Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Felix Royo.
The human CERS2 gene encodes a ceramide synthase enzyme, known as CERS2 (ceramide synthase 2). This protein is also known as LASS2 (LAG1 longevity assurance homolog 2) and TMSG1 (tumor metastasis-suppressor gene 1). Bladder cancer (BC) is a significant cause of cancer-related deaths globally, ranking as the second-most-common reason for genitourinary cancer-related mortality [1,2]. The treatment of non-muscle invasive bladder cancer includes transurethral resection followed by chemotherapy to reduce recurrence chances, while muscle-invasive bladder cancers are associated with high rates of progression and metastasis and are usually treated via radical cystectomy if the tumor is organ-confined.
- tumor suppressor
- cell metabolism
- ceramides
- biomarker
- extracellular vesicles
1. CERS2 Expression in BC
The regulation through miRNAs points toward the silencing of the gene in tumors, and indeed, different studies have investigated the correlation between CERS2 expression and BC progression. Regarding human bladder carcinoma cell lines, it has been described that the most malignant cells express lower amounts of CERS2 at both the mRNA and protein levels [38,39][1][2]. However, gene expression level assessment in a panel of bladder cell lines offers wide variation. The cell lines showing higher expression of CERS2 were a non-tumoral SV40 immortalized cell line named SVHUC1 and the cell line UMUC10, described as non-tumorigenic. On the contrary, the lowest expression was observed in UBLC1, a tumorigenic cell line. However, the second lowest expression was found in HTB4, which is described by the American type culture collection (ATCC) as non-tumorigenic in nude mice [3]. There is large agreement between that report and the classification in information obtained from The Human Protein Atlas webpage (https://www.proteinatlas.org/ accessed 13 October 2023) [58][4], and there is only disagreement for the bladder cell line 5637, since in this database it is recorded as having the highest CERS2 expression in spite of being quite tumorigenic according to ATCC (Figure 31).
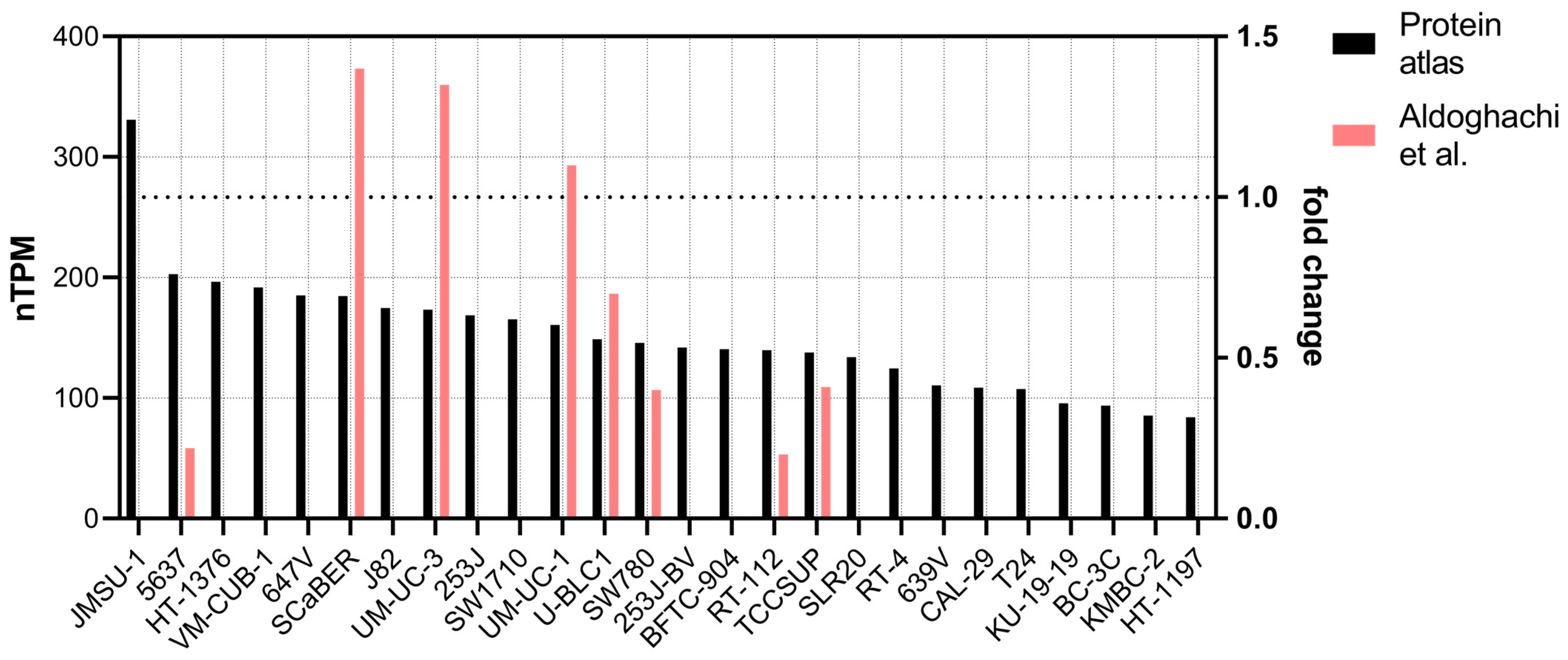
Figure 31. Transcript expression of CERS2 in bladder cancer cell lines. Black bars present the values obtained from The Human Protein Atlas (see text), in decreasing order. The red bars show the expression of CERS2 in some of the cell lines, normalized against the expression in UM-UC-13 cell line obtained in [3] (right axis, 1 denotes the expression of UM-UC-13).
