parameters without interfering with the patient’s routine activities. As a result, this kind of technology needs to be adaptable, small, and capable of integrating with a variety of functional modules, such as wireless communicators, sensors, powers, displays, and other micro-components [21][68].
The best technique to transmit signals in wearable electronics is through a wireless system as opposed to more conventional cable data transmission methods. To avoid the hassle of cable transmission, Chiou et al. created a wireless SCL system made up of a wireless communicator [22][69]. For real-time biomarker monitoring, SCLs require a reliable, long-lasting power source. However, due to their diminutive size, contact lenses have a limited capability for storing energy. Consequently, external sources of power (such as inductive power, radio frequency [RF] power, or optical power) must be used to wirelessly power biosensors. Radio waves are used by RFID, a type of identification technology, to identify individuals or things. Power harvesting from radio waves became possible with the development of passive RFIDs [23][70]. A strategy for recovering energy from the outside environment is power harvesting. This method enables the replacement of tiny batteries in low-power electrical appliances.
In the past decade, the pursuit of next-generation electronics, spurred by breakthroughs in materials science, has catalyzed the emergence of stretchable and transparent electronics [24][25][71,72]. This technological evolution has paved the way for innovative applications like SCLs and wearable sensors, which demand a more extensive investigation into the materials and manufacturing methods involved. Consequently, numerous research endeavors have been directed towards creating materials and devices that possess both mechanical stretchability and optical transparency. Utilizing flexible and biologically stable electrode materials is important for smart lenses to show pertinent information [26][73]. In this context, Lee et al. demonstrated a straightforward microscale light-emitting diode (LED) device made of graphene, a type of 2D carbon nanomaterial with superior optical, electrical, and mechanical capabilities, and constructed on a contact lens [18][66]. Additionally, graphene exhibits good air permeability and electromagnetic wave absorption, making it a suitable candidate for electromagnetic interference shielding. Another sort of wireless display was created by Park et al. [27][31]. It has three electronic parts: a rectifier, an antenna, and an LED pixel. These components were created on a Si wafer that had an 800 nm-thick Cu sacrificial layer placed on top of it. An inductively coupled alternating current (AC) was consequently wirelessly received by the contact lens from a transmission coil (50 MHz) within a 5 mm range.
Developing wearable devices capable of wirelessly monitoring IOP and facilitating precise medication administration is a pressing need in glaucoma treatment. However, this endeavor poses considerable challenges related to size constraints, wireless functionality, and interference issues. To address these challenges, an integrated wireless theragnostic contact lens is proposed for the real-time electrical monitoring of IOP and on-demand delivery of anti-glaucoma drugs [28][74]. This groundbreaking wireless theragnostic contact lens adopts an exceptionally compact structural design, allowing for seamless integration and efficient frequency separation on the curved and limited surface area of the lens. The IOP sensing component exhibits remarkable sensitivity, owing to its innovative cantilever configuration within the capacitive sensing circuit. Meanwhile, the drug delivery mechanism utilizes an efficient wireless power transfer circuit to trigger the release of anti-glaucoma medication into the aqueous chamber through iontophoresis. With its minimally invasive, intelligent, wireless, and theragnostic capabilities, the wireless theragnostic contact lens emerges as a highly promising solution for advancing glaucoma treatments [28][74].
Connecting the electrical components within SCLs requires microwires. For printing 2D or 3D flexible electronics, these wires are commonly constructed out of conductive silver or carbon nanotube inks [29][75]. These are used frequently because they are more affordable than gold and platinum. Silver ink is very adaptable for a wide range of applications due to its strong electrical and thermal properties. In recent years, a variety of conductive silver inks have been created for use as conductors for flexible paper displays, silver art inks with luminous LEDs, and miniature antennas that can be 3D-printed. Similar to this, printing techniques for numerous electronic components, such as emitters, radiofrequency inductors, and transistors, have extensively utilized carbon nanotubes with excellent mechanical and electrical qualities. Integrating wireless communication, power systems, displays, and various other elements, intelligent contact lenses facilitate effortless and non-intrusive physiological monitoring. These advancements surpass traditional methods dependent on inflexible circuit boards, cables, needle electrodes, and terminal connections.
