Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Lingling Zhao.
Hydrogels prepared from natural polymer have attracted extensive attention in biomedical fields such as drug delivery, wound healing, and regenerative medicine due to their good biocompatibility, degradability, and flexibility.
- hydrogel
- natural polymer
- drug delivery
- tissue engineering
- wound healing
1. Introduction
Natural polymers derived from materials in the natural world, such as polysaccharides and proteins, have a good biocompatibility and biodegradability, making them an ideal choice for the fabrication of hydrogels. Polysaccharides used for hydrogel fabrication include cellulose, chitosan, dextran, alginate, hyaluronic acid, as well as their derivatives. There are abundant hydroxyl groups and/or other functional groups (amino, carboxyl groups, and so on) on the chains of polysaccharides, offering versatile opportunities to prepare polymer-based hydrogels via chemical or physical cross-linking. Proteins including collagen, gelatin, and fibrin are essentially polymers of amino acids. It is thought that proteins can form fibrils in nanometer width and micrometer length via intermolecular and/or intramolecular forces (such as hydrogen bonds, electrostatic interactions, and hydrophobic effects) and further form three-dimensional hydrogels via self-organization and entangling under appropriate conditions [20][1].
2. Cellulose and Cellulose-Based Hydrogels
Cellulose is the most abundant natural polymer compound on earth, consisting of β (1-4)-glycosidic-linked glucose units (Figure 1). Cellulose organizes in a rather intricate supramolecular structure formed by the intermolecular cohesion of cellulose molecules, which is an extended intra/intermolecular network of hydrogen bonds. In the dissolution process of cellulose, the intramolecular hydrogen bonds are broken, and the supramolecular structure of cellulose is disintegrated, enhancing the activity of hydroxyl in cellulose, and making it easy to combine with other natural or synthetic polymers by reconstructing hydrogen bonds. Therefore, cellulose is one of the ideal candidates for hydrogels’ preparation, endowing cellulose composite hydrogels with specific performance, such as biodegradability, renewability, flexibility, and high mechanical strength [21][2].
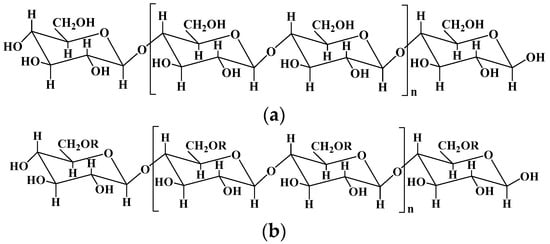
Figure 1.
The chemical structures of cellulose (
a
) and some important derivatives of cellulose (
b
).
Methylcellulose: R = -H, -CH3; ethylcellulose: R = -H, -CH2CH3; hydroxyethyl cellulose: R = -H, -CH2CH2OH; carboxymethylcellulose: R = -H, -CH2COOH; hydroxypropyl cellulose: R = -H, -CH2CH(OH)CH3; hydroxy propyl methyl cellulose: R = -H, -CH3, CH2CH(OH)CH3.
Cellulose can be divided into plant cellulose and bacterial cellulose. Plant cellulose is widely sourced from cotton, wood, and other plants, such as cardamom fiber, seed fiber, and wood fiber, and is the most abundant organic substance in nature [22][3]. Plant cellulose is mainly produced in a nonpure form as lignocellulose, and can be purified using an ecofriendly biological method and a non-ecofriendly chemical method [23][4]. The biological method depends on microbial enzymes and has a low productivity, while the chemical method has many steps and types of equipment and a high productivity. Compared with plant cellulose, bacterial cellulose produced via certain types of bacteria has a high purity and functionality, which is a major alternative source of plant cellulose [24][5]. Bacterial cellulose can be produced by the biosynthesis of certain types of Gram-negative and Gram-positive strains in high-glucose-containing media.
Nanocellulose (NC) is a mesoscopic material formed in the regeneration process of cellulose, which combines the advantages of both cellulose and nanomaterials. NC, including cellulose nanocrystals (CNCs) and cellulose nanofibrils (CNFs), has been widely used in the field of functional materials due to its unique morphology of nanostructures and featuring many advantages like excellent mechanical properties, biodegradability, and environmental friendliness [25,26][6][7]. For example, CNFs prepared by TEMPO (2, 2, 6, 6-Tetramethylpiperidine-1-oxyl)-mediated oxidation has plenty of hydroxyl groups and carboxyl groups on their surface, endowing CNFs with a good stability and dispersibility without aggregation in water, and making them easy to combine with other polymers or nanoparticles to construct novel reinforced composite materials and hydrogels [27,28][8][9].