As in most of cell bladder cell lines, a study with 44 samples observed that CERS2 expression decreases as the stage of malignancy increases for both protein and mRNA level [59][5]. This correlation has also been tested experimentally, using nude mice to study the evolution of xenografts with a highly invasive human BC cell line. Indeed, when CERS2 was silenced in the xenografts, the resulting tumors significantly increased in volume [60][6]. Also supporting the tumor suppressor activity of CERS2, an epidemiology study based on SNPs showed that a particular substitution, which favors RNA instability and reduces transcript abundance, acts as an independent risk factor of BC susceptibility and clinical prognosis in the Chinese population [24][7].
However, and in spite of the previous studies supporting the idea that lower CERS2 expression is associated with poor prognosis in BC, there are cases in which an increase in CERS2 mRNA expression is observed early in tumor development [39][2]. Moreover, in silico studies and confirmation via qPCR show an increase of CERS2 in BC [10][8]. The ATCG database showed that 10% of cases profiled show gene amplification for CERS2 in bladder cancer (data obtained through cBioPortal [61][9]), and regarding gene transcription, the expression is slightly higher in cancer samples (Log2 foldchange 0.2, with a p value of 0.025) among 432 samples (404 tumor vs. 28 non-tumor, data obtained through GEPIA2 [62][10]).
2. CERS2 and Extracellular Vesicles
Extracellular vesicles (EVs) are membrane-bound entities released by cells into the extracellular environment. They play an important role in intercellular communication by transporting proteins, nucleic acids, and metabolites to recipient cells, providing information about their parent cells [69][11]. This transfer of cargo can modulate recipient cell functions, influencing processes such as immune response regulation, cell proliferation, and tissue repair. There are three main types of EVs: exosomes, microvesicles, and apoptotic bodies [70][12]. Exosomes are the smallest, ranging from 30 to 150 nanometers in diameter, and are formed within the endocytic pathway. Microvesicles, also known as shedding vesicles or ectosomes, are larger, typically ranging from 100 to 1000 nanometers, and are formed through direct budding from the cell membrane. Apoptotic bodies are the largest and are released during programmed cell death. EVs have also been implicated in various diseases, including cancer, neurodegenerative disorders, and cardiovascular diseases, making them attractive targets for diagnostic and therapeutic applications [71][13]. EVs can be found in all body fluids, such as blood, breast milk, cerebrospinal fluid, and urine [72][14]. Specifically, their presence in urine has been described and well characterized for a long time [73][15], and their value as a diagnostic tool well documented (reviewed in [74][16]), including their use in BC diagnosis [75,76][17][18].
In a comparative analysis between transcripts associated with urinary EVs, the detection of CERS2 mRNA was achieved in different preparations from BC patients via both microarray and PCR technologies. In contrast, it was not detected in urinary EVs from patients with benign hyperplasia [5][19]. More recently, a study confirmed increase in this transcript in the urine of BC patients [10][8].
This result, which seems to contradict the role of CERS2 as a tumor suppressor, is nevertheless in agreement with the results observed in different gene expression data sets in BC biopsies. However, it should be noted that the presence of mRNA in EVs does not necessarily imply increased gene or protein expression in tumor tissue. In fact, the fate of some miRNA-silenced mRNAs may be their release through EVs by the binding of a zip code to a central domain in the stem–loop structure. For example, miR-1289 binds directly to this zip code of its target mRNA and orchestrates its transfer to microvesicles [77][20].