Moreover, multiplexed organic electrochemical transistor-based sensors are shown to be self-powered by organic solar cells (OSCs) [30][76]. The integrated device was created using a straightforward technique that included heat evaporation and solution blade coating. Without any peripheral circuitry, OSCs were tuned to produce the best operating voltage for sensors that respond semi-log-linearly to the calcium and glucose ions in tear fluids. A near-field communication unit can wirelessly communicate the sensing signals to the laptop. Real-time monitoring of the biomarkers in tears will be provided by an integrated self-powered multiplexed sensing device, which is anticipated to be put on SCLs for the early identification and diagnosis of diabetes. The design of the integrated system of multiplexed sensing devices for glucose and Ca2+ monitoring driven by OSCs is shown in Figure 13a. The two components of this system are (i) two groups of OSCs with one inverted cell powering the gate electrode, and (ii) two separate PEDOT: PSS-based OECT (p-OECT) sensors. The bottom electrodes of the OSCs are connected to the source electrodes of the sensors. The platinum gate is changed by a mixture of chitosan and glucose oxidase for glucose sensing, and the Ca2+ selective membrane is coated on the PEDOT: PSS channel for Ca2+ selective monitoring (Ca2+ OECT, c-OECT). When a positive bias is provided, enzymes immobilized on the gate electrode catalyze the electrochemical oxidation of H2O2 by the platinum electrode. This produces peroxide (H2O2) from glucose. By transferring electrons to the gate electrode and altering the electrical double layer at the gate/electrolyte interface, the oxidation process lowers the voltage drop at the interface, which raises the potential applied to the active channel and lowers the drain current [30][76].
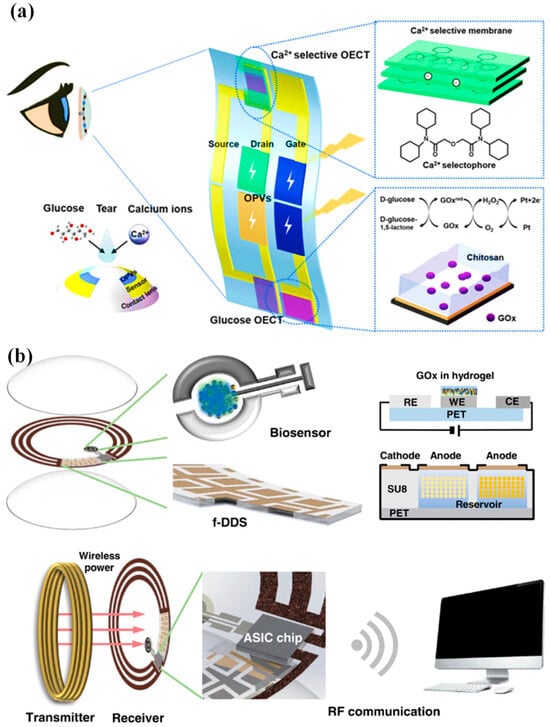
Figure 13. (a) Device and contact lens integration schematic. The electrode for the Ca2+ and glucose sensors has been modified, as shown in the insets [30][76]. (b) A visual representation of the innovative SCL engineered for both diabetic diagnosis and therapy. This advanced contact lens seamlessly integrates multiple key components, including a cutting-edge biosensor, a flexible on-demand drug delivery system (f-DDS), a wireless power transmission system with transmitter and receiver coils, an application-specific integrated circuit (ASIC) chip, and a versatile remote communication system. Together, these components form a versatile and ubiquitous platform with the potential to cater to a wide range of diagnostic and therapeutic applications, marking a significant advancement in the field of healthcare technology [31][24].
An innovative remotely controllable SCL designed to revolutionize healthcare through noninvasive glucose monitoring and targeted drug delivery for diabetic retinopathy treatment is proposed in [31][24]. This multifunctional marvel comprises five integral components, each contributing to its remarkable functionality: a real-time electrochemical biosensor, an adaptable on-demand flexible drug delivery system (f-DDS), an efficient resonant inductive wireless energy transfer system, a highly integrated circuit (IC)-based microcontroller chip with an adept power management unit (PMU), and a sophisticated remote radio frequency (RF) communication system, as depicted in Figure 13b. The real-time amperometric biosensor’s pivotal role is to detect glucose levels in tears, obviating the need for invasive and uncomfortable blood tests. Meanwhile, the self-regulated pulsatile f-DDS allows for controlled drug release, with remote communication as its modus operandi. The lens’s wireless powering is facilitated by resonant inductive coupling to a copper (Cu) receiver coil, drawing energy from an external power source equipped with a transmitter coil. The device seamlessly communicates with an external controller via RF communication.