The fabrication of cellulose-based hydrogels is challenging because cellulose is hardly soluble in common solvents due to its highly extended hydrogen-bonded structure. One of the strategies to resolve this issue is the development of several solvent systems, for example dimethylacetamide, alkali/urea (or thiourea) aqueous systems, and ionic liquids [29,30][10][11]. For instance, several solvent systems including LiCl/dimethylacetamide, paraformaldehyde/dimethyl sulfoxide, and triethylammonium chloride/dimethyl sulfoxide have been used to directly fabricate cellulose hydrogels [31][12], and some hydrophilic ionic liquids, such as 1-butyl-3-methylimidazolium chloride and 1-allyl-3-methylimidazolium chloride, have been used to dissolve cellulose [32][13]. Chemical modification is another effective method to resolve the poor solubility of cellulose. Hydroxyl and carboxyl groups are the most commonly used active groups introduced into the skeleton of cellulose, obtaining various cellulose derivatives including carboxymethyl cellulose, hydroxyethyl cellulose, hydroxypropyl cellulose, and hydroxypropyl methyl cellulose [33,34][14][15]. Cellulose-based hydrogels can be formed via the cross-linking between these functional groups, for instance, the esterification of hydroxyl and carboxyl groups by carbodiimide-condensing agents, Michael’s addition reaction between hydroxyl groups and carbon–carbon double bonds under alkaline conditions, epoxide and alkyl halide cross-linking under strong basic and high-temperature conditions, and free radical polymerization [22,35][3][16].
Cellulose–gelatin hydrogels with high strength and pH-responsiveness were prepared using the cyclic freezing–thawing method [36][17]. The repeated freezing–thawing cycles played a vital role in the formation of the supramolecular network structure via physical cross-linking between cellulose and gelatin. The superior mechanical performance contributed to the combination effect of the hydrogen bond and the reinforcement of CNFs. A bacterial cellulose-based hydrogel with good mechanical properties and improved ionic conductivity was prepared for thermo-electrochemical cells in practical wearable electronics by introducing highly soluble urea and the thermodiffusion effect of NaCl [37][18]. The bacterial cellulose hydrogel-based electrolyte had a nanofiber-porous 3D network structure, and its nanochannels contained plenty of surface hydroxyl groups, which favored the transport of positive ions.
3. Chitosan and Chitosan-Based Hydrogels
Chitosan is a natural polycationic polymer with hydrophilic properties, consisting of the repeating residues of D-glucosamine and N-acetyl-D-glucosamine (Figure 2) [10][19]. It is obtained by the partial deacetylation of chitin, one of the most abundant polymers after cellulose, extracted from the fungal cell walls and the exoskeleton of crustaceans/insect [38][20]. Generally, chitosan should have a deacetylation degree of 60, containing at least 60% of D-glucosamine for the deacetylated chitin [10][19]. Chitosan is an analogous of glucosaminoglycan, one of the main components found in the extracellular matrix (ECM) of some living tissues, so chitosan can be used to mimic the ECM in regenerative medicine and has been widely studied in the areas of biotechnology [39,40][21][22]. As the only cationic polysaccharide found in natural polysaccharides to date, chitosan is well known for its antimicrobial and antifungal properties. It is thought that the positively charged chitosan can interact with the negatively charged surfaces of cells and microbes, thereby inhibiting the absorption and excretion of substances [41][23]. Chitosan exhibits a degradability in vivo by human protease such as lysozyme due to its multiple amino groups [42][24]. Recently, studies have highlighted the antitumor activity, mucoadhesive, and hemostatic properties of chitosan, which render its applications in biomedicine and pharmaceuticals promising. What is more, due to the abundant hydroxyl and amine groups on the chitosan backbone, its chemical properties can be further tailored by adding functionalities to develop functionalized chitosan for biomedical applications.
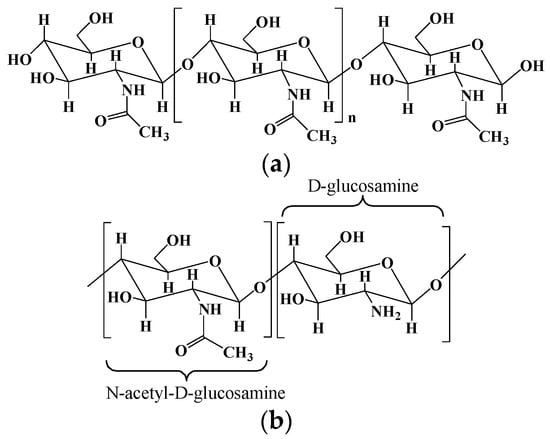
Figure 2.
The chemical structures of chitin (
a
) and chitosan (
b
).
The two main sources of chitosan are animal sources (crustaceans and fungal mycelia) with seasonal supplies and mushroom sources with a better reproducibility. Chitosan is derived from the partial deacetylation of chitin using a chemical or biological approach. The chemical hydrolysis is performed under severe alkaline conditions (i.e., concentrated sodium hydroxide and sodium borohydride), and the biological method is performed by particular bacteria and/or enzymes [11][25].
Chitosan-based hydrogels are formed by physical association or chemical cross-linking [43][26]. In physically cross-linked hydrogels, non-covalent interactions, namely, electrostatic interactions, hydrophobic interactions, and hydrogen bonding between polymer chains, are used to fabricate the gel network. In chemically cross-linked hydrogels, cross-linker agents, secondary polymerizations, click chemistry, or irradiation chemistry is used to form the chitosan-based hydrogels. Many bifunctional/polyfunctional molecules with aldehyde, including glutaraldehyde, glycidyl ether, isocyanate, acrylate, azides, and so on, have been used to cross-link chitosan polymers and fabricate chitosan-based hydrogels, especially hydrogels with environmental sensitivity [44,45][27][28]. Chitosan hydrogels with self-healing ability were prepared using natural vanillin as a cross-linking agent [46][29]. The hydrogel network contained hybrid linkages of Schiff base bonds and hydrogen bonds. The concentration of vanillin has an important effect on the self-healing ability of the hydrogel because the self-healing ability mainly comes from the reconstruction of the dynamic Schiff base bond. Since chitosan itself has poor solubility, chemical modification can be used to produce chitosan derivatives with good water solubility. Succinylation is a simple and effective way to improve the water solubility of chitosan and obtain new functional groups, and succinylated chitosan can be ionically cross-linked with glucose-6-phosphate to create a pH-sensitive hydrogel [47][30]. The succinylated chitosan hydrogel produces the rapid gelation and efficient release of drug, and the drug release can be controlled by adjusting the succinylation and temperature, suggesting a bright and promising future for cancer and inflammation treatments. Ion-cross-linked succinylated chitosan hydrogels via glucose-6-phosphate were prepared as bone graft material carriers for the repair of bone defects [48][31]. The succinylated chitosan hydrogels had a high decomposition rate and good biocompatibility, could hold bone graft materials in the transplantation area, and showed a high cell-growth rate and bone differentiation rate.