Regarding the possible role of circulating CERS2 in EVs as a biomarker, there are several reports on the presence of the protein and mRNA in EV preparations registered in Vesiclepedia archives (http://microvesicles.org/ accessed 6 June of 2023) isolated from different tumor tissues such as glioblastoma, colorectal, breast, or brain cancer. Interestingly, dendritically derived EVs loaded with CERS2 had an anti-apoptotic effect in neurons in and age-driven inflammatory context [78][21]. Moreover, ceramides synthesized by CERS2 are also released and transported by microvesicles [79][22] and the lack of function of the protein in some tumors probably modifies the composition of their EVs. Therefore, both the presence of circulating CERS2 mRNA or protein as cargo in EVs, as well as the composition of these EVs, have great potential for establishing not only the diagnosis but also the prognosis of patients with BC.
3. CERS2 as a Therapeutical Target
Regarding chemotherapy, the standard approach for treating patients with advanced urothelial cancer is the so-called methotrexate, vinblastine, doxorubicin, and cisplatin regimen. Unfortunately, due to high toxicity and suboptimal responses, there has been a need to explore newer treatment combinations. For example, the regimen using gemcitabine and cisplatin was found to yield similar survival outcomes with fewer side effects. These chemotherapies demonstrate high initial response rates, with a median survival of 15 months (reviewed in [80][23]). A recent approach is based on immune checkpoint inhibitors, which are approved as a second-line treatment for patients who experience progression after initial cystatin treatment [81][24]. These drugs are effective in tumor cells that express PD-L1 proteins, blocking the binding of the ligand to the associated checkpoint receptor. In this context, it is worth mentioning that basal subtypes of bladder cancer, characterized by p63 activation, squamous differentiation, and a more aggressive disease, also express higher levels of immune checkpoint ligands, specifically programmed-death ligand 1 (PD-L1), compared to luminal tumors [82][25]. Hence, stratifying bladder cancer is essential for guiding therapeutic decisions.
To address this objective, the integration of genetic and molecular information can provide valuable guidance for treatment decisions in newly diagnosed bladder cancer (BC) patients. Considering the dual role observed for CERS2, this gene emerges as a promising candidate for molecular markers to inform therapy decisions. CERS2 plays a pivotal role in multiple survival mechanisms, and manipulating its expression and activity can significantly affect cell proliferation and tumor progression. Numerous studies have delved into the molecular implications of this gene’s involvement. As a key player in the synthesis of long ceramides, CERS2’s role in the context of chemotherapy appears to promote chemoresistance. The upregulation of CERS2 induced by treatment with C2-ceramide increases the protein levels of CERS2, thereby modulating sphingolipid metabolism to favor the conversion of C2-ceramide into pro-survival sphingolipids in hepatocarcinoma cells. This mechanism is associated with autophagy and a reversible senescence phenotype, ultimately contributing to C2-ceramide resistance in these cells. However, co-treatment with polyphenols downregulated the protein level of CERS2 and increased oxidative and endoplasmic reticulum stress, leading hepatocarcinoma cells toward apoptosis [83][26]. In the context of renal malignancies, the downregulation of CERS2 reduces doxorubicin chemoresistance. This action is mediated by the reduction in the protein amount and plasma membrane localization of ABCB1, a transmembrane transporter that extrudes both proapoptotic substrates and drugs out of the cell. Again, this effect is caused by an imbalance in ceramide species [67][27].
On the contrary, in the case of some solid tumors, the activity of proteins such as BCL2, with the capacity to bind to and inhibit CERS2, takes the opposite course. For instance, in glioblastoma tumor cells with high expression of BCL2 like 13 (BCL2L13), CERS2 activity is blocked, resulting in the prevention of apoptosis in response to both conventional and targeted therapies. It is noteworthy that pharmacological inhibition of BCL2L13 may offer the potential to increase proapoptotic ceramide levels in cancers by restoring CERS2 activity [84][28]. In the same direction, treating cells with diterpenoids that reduce BC2 and increase CERS2 activity also increases apoptosis in glioma cells [68][29]. In different contexts, the activity of CERS2 can either promote apoptosis or inhibit it, depending on various factors, highlighting its versatile role in cancer biology.