3. Fabrication Methods
The science and technology of systems with integrated channels on the microscale (from tens to hundreds of micrometers) allow for the controlled and systematic manipulation of small amounts of fluid flow in specific configurations [32][77]. The widespread use of microfluidics in current organ-on-a-chip systems, multiphase flow manipulation, chemical synthesis, and bioanalysis makes it especially appealing for contact lens applications. Microscale forces, including fluid surface tension, capillary forces, energy dissipation, and fluid resistance, are the basis for the principles regulating microfluidics in contact lenses [33][78]. When the eyelids blink, the person’s tears will cover the surface of the contact lens. Then, due to the capillary force, they will enter microchannels during ocular fluid collection. When liquid moves via a small opening, capillary phenomena take place. A crucial parameter in microchannels that gauges the degree of fluid flow resistance is the Reynolds number [34][25].
SCLs incorporate numerous microelectronic components and microchannels, which are created either directly or indirectly through the utilization of photolithography technology. Photolithography is a manufacturing process that employs light to transfer patterns from a photomask onto the surface of a silicon wafer. The use of photolithography in SCLs encompasses two primary aspects. Firstly, it involves replicating the desired microstructures within the contact lens material. Secondly, it entails the fabrication of microelectronics, including flexible wires, microelectrodes, and other microsensor components integrated into the contact lenses.
When designing SCLs, it is important to consider the peculiarities of microfluidic systems. For instance, the size/shape and flow rate of microchannels can influence the collection of ocular fluid. As it impacts the fluid absorption efficiency of microsensors and could alter the detection accuracy of microsensors, the flow velocity of the ocular fluid is crucial for optimizing the properties of SCLs. Wu et al. used MEMS technology to directly integrate a flow velocity sensor into microchannels to monitor the flow velocity and analyze the microfluidic flow velocity in SCLs. They employed a microchannel wall that served as both a heating element and a velocity sensor to produce a boron-doped polysilicon sheet [35][79].
Microfluidics stands at the forefront of innovation in the realm of SCLs, ushering in a transformative era in wearable technology and elevating the visual capabilities to new heights [36][80]. Nestled within the intricate structure of these lenses are minuscule, precisely engineered channels and chambers, enabling the meticulous control and manipulation of fluids [37][81]. The integration of microfluidic systems into SCLs unlocks a spectrum of functionalities, from administering targeted drug delivery for ocular conditions to dynamically adjusting focal length for individuals grappling with presbyopia. Moreover, it facilitates the real-time monitoring of biomarkers within the tears, revolutionizing health diagnostics [38][82]. Beyond these impressive features, microfluidics is the linchpin for ensuring user comfort and safety, maintaining a stable tear film and shielding against discomfort or dryness.
Injection molding is a method of producing SCLs that combines conventional contact lens production methods with the incorporation of smart technology components. The preferred lens substance is manufactured as a liquid or pellet. It might be a hydrogel made of silicone or another appropriate polymer. When the material is heated and poured into the heated mold cavity, which is then sealed shut under intense pressure, the material takes on the shape of the lens mold. In the mold, the substance is allowed to cool and harden. The lens is released from the mold once it has been set and is then subjected to a few post-processing procedures. To get rid of any flaws and guarantee a smooth surface for pleasant usage, the lenses are polished.
In [22][69], a conventional cast molding technique was employed to seamlessly integrate the chip, receiving antenna, and biosensor onto a soft contact lens using readily available biocompatible materials. To enhance comfort and user adherence, it was imperative to conform the contact lens design to standard specifications, with thicknesses of 200 μm and 100 μm for the peripheral and optical regions of the lens, respectively. This study utilized a conventional contact lens manufacturing process to create the proposed SCL packaging, employing hydrogel-based materials. Figure 24a illustrates the procedural steps involved in crafting the hydrogel-based contact lens biosensor system packaging, employing a set of convex and concave contact lens molds [22][69]. During this process, the biosensor component was positioned onto a concave mold, ensuring alignment with the mold’s center. Subsequently, a hydrogel material was poured into the concave mold, and the convex and concave molds were combined. The contact lens, along with the molds, was then subjected to ultraviolet (UV) light for curing. Finally, the mold underwent immersion in a hydration solution to facilitate the demolding process. Throughout the reaction, the contact lenses within the molds absorbed water, rendering them pliable and easily detachable from the molds. This assembly process seamlessly integrated with the standard contact lens manufacturing process, resulting in improved edge smoothness and flatness of the contact lens. This method played a crucial role in achieving a wrinkle-free SCLs, as depicted in Figure 24b [22][69].