Chitosan-based hydrogels have good biocompatibility, biodegradability, and antimicrobial properties and are ideal candidates for drug delivery and tissue engineering. However, the applications of chitosan scaffolds in tissue regeneration have been limited due to their insufficient mechanical strength and inadequate degradation rate, so the composite scaffolds prepared by mixing chitosan with other functional substances will have better tissue regeneration effect [49][32]. Liu et al. reported a novel type of homogeneously structured polyelectrolyte complexes (PEC) hydrogel with electro-responsive performance and high mechanical strength based on chitosan and carboxymethylcellulose using the cyclic freezing–thawing method [50][33] The PEC hydrogel underwent a variety of programmable 3D shape transformations, such as helix, flower, V- and M-like shapes and other intermediate variations owing to the asymmetric deformation of gel strips caused by the uneven osmotic stress on both sides of the hydrogel.
4. Collagen/Gelatin and Collagen/Gelatin-Based Hydrogel
Collagen, as one of the most abundant renewable natural polymers along with cellulose and chitosan, has significant applications in the biomedical field [51,52][34][35]. It plays an important role in structural proteins for most tissues, i.e., skin, bones, muscles, blood vessels, and cartilages, and contains plenty of functional groups, i.e., hydroxyl, amino, carboxyl, guanidyl, and imidazoles, endowing collagen with many physical and chemical properties. Collagen is able to self-assemble into a triple-helical fibrous structure under physiological conditions (Figure 3), giving collagen a great tensile strength and durability [53][36]. Collagen has many excellent natural characteristics, such as hydrophilicity, biocompatibility, biodegradability, nonimmunogenicity, and mechanical durability, making it an essential component in investigations in the biomedical field [54][37]. For instance, in engineered tissues, collagen can provide mechanical strength to other amorphous hydrogels and modulate the hydrogel to mimic native tissues, providing critical recognition sites for cellular migration and attachment and long-term structural support for tissues [55][38]. In addition, degradability is another crucial factor to be considered in tissue engineering applications. Due to the collagenase activity in the body, the intrinsic degradability of collagen enables collagen-based hydrogels to have a bright application prospect in the biomedical field including smart drug delivery and tissue regeneration.
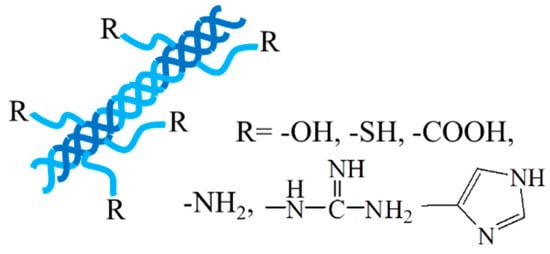
Figure 3.
The triple-helical fibrous structure of collagen.
Animal is the main source of collagen, and the animal-derived collagen can be obtained from porcine skin, bovine tendon, rat tail, or marine sources by extraction and purification using a chemical or enzyme method. The commonly used agents for the chemical treatment include a neutral salt solution, an alkali solution, dilute acetic acid, and hydrochloric acid. Moreover, recombinant collagen produced by recombinant technology and biosynthesis is an alternative source of collagen, which expressed in yeast, Escherichia coli, mammalian cells, insect cells, tobacco plants, corn seeds, and so on [12][39].
The chemical modification of collagen is generally applied to create desired properties since the direct usage of collagen in specific applications may sometimes lead to several problems like calcium deposition, high thrombogenicity, uncontrollable degradation rate, inadequate mechanical properties, and so on. The chemical modification can be achieved through structural decoration by the insertion of new functional groups and/or by the combination of novel materials containing new functional groups [51,56][34][40]. Hydrogels with M2 macrophage-polarization and anti-inflammatory properties were prepared through enzymatic cross-linking of tyramine-grafted collagen and gallate dimer-grafted hyaluronic acid [57][41]. The hydrogels integrated with deferoxamine-loaded mesoporous polydopamine nanoparticles have enhanced mechanical strength and desirable tissue adhesion and injectability, showing an improved repair effect of diabetic wounds.