References
- Zhao, Q.; Wang, H.; Yang, M.; Yang, D.; Zuo, Y.; Wang, J. Expression of a tumor-associated gene, LASS2, in the human bladder carcinoma cell lines BIU-87, T24, EJ and EJ-M3. Exp. Ther. Med. 2013, 5, 942–946.
- Wang, H.; Wang, J.; Zuo, Y.; Ding, M.; Yan, R.; Yang, D.; Ke, C. Expression and prognostic significance of a new tumor metastasis suppressor gene LASS2 in human bladder carcinoma. Med. Oncol. 2012, 29, 1921–1927.
- Aldoghachi, A.F.; Baharudin, A.; Ahmad, U.; Chan, S.C.; Ong, T.A.; Yunus, R.; Razack, A.H.; Yusoff, K.; Veerakumarasivam, A. Evaluation of CERS2 Gene as a Potential Biomarker for Bladder Cancer. Dis. Markers 2019, 2019, 3875147.
- Karlsson, M.; Zhang, C.; Mear, L.; Zhong, W.; Digre, A.; Katona, B.; Sjostedt, E.; Butler, L.; Odeberg, J.; Dusart, P.; et al. A single-cell type transcriptomics map of human tissues. Sci. Adv. 2021, 7, eabh2169.
- Yegin, Z.; Aydin, O.; Koc, H.; Buyukalpelli, R. Expression profiles of proto-oncogene TWIST1 and tumor metastasis suppressor gene LASS2 in bladder cancer. Cell Mol. Biol. (Noisy-le-grand) 2018, 64, 66–73.
- Chen, Y.; Wang, H.; Xiong, T.; Zou, R.; Tang, Z.; Wang, J. The role of LASS2 in regulating bladder cancer cell tumorigenicity in a nude mouse model. Oncol. Lett. 2017, 14, 5149–5156.
- Huang, Y.; Wang, H.; Fu, S.; Luan, T.; Zuo, Y.; Li, N.; Ding, M.; Chen, Y.; Wang, J. Association of rs8444 polymorphism in the LASS2 3′-UTR and bladder cancer risk in Chinese population. Eur. J. Cancer Prev. 2020, 29, 329–337.
- Ren, R.; Wang, H.; Xie, L.; Muthupandian, S.; Yang, X. Identify Potential Urine Biomarkers for Bladder Cancer Prognosis Using NGS Data Analysis and Experimental Validation. Appl. Biochem. Biotechnol. 2023, 195, 2947–2964.
- de Bruijn, I.; Kundra, R.; Mastrogiacomo, B.; Tran, T.N.; Sikina, L.; Mazor, T.; Li, X.; Ochoa, A.; Zhao, G.; Lai, B.; et al. Analysis and Visualization of Longitudinal Genomic and Clinical Data from the AACR Project GENIE Biopharma Collaborative in cBioPortal. Cancer Res. 2023. Online ahead of print.
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560.
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383.
- Jeppesen, D.K.; Zhang, Q.; Franklin, J.L.; Coffey, R.J. Extracellular vesicles and nanoparticles: Emerging complexities. Trends Cell Biol. 2023, 33, 667–681.
- Beetler, D.J.; Di Florio, D.N.; Bruno, K.A.; Ikezu, T.; March, K.L.; Cooper, L.T., Jr.; Wolfram, J.; Fairweather, D. Extracellular vesicles as personalized medicine. Mol. Asp. Med. 2023, 91, 101155.
- Urabe, F.; Kosaka, N.; Ito, K.; Kimura, T.; Egawa, S.; Ochiya, T. Extracellular vesicles as biomarkers and therapeutic targets for cancer. Am. J. Physiol. Cell Physiol. 2020, 318, C29–C39.
- Pisitkun, T.; Shen, R.F.; Knepper, M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373.
- Erdbrugger, U.; Blijdorp, C.J.; Bijnsdorp, I.V.; Borras, F.E.; Burger, D.; Bussolati, B.; Byrd, J.B.; Clayton, A.; Dear, J.W.; Falcon-Perez, J.M.; et al. Urinary extracellular vesicles: A position paper by the Urine Task Force of the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2021, 10, e12093.
- Smalley, D.M.; Sheman, N.E.; Nelson, K.; Theodorescu, D. Isolation and identification of potential urinary microparticle biomarkers of bladder cancer. J. Proteome Res. 2008, 7, 2088–2096.