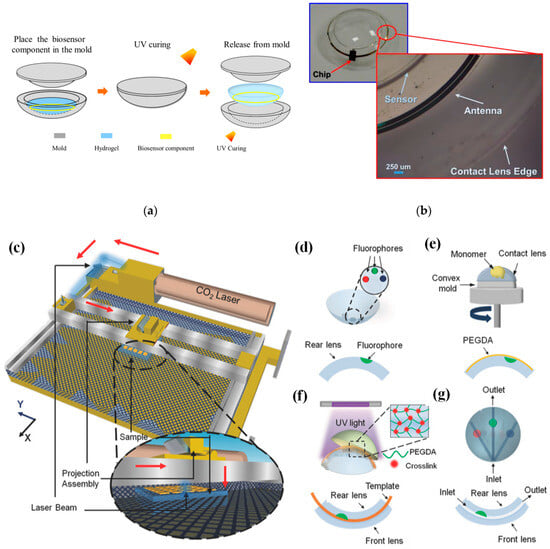
Figure 24. (a) Contact lens assembly process [22][69]. (b) Assembled wrinkle-free soft contact lens [22][69]. The process of fabricating microfluidic contact lenses involves several critical steps: (c) to begin, CO2 laser patterning is employed, as depicted schematically, with arrows illustrating the laser path [39][84]; (d) subsequently, fluorophores are meticulously deposited within the intricately patterned microconcavities found on the contact lens [39][84]; (e) following this, a PEGDA monomer layer is uniformly applied to the contact lens through a spin-coating process [39][84]; (f) to establish microchannels spanning the microconcavities, fiber templates are strategically positioned, and the combination of these templates with the microconcavities is achieved using UV-initiated free-radical polymerization, ultimately fusing them with a pristine contact lens [39][84]; (g) the final step involves the careful extraction of the fiber templates from the contact lens, resulting in the successful creation of a fully functional microfluidic contact lens [39][84].
In [39][84], CO2 laser ablation (10.64 µm wavelength, 30 W power) with a spot size of approximately 180 µm was opted to create microconcavities in contact lenses due to its advantages of rapid fabrication and precise programmable beam speed and accuracy (as illustrated in Figure 24c). To craft the microfluidic contact lens, two standard commercial contact lenses were employed. The fabrication process commenced by using laser ablation to create microconcavities on a contact lens, followed by the introduction of fluorophores into these microconcavities, as shown in Figure 24d. Subsequently, a layer of poly(ethylene glycol) diacrylate (PEGDA) monomer was evenly applied through spin-coating onto the contact lens, which was affixed onto a convex mold (as depicted in Figure 24e). Within the PEGDA layer positioned above the patterned microconcavities, silica fiber templates were inserted. These templates were then enclosed between another unaltered contact lens, which served as the front surface of the assembly. The front and rear contact lenses were securely bonded together through a process of UV-initiated free-radical polymerization (depicted in Figure 24f). To complete the creation of the microfluidic device, the silica fiber templates were carefully removed from the contact lens, resulting in the final product, as illustrated in Figure 24g.
Laser ablation is a frequently employed method in the production of microfluidic devices. This technique involves directing a high-intensity laser beam at specific locations on materials, allowing the laser’s energy to remove material at the targeted points. The creation of a microstructure is achieved by adjusting the laser source’s position or projecting the laser through a mask onto the substrate, considering factors such as the substrate material, laser intensity, and wavelength. For the first time, femtosecond laser ablation was harnessed as a straightforward, one-step, and incredibly precise method for crafting NFC antennas using conventional flexible printed circuit board materials [40][85]. This innovative approach allowed us to create antenna lines with a depth of 9 μm and a width of 35 μm. The resulting antenna, boasting a compact footprint of 19.5 mm2, was subjected to rigorous testing in biological solutions, enduring aging, and bending trials. Remarkably, the antenna exhibited a frequency deviation of less than 1%. In a real-world application, the potential of this technology is showcased by fabricating a SCL integrated with the NFC antenna, an NFC chip, and an electrochemical sensor. This SCL enabled the wireless monitoring of glucose levels in an artificial tear solution via a smartphone. Impressively, the device demonstrated its capability to accurately quantify biologically relevant glucose concentrations within the range of 0.2 to 1 mM, with a limit of detection as low as 66 μM. Furthermore, the device exhibited a minimal response to interfering molecules, with a variation of less than ±1 nA, and successfully passed a spike-and-recovery test [40][85].