Gelatin is a denatured, water-soluble polypeptide derived from collagen being irreversibly hydrolyzed [58][42]. The hydrolysis of collagen dissociates the triple helix into three peptide chains and decomposes collagen to gelatin, making the material biocompatible and biodegradable for cell growth [59][43]. Gelatin aqueous solution at a concentration of 0.5–50 wt% is in a sol state above the melting temperature (31.7–34.2 °C) and forms a thermoreversible gel after cooling [60][44]. However, the low melting temperature limits its application under physiological conditions. The addition of salt or other small soluble compounds could lead to a structural reorganization of the gelatin hydrogels due to the change in the interactions between gelatin molecules, such as hydrogen bonding, hydrophobic forces, and electrostatic forces [61,62][45][46]. Gelatin methacrylate (GelMA) is a photo cross-linkable gelatin derivative prepared by modifying the reactive side groups of gelatin using glycidyl methacrylate and has received attention in biological fields [63,64][47][48]. GelMA can be cross-linked to form hydrogel via photo-polymerization due to the presence of methacrylate, and the stiffness and porosity of the GelMA hydrogel can be regulated by controlling the production parameters such as hydrogel concentration, degree of functionalization, UV intensity, and additive supplementation [65][49].
5. Alginate and Alginate-Based Hydrogel
Alginate is a commonly available natural biopolymer, which is a linear anionic polysaccharide consisting of repeated residues of α-L glucuronate (G) and β-D mannuronate (M) (Figure 4) [66][50]. It provides biocompatibility, biodegradability, non-antigenicity, chelating ability as well as a good stability for a long time [67][51]. Alginate can absorb large quantities of biological liquids and be purified to prevent immunogenicity, rendering alginate an ideal polymer for hydrogel preparation under mild conditions [68][52].
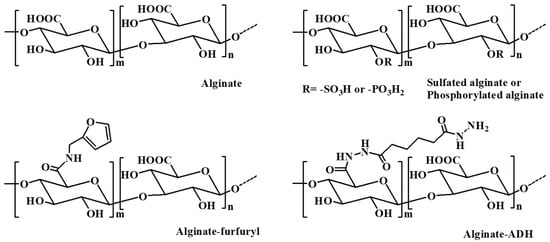
Figure 4.
The chemical structure of alginate and some alginate derivatives.
Alginate can be extracted from brown algae using alkali solutions (typically a sodium hydroxide solution). The extraction liquid is filtered, and then alginate is precipitated by adding sodium or calcium to the filtrate. In addition, bacterial alginate produced by bacterial biosynthesis from Azotobacter and Pseudomonas is another source of alginate, which provides alginate with more defined chemical structures and physical properties than that of seaweed-derived alginate [13][53].
Naturally obtained alginate may contain various impurities, including heavy metals, proteins, and endotoxins. These impurities can affect its application in the field of pharmaceuticals, especially for parenteral administration. Alginate can be obtained in an ultrapure form by a multistep extraction procedure, which has a controlled pyrogenicity and is suitable for implants in combination with drugs [69,70][54][55]. It has been reported that ultrapure alginate is effective in hemostasis, antiadhesion, and wound healing. Ultrapure alginate bilayer sponges were prepared by cross-linking two distinct ultrapure alginates with different molecular weight with calcium ions and lyophilized [71][56]. The bilayer sponges reduced adhesion by nearly 38% compared to the control group in the Pean crush hepatectomy model in rats. Simultaneously, by covering trauma with endotoxin-free sponges, the ultrapure alginate promoted the regeneration of mesothelium and supported wound healing. Further, a compression process was used to regulate the thickness and improve the mechanical property of ultrapure alginate bilayer sponges after preparing the initial sponges through lyophilization [72][57]. The results showed that the compressed ultrapure alginate bilayer sponges with the optimum thickness (100 μm) could balance the antiperitoneal adhesion and hemostasis simultaneously.
Alginate hydrogels can maintain a structural similarity to the ECM in tissues and be administrated to play several vital roles; thus, these hydrogels have attracted remarkable interest in biomedical applications, such as wound repair, drug delivery, and tissue regeneration [66][50]. Various approaches have been applied to prepare alginate-based hydrogels, including ionic cross-linking with divalent cations (i.e., Ba2+, Fe3+, Ca2+) [73[58][59],74], covalent cross-linking with multifunctional molecules (i.e., poly(acrylamide-co-hydrazide, poly(ethylene glycol)-diamines)) [75[60][61],76], and thermal gelation and in situ copolymerization of thermosensitive polymers (i.e., N-isopropylacrylamide (NIPAAm)) by UV irradiation [77][62]. In addition, various alginate derivatives including amphiphilic alginate and cell-interactive alginate have also been used in the fabrication of hydrogels for biomedical applications. Amphiphilic alginate derivatives obtained by coupling hydrophobic moieties, such as alkyl chains and hydrophobic polymers, to an alginate skeleton can self-assemble to form hydrogels in aqueous solution, which have promising prospect in drug delivery systems [78][63].
The mechanical properties of alginate hydrogels formed through intramolecular cross-linking via divalent cations are directly related to the concentration of the cross-linking cation, the molecular weight and length of the G-blocks [79][64]. Alginate hydrogels prepared with high cation concentrations, a high molecular weight, and a high G-content alginate have a relatively high stiffness [80][65]. The mechanical properties of alginate hydrogels significantly control the stability of the gel and thus affect their biomedical applications, including the drug release rate from the gel, as well as the phenotype and function of the cells encapsulated in the alginate gels [13][53].