- Hiltbrunner, S.; Mints, M.; Eldh, M.; Rosenblatt, R.; Holmstrom, B.; Alamdari, F.; Johansson, M.; Veerman, R.E.; Winqvist, O.; Sherif, A.; et al. Urinary Exosomes from Bladder Cancer Patients Show a Residual Cancer Phenotype despite Complete Pathological Downstaging. Sci. Rep. 2020, 10, 5960.
- Perez, A.; Loizaga, A.; Arceo, R.; Lacasa, I.; Rabade, A.; Zorroza, K.; Mosen-Ansorena, D.; Gonzalez, E.; Aransay, A.M.; Falcon-Perez, J.M.; et al. A Pilot Study on the Potential of RNA-Associated to Urinary Vesicles as a Suitable Non-Invasive Source for Diagnostic Purposes in Bladder Cancer. Cancers 2014, 6, 179–192.
- Bolukbasi, M.F.; Mizrak, A.; Ozdener, G.B.; Madlener, S.; Strobel, T.; Erkan, E.P.; Fan, J.B.; Breakefield, X.O.; Saydam, O. miR-1289 and “Zipcode”-like Sequence Enrich mRNAs in Microvesicles. Mol. Ther. Nucleic Acids 2012, 1, e10.
- Casadome-Perales, A.; Naya, S.; Fernandez-Martinez, E.; Mille, B.G.; Guerrero-Valero, M.; Peinado, H.; Guix, F.X.; Dotti, C.G.; Palomer, E. Neuronal Prosurvival Role of Ceramide Synthase 2 by Olidogendrocyte-to-Neuron Extracellular Vesicle Transfer. Int. J. Mol. Sci. 2023, 24, 5986.
- McNally, B.D.; Ashley, D.F.; Hanschke, L.; Daou, H.N.; Watt, N.T.; Murfitt, S.A.; MacCannell, A.D.V.; Whitehead, A.; Bowen, T.S.; Sanders, F.W.B.; et al. Long-chain ceramides are cell non-autonomous signals linking lipotoxicity to endoplasmic reticulum stress in skeletal muscle. Nat. Commun. 2022, 13, 1748.
- Hui, G.; Stefanoudakis, D.; Zektser, Y.; Isaacs, D.J.; Hannigan, C.; Pantuck, A.J.; Drakaki, A. Do Cancer Genetics Impact Treatment Decision Making? Immunotherapy and Beyond in the Management of Advanced and Metastatic Urothelial Carcinoma. Curr. Oncol. 2023, 30, 7398–7411.
- Cheng, W.; Fu, D.; Xu, F.; Zhang, Z. Unwrapping the genomic characteristics of urothelial bladder cancer and successes with immune checkpoint blockade therapy. Oncogenesis 2018, 7, 2.
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2018, 174, 1033.
- Chiu, C.C.; Chen, Y.C.; Bow, Y.D.; Chen, J.Y.; Liu, W.; Huang, J.L.; Shu, E.D.; Teng, Y.N.; Wu, C.Y.; Chang, W.T. diTFPP, a Phenoxyphenol, Sensitizes Hepatocellular Carcinoma Cells to C(2)-Ceramide-Induced Autophagic Stress by Increasing Oxidative Stress and ER Stress Accompanied by LAMP2 Hypoglycosylation. Cancers 2022, 14, 2528.
- Lee, W.K.; Maass, M.; Quach, A.; Poscic, N.; Prangley, H.; Pallott, E.C.; Kim, J.L.; Pierce, J.S.; Ogretmen, B.; Futerman, A.H.; et al. Dependence of ABCB1 transporter expression and function on distinct sphingolipids generated by ceramide synthases-2 and -6 in chemoresistant renal cancer. J. Biol. Chem. 2022, 298, 101492.
- Jensen, S.A.; Calvert, A.E.; Volpert, G.; Kouri, F.M.; Hurley, L.A.; Luciano, J.P.; Wu, Y.; Chalastanis, A.; Futerman, A.H.; Stegh, A.H. Bcl2L13 is a ceramide synthase inhibitor in glioblastoma. Proc. Natl. Acad. Sci. USA 2014, 111, 5682–5687.
- Kong, T.; He, Z.; Wang, S.; Jiang, C.; Zhu, F.; Gao, J.; Li, L.; Wang, Y.; Xie, Q.; Li, Y. Diterpenoid DGA induces apoptosis via endoplasmic reticulum stress caused by changes in glycosphingolipid composition and inhibition of STAT3 in glioma cells. Biochem. Pharmacol. 2022, 205, 115254.
More