Chiou et al. detailed the creation of a graphene-based thin-film supercapacitor designed for use as an energy storage system in a SCL [41][86]. The fabrication process involved several steps: the initial deposition of copper and parylene-C layers on a silicon wafer as sacrificial layers; the deposition and patterning of Ti (40 nm) and Au (200 nm) to form the current collector; the application, baking, and patterning of graphene; the coating of a PVA-H3PO4 gel electrolyte through drop-casting; the subsequent deposition of parylene-C via chemical vapor deposition (CVD) for electrolyte insulation; the release of the components from the silicon wafer and their incorporation into a standard hydrogel soft contact lens using a cast-molding process. This innovative approach resulted in a SCL characterized by stability and flexibility, making it a potential substitute for RF-based power systems.
Each lens is thoroughly examined for flaws, shape accuracy, and the integration of smart components before being packaged in a sterile setting to preserve its safety and quality until it is worn. It may be necessary to calibrate and test the electronic components of SCLs. This can entail making sure that the sensor readings are correct, the wireless connection is effective, and the power usage is minimized. To provide optimal vision correction and comfort, SCLs must be fitted to the wearer’s eye and prescribed by an eye care specialist. The guidelines for wearing and caring for SCLs must be followed by the wearers. This entails treating them gently, charging them if they have power, and replacing them according to the manufacturer’s instructions.
4. Applications
Diabetes stands as one of the most prevalent lifelong chronic conditions afflicting humans. Its primary causes are rooted in genetic predisposition, immune system dysregulation, and other factors impacting the human body. These factors collectively contribute to the decline in islet function and the emergence of insulin resistance, culminating in an imbalance of glucose levels within the body. This manifests as a disruption in glucose metabolism and the onset of hyperglycemia. Diabetes encompasses two distinct categories: type 1 and type 2. Type 1 diabetes arises from a deficiency in insulin secretion by the pancreas [42][87]. Conversely, type 2 diabetes predominantly stems from the ineffective utilization of insulin, driven by insulin resistance and diminished insulin sensitivity in affected individuals. The prevalence of diabetes is substantial, with a wide array of complications and multifaceted underlying causes. It poses significant challenges in terms of treatment and presents considerable threats to human health. As a result, numerous fields of research have been actively engaged in the study and exploration of diabetes-related aspects [43][88].
The measurement of glucose concentration plays a crucial role in diagnosing diabetes mellitus. Nevertheless, the conventional method of obtaining a single-time-point blood glucose reading necessitates a painful finger puncture to collect a blood sample. Biological fluids, such as tears [44][89], saliva [45][90], interstitial fluid (ISF) [46][47][91,92], and sweat [48][49][93,94], offer non-invasive or minimally invasive methods for obtaining samples, as shown in Figure 35 [50][95]. These fluids contain glucose concentrations that correlate with blood glucose levels, making them intriguing candidates for painless glucose monitoring within the body. Nevertheless, ensuring the accuracy and reliability of glucose measurements from these alternative biofluids requires careful consideration. One critical aspect to note is that the glucose levels in these biofluids tend to be lower compared to blood.
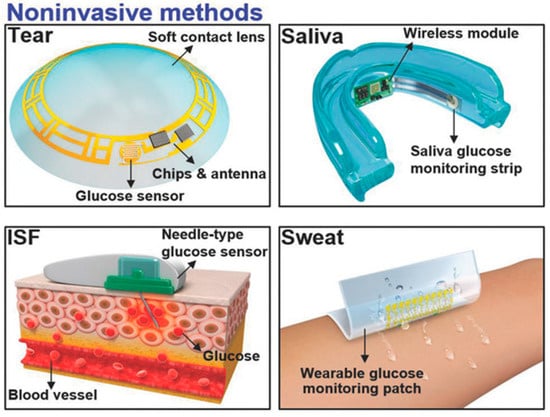
Continuous glucose monitors (CGMs), often referred to as diabetes monitors, represent wearable technological advancements designed to simplify the continuous tracking of blood sugar levels [51][96]. These FDA-approved medical devices regularly assess glucose levels in your bloodstream while they are worn. The functioning of a CGM relies on a minuscule sensor that is discreetly inserted beneath the skin, typically on the abdomen or arm. This sensor gauges the interstitial glucose concentration, which refers to the glucose present in the fluid that surrounds cells. Every few minutes, this sensor dutifully records the glucose levels, with the collected data being wirelessly transmitted to a monitoring device. The monitoring device can be conveniently carried in a pocket or purse as a standalone unit or as an integral component of an insulin pump. In some instances, certain CGMs can transmit data directly to a tablet or smartphone [52][97].