Ionically cross-linked alginate hydrogels can be dissolved by releasing the divalent ions cross-linking agent into the surrounding medium. However, due to the lack of corresponding enzymes in mammals, such as alginase, and the fact that the average molecular weight of many commercially available alginates is higher than the clearance threshold of the kidneys, alginate cannot be completely cleared from the body [81][66]. The partial oxidation of alginate chains is an attractive method to make alginate degradable in physiological conditions. The slight oxidization of alginate alters the chain conformation to an open-chain adduct, enabling the degradation of the alginate backbone [13][53]. In the presence of divalent cations, the partial oxidation of alginate does not significantly influence its gel-forming ability, and the degradation rate of the resultant hydrogels is largely dependent on the degree of oxidation, the pH, and the temperature of the medium [82][67].
In situ cross-linked alginate hydrogels with calcium ions were designed for myocardial infarction repair [83,84][68][69]. A partially cross-linked alginate solution injected into the infarct zone underwent a rapid gelation and phase transition to hydrogel because of the elevated calcium concentrations at the acute infarct site. The calcium cross-linked alginate hydrogel degraded within six weeks after administration via the ion exchange between cross-linking calcium ions and sodium ions from the surrounding tissue. Beneficial therapeutic effects of the calcium cross-linked alginate hydrogels were observed in rat and pig models of acute myocardial infarction, where the hydrogels were replaced by host tissues composed of myofibroblasts and blood capillaries. Hydrogels prepared by cell-interactive alginate, which was synthesized by chemically coupling cell-adhesive peptides (i.e., RGD) as side-chains, are crucial for cell growth and tissue regeneration due to the promotion and regulation of cellular interactions [85][70]. Hong et al. developed a polymeric hydrogel based on an alginate–boronic acid conjugate through borate ester cross-linking between the intrinsic cis-diol at the alginate backbones and boronic acid [86][71]. The prepared hydrogel demonstrated unprecedented multifunctionalities simultaneously, such as self-healing capacity, stretchability, shear-thinning, stimuli sensitivity, and adhesive and reshaping properties, which were owing to the reversible intermolecular/intramolecular interactions resulting from the dynamic complexation and dissociation of the borate ester.
6. Hyaluronic Acid and Hyaluronic Acid-Based Hydrogel
Hyaluronic acid is a natural polysaccharide found in the ECM prominently throughout the body, comprising N-acetyl-glucosamine and D-glucuronic acid (Figure 5) residues [87][72]. Hyaluronic acid is well known for its bioactivity, which is the main nonsulfuric glycosaminoglycan that regulates many cellular responses in the ECM [2,88][73][74]. As a main component of the ECM, hyaluronic acid plays a significant role in many physiological functions, including lubrication, water absorption, and retention for tissue and the ECM, and structural and space-filling functions, and interacts with various cell receptors to coordinate cell communication and behavior [89][75]. Because of its nontoxicity, nonallergy, biocompatibility, and biodegradability, hyaluronic acid has been widely used as biomedical materials including tissue engineering scaffolds, wound dressings, and drug carriers [90,91][76][77]. Hyaluronic acid degrades rapidly in the body and is often used in combination with other materials. For instance, the chemical modification and cross-linking of hyaluronic acid can extend the retention time in vivo [2][73]. The chemical modification of hyaluronic acid is mainly focused on three distinct functional groups: the glucuronic carboxylic acids, the primary and secondary hydroxyl groups, and the N-acetyl groups. Altering any of these functional groups may result in changes in the mechanical properties, chemical properties, and biological activity of subsequent hyaluronic acid biomaterials [91][77]. In addition, hyaluronic acid hydrogel can be formed by the auto-cross-linking of intra- and intermolecular ester bonds between the reaction of carboxyl groups and hydroxyl groups. The auto-cross-linking improves the viscoelasticity of hydrogels, and this property can be tuned by changing the esterification reaction conditions [70][55].
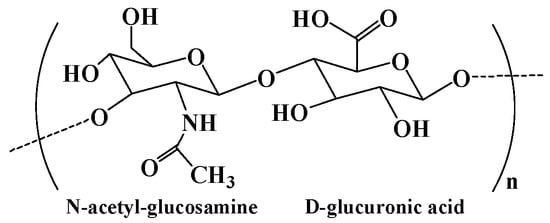
Figure 5.
The chemical structure of hyaluronic acid.
Hyaluronic acid is mainly manufactured via extraction from animal tissues (including human umbilical cords, vitreous humor of cattle, bovine synovial fluid, and rooster combs) and from microbial/bacterial fermentation using pathogenic bacteria and nonpathogenic bacteria [14][78]. Recently, a novel enzymatic technology for hyaluronic acid manufacture has been developed, and hyaluronic acid with a high molecular weight, defined chain length, and low polydispersity can be enzymatic polymerized using UDP-sugar monomers [92][79].
Hyaluronic acid contains hydroxyl and carboxyl functional groups in the main chain, which can be chemically modified to obtain hyaluronic acid derivatives with unique biological and physicochemical properties. The carboxyl group can be modified to synthesize hyaluronic acid derivatives with ester (by alkyl halides, diazomethane, and tosylate activation) and amide (using carbodiimides or carbonyldiimidazole) [90][76]. For example, adipic dihydrazide functionalized hyaluronic acid (HA-ADH) was obtained by coupling adipic dihydrazide to hyaluronic acid through the amidation between the carboxyl group of hyaluronic acid and the amine group of adipic dihydrazide. Hydrogels integrated with sanguinarine-loaded gelatin microspheres were prepared based on HA-ADH and oxidized dextran through Schiff’s base cross-linking for wound healing, exhibiting improved re-epithelialization and ECM remodeling, enhanced antibacterial activities, and decreased inflammatory responses [93][80]. In addition, the vicinal diol in hyaluronic acid can be oxidized to aldehyde groups by sodium periodate to form aldehyde–hyaluronic acid, and chitosan–hyaluronic acid hydrogel was formed through Schiff’s base cross-linking for the immobilization of insulin-like growth factor 1, which improved stem cell therapy for limb ischemia [94][81]. Moreover, hyaluronidase can promote the decomposition of hyaluronic acid, and its concentration was found to be remarkably higher in various cancerous cells [95][82]. Thus, hyaluronic acid-based hydrogels are commonly degradable and can be used as a local therapy carrier. In situ-forming hyaluronic acid-based hydrogel was prepared as a carrier to locally deliver adipose-derived stem cells (ASCs) into the salivary gland [96][83]. The results showed that the retention of locally delivered ASCs by the hyaluronic acid-based hydrogel could enhance the paracrine effect of ASCs and provide a more efficient alleviation of radiation-induced salivary gland damage.
7. Starch and Starch-Based Hydrogel
Starch is a natural polysaccharide composed of glucose repeating units through α-D-(1-4) and α-D-(1-6)-glycosidic linkage (Figure 6) [97,98][84][85]. It has a wide range of applications in the food, agriculture, biomedical, and pharmaceutical fields due to its low cost, renewability, biodegradability, and biocompatibility. Starch exhibits a granular appearance in nature, which is called starch granule, containing a small number of proteins, fatty acids, and minerals [15][86]. Amylose and amylopectin represent the primary two types of polysaccharides in the starch structure. Amylose with a linear structure is known for the double-helix formation due to its left-handed helical conformation and can form supramolecular inclusion complexes with guest molecules [99][87]. Amylopectin has a more highly branched structure than that of amylose and can form a helical and crystallized structure, playing an important role in stabilizing starch granules’ structure [100][88]. Variations in amylose and amylopectin have a great influence on the gelatinization and degradation properties of starches, and in turn, play a substantial role in starch hydrogels’ elaboration. Hydrogels produced by low amylose starches are partially or completely water-soluble, while those produced by high amylose starches have a higher structural integrity [101][89]. Containing plenty of hydroxyl groups, starch has excellent hydrophilic properties and is an ideal candidate for the development of hydrogels with a higher swelling capacity and enhanced biodegradation properties [102][90]. Compared to native ones, modified starch is a superior material for preparing hydrogels with better properties. For instance, ozonated cassava starch hydrogels have good pasting properties and printability, and an ozonization for 30 min can improve the performance of hydrogels, especially their printability [103][91].
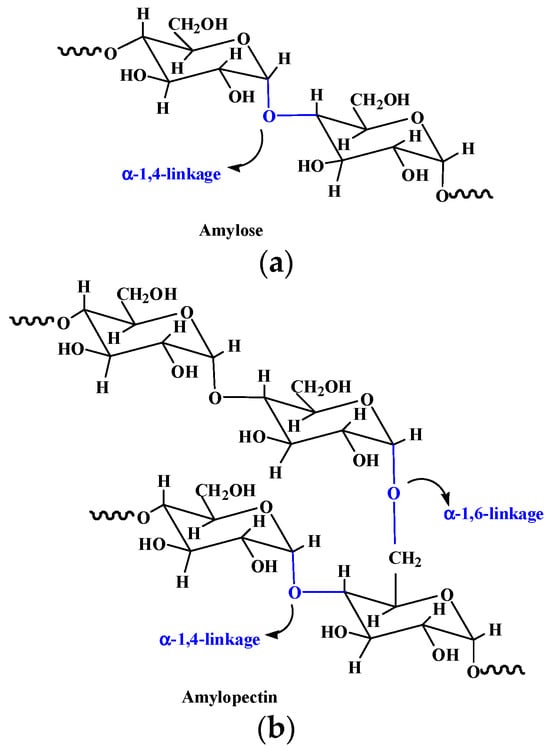
Figure 6.
Chemical structures of amylose (
a
) and amylopectin (
b
) in starch.
Starch can be extracted from seeds (such as beans, peas, grasses, cereal grains, etc.), roots, tubers, stems, fruits, and all leaves. The component of amylose and amylopectin in starch varies depending on the plant source. For instance, cornstarch contains about 28 wt% of amylose, and amylose in cassava starches is about 17 wt%, while amylose in waxy potato starches is only 8 wt% [104][92].
Starch-based hydrogels can be formed by environmentally and friendly physical methods including starch retrogradation and extrusion [15][86]. The formation of starch hydrogels by retrogradation is due to the rearrangement of amylose during the heating–cooling process. In addition, various chemical methods including etherification and grafting can be adopted to fabricate starch-based hydrogels [98][85]. The hydroxy groups in the starch structure can be substituted by various ether groups like carboxy-methyl to form etherified starches, and different vinyl monomers (such as acrylamide, acrylic acid, and so on) can be grafted on starch in the grafting method [15][86]. Light, curable, starch-based hydrogels with Fe3O4 nanoparticles encapsulation were prepared by cross-linking of acrylic-glycol modified starch under blue light, exhibiting a controllable release of quercetin, improved bioavailability, and enhanced mechanical properties [105][93].