Extensive research and data have consistently demonstrated a positive correlation between the glucose concentration in tears and that in the blood [53][98]. To mitigate the discomfort of blood sampling, SCLs equipped with an integrated glucose sensor offer an innovative solution. These lenses can continually monitor the glucose concentration in tears, providing a less invasive and more user-friendly alternative for managing diabetes. Within human tears reside a multitude of compounds encompassing proteins, lipids, electrolytes, urea, ascorbic acid, L-lactic acid, cholesterol, and other pivotal metabolites. Remarkably, the chemical makeup of tears closely mirrors that of blood. The real-time assessment of the concentrations of these substances furnishes essential physiological insights, thereby contributing to the enhancement of approaches aimed at treating and preventing certain illnesses. The most vital analytes and their concentration in the tears along with the related diseases are listed in Table 2.
| Analytes | Tear Conc. (mM) | Diagnostic Disease | References |
|---|---|---|---|
| Lactate | 2.0–0.05 | Cancer; sepsis; ischemia; liver disease | [55][99[56],100] |
| Glucose | 0.01–0.05 | Diabetes | [57][101[58],102] |
| Urea | 3.0–6.0 | Renal function | [59][103] |
| Dopamine | 0.37 | Glaucoma | [60][61][36,104] |
| Cortisol | 1–40 ng/mL | Stress levels and brain injuries | |
| Mg2+ | 0.5–0.9 | Hyper/hypomagnesemia | [62][105] |
| K+ | 20–42 | Hyper/hypokalemia and an indicator of ocular disease | [63][106] |
| Ca2+ | 0.4–1.1 | Hyper/hypocalcemia | [64][107] |
| Cl− | 118–135 | Hyper/hypochloremia | [65][108] |
| Na+ | 120–165 | Hypo/hypernatremia | [66][109] |
| Total protein | 7 g/L | Dry eye syndrome | [67][110] |
Moreover, research has underscored the significant role of substantial fluctuations in IOP as a contributing factor to the development of glaucoma [68][69][111,112]. However, the routine measurement of IOP using the Goldmann applanation tonometer, a conventional clinical tool, is a complex procedure. Consequently, contact lens sensors have emerged as a promising avenue for IOP monitoring by gauging changes in corneal curvature. Leonardi et al. devised a wireless SCL engineered to detect IOP. This innovative lens comprised components such as an antenna, passive gauges, a microprocessor, and other electrical elements. Notably, this device facilitated prolonged and minimally invasive IOP monitoring, delivering both diagnostic and therapeutic advantages for effective glaucoma management. In the realm of IOP monitoring using contact lenses, four fundamental types of sensors have emerged: capacitance sensors [70][113], piezoresistive sensors [71][114], strain gauge sensors [72][115], and micro-inductor sensors [20][37]. Each of these sensor types holds the potential to contribute to enhanced IOP monitoring techniques.
Lactate stands as a pivotal biomarker with considerable significance in clinical diagnostics and health surveillance [73][116]. Its utility spans the identification of hypoxia or elevated salt levels arising from physiological or pathological circumstances. Broadly speaking, a human blood lactic acid level surpassing 2 mmol/L is indicative of lactic acidosis, a condition associated with potential complications, such as lactic acid poisoning, stemming from factors like toxins, shock, anemia, sepsis, and organ failure. In the contemporary landscape, tears have emerged as a viable alternative sampling medium due to their ease of extraction, offering a more comfortable and less burdensome substitute for the traditionally uncomfortable blood sampling method [27][55][56][31,99,100]. The realm of real-time lactate concentration monitoring within the body has gained traction through the utilization of contact lens sensors. This avenue holds promise, presenting an exciting prospect for the future.