8. Guar Gum and Guar Gum-Based Hydrogel
Guar gum is a nonionic natural polysaccharide isolated from the seed of leguminous plant called Cyamopsis tetragonoloba. It comprises linear main chains of (1-4)-β-D-mannopyranosyl units and branch chains of α-D-galactpyanosyl units connected by (1-6) linkages (Figure 7) [16][94]. Guar gum has attracted great attention in the biomedical field due to its hydrophilicity, nontoxicity, easy availability, biocompatibility, and biodegradability. Guar gum contains primary and secondary hydroxyl groups, and its physicochemical and biological properties can be adjusted by chemical modification or grafting with other functional materials to suit desired biomedical applications. Guar gum-based biomaterials have aroused great interest in drug delivery systems due to their enhanced drug loading, sustained drug delivery behavior, microbial degradability, improved firmness over a wide pH range, and pH-dependent hydration [106][95].
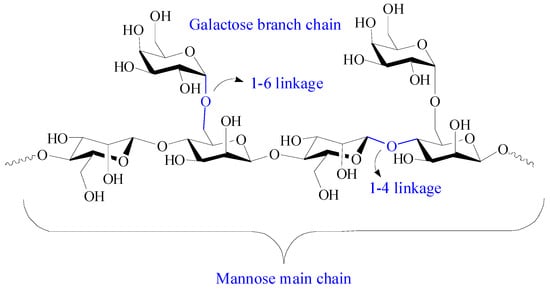
Figure 7.
Chemical structure of guar gum.
With abundant hydroxyl groups, guar gum can form adhesive and stimuli-responsive hydrogels with borate by chemical cross-linking of borate–diol interactions [107][96]. However, due to the reversibility of the borate ester bond, neat guar gum-based hydrogel has an instability and low strength which restrain its wide application. In addition, galactomannan is sensitive to temperature-based degradation, and the rheological value of a guar gum solution decreases with the storage time, indicating the degradation of polymers [108,109][97][98]. In fact, the mechanical strength of borate-based guar gum hydrogel showed a change between 25 °C and 37 °C, which is an unsatisfactory property for wound healing and cellular scaffolds [110][99]. An attractive approach to overcome this deficiency is the involvement of other polymers, such as alginate and polyacrylic acid, to form dual-network hydrogels [111,112][100][101].
pH responsive and self-healable guar gum–borate hydrogels were developed for muco-adhesion [113][102]. The results of burst pressure tests showed that 1% w/v guar gum-borated hydrogel could resist an 8 ± 2 kPa pressure at pH 7.4, which was comparable to fibrin glue and higher than that at solution (pH 5) and brittle gel (pH 10) conditions. Guar gum-based hydrogel with good self-healing capacity, injectability, and adhesion was developed by the introduction of the gel-polydopamine complex and CNCs [109][98]. Dopamine self-polymerizes to polydopamine on gelatin chains under alkaline condition, and gelatin–polydopamine is further cross-linked to guar gum and CNC via a borate–diol bond, intramolecular Schiff base, and Michael’s addition reaction. CNC also acts as a reinforcer to contribute mechanical strength to the hydrogel. The obtained guar gum-based hydrogels have excellent cytocompatibility and hemolysis ratio, suggesting broad prospects in the biomedical field.
9. Agarose and Agarose-Based Hydrogel
Agarose is a natural oxygen-rich polysaccharide extracted from seaweed, consisting of alternating 1,3-linked β-D-galactose and 1,4-linked 3,6-anhydro-α-Lgalactose units (Figure 8) [17][103]. Agarose has good biodegradability, biocompatibility, antibacterial properties, perfect cell/matrix interaction, and high hydrophilicity and is widely used in biomedicine and bioengineering. Agarose has an effective porosity with interconnected pores and channels, facilitating the transportation of the material. Moreover, the uniform pore-size distribution and the compatibility of the porous architecture with tissues/organs endow agarose materials with a high ability for controlled drug release [114,115][104][105]. A notable feature of agarose is its self-gelling behavior at low temperatures, where the polysaccharide forms a double-helix structure, creating a water-insoluble, gel-type structure, and the melting temperature of agarose gel ranges 80–90 °C [116][106]. The agarose gel strength is related to the content of 3,6-anhydrogalactose in agarose, and the higher the content, the greater the gel strength [117][107]. However, the application of agarose gels has been limited due to its high gelling temperature and high rigidity [118][108]. The high gelling temperature of agarose would inactivate the heat-sensitive tissues or drugs during the gelling process. One effective method to lowering the gelling temperature of the agarose is to introduce functional groups into agarose to hinder the formation of a helical structure at low temperatures, such as agarose modification through acetylation, alkylation, alkenylation, acylation, and oxyalkylation [119][109]. In addition, agarose can form a stable gel even when other biopolymers are added to the matrix, so by mixing different biopolymers as regulators, agarose has the ability to form stable hybrid hydrogels with tunable selective profiles [120][110].
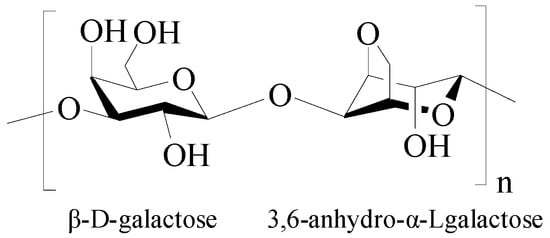
Figure 8.
Chemical structure of agarose.
β-cyclodextrin functionalized agarose (CFA) gel with low gelling temperature was developed for controlled drug delivery [121][111]. The gelling temperature of CFA dramatically decreased to 26.7 °C from 65 °C. CFA gel can be used for the sustained release of doxorubicin via the inclusion complexation of β-cyclodextrin, as well as for the complete release of bovine serum albumin. Agarose-based hybrid hydrogels with tunable, charge-selective permeability properties were prepared by mixing a series of (bio-)macromolecules with agarose [122][112]. These hybrid hydrogels can selectively retard the diffusion and translocation of positively or negatively charged dextran at both acidic and neutral pH, according to the type and concentration of the incorporated macromolecules. What is more, the agarose matrix provides a high level of versatility, and their permeability profiles can be tailored by integrating macromolecules with the desired physicochemical properties. Human-derived cytokine functionalized sericin/agarose hybrid hydrogels based on the hydrogen bond network structure of sericin and agarose were fabricated [123][113]. The hybrid hydrogels showed improved stability and mechanical performance and improved the cell-proliferation activity, suggesting a promising functional biomaterial for application in the clinic. A triple-network carboxymethyl chitosan/sodium carboxymethyl cellulose/agarose hydrogel containing silk fibroin/polydopamine nanoparticles was fabricated as an antibacterial dressing [124][114]. The hydrogel showed effective inhibition in the growth of Pseudomonas aeruginosa and supported the proliferation and growth of the fibroblast cells, suggesting a promising material to treat wound infection.
10. Dextran and Dextran-Based Hydrogel
Dextran is a branched neutral glucan composed of α-1, 6 glycosidic linkages between glucose monomers, with branches from α-1, 2, α-1, 3, and α-1, 4 linkages (Figure 9) [18][115]. It has excellent solubility, biocompatibility, biodegradability, and nonimmunogenicity, making dextran a suitable material in biomedicine [19][116]. Dextran is physiologically harmless as it can be depolymerized by digestive enzymes in the lumen of the body, such as the large intestine, liver, spleen, and kidney. Moreover, dextran is an excellent starting biopolymer for structural design. It has a narrow molecular weight distribution and abundant active hydroxyl groups, which is advantageous for chemical modification. Various dextran derivatives can be prepared by reactions including esterification, etherification, and oxidation [125][117].
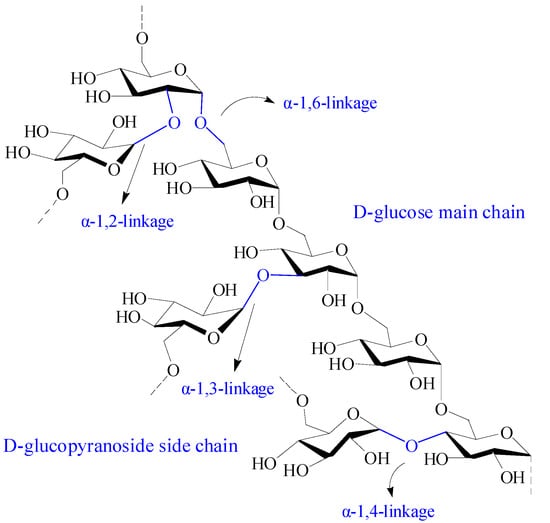
Figure 9.
Chemical structure of dextran.
Dextran was first discovered by Louis Pasteur in 1861 from slime-producing bacteria called Leuconostoc Lesenteroides. Subsequently, researchers found that several Gram-positive, facultative anaerobic bacteria, such as Leuconostoc and Streptococcus strains, can also produce dextran [126][118]. The extracellular catalyzation of D-glucopyranyl residues from sucrose to dextran by several lactobacilli via dextransucrase is the main source of dextran in nature [18][115]. The degree of branching units in the 2, 3, and 4 positions are related to the dextran-producing bacterial strain. In addition, dextran can be synthesized by the cationic ring-opening polymerization of levoglucosan, which is a pyrolysis product of polyglucans.
Dextran-based hydrogels have been widely used in biomedicine because of their good mechanical strength and swelling degradation properties. The methods for preparing dextran hydrogels include crystallization, physical cross-linking, chemical cross-linking, radiation cross-linking copolymerization, and so on [127][119]. Bioresponsive diselenide-functionalized dextran-based hydrogels with GPx-like catalytic activity was developed via Schiff base cross-linking for GSH consumption-enhanced local starvation- and hypoxia-activated melanoma therapy [128][120]. The dextran-based hydrogel is pH- and dual redox-responsive and could degrade sensitively and excite drug release with the increasing acid and H2O2, resulting in remarkably enhanced local anticancer efficacy. Gallic acid modified chitosan/oxidized dextran hydrogels were prepared via Schiff base cross-linking for wound healing [129][121]. The hydrogel showed an excellent self-healing ability, good injectability, strong antioxidant activity, favorable biocompatibility, and accelerated wound healing through the elimination of ROS in burn injury.
References
- Ahn, W.; Lee, J.H.; Kim, S.R.; Lee, J.; Lee, E.J. Designed protein- and peptide-based hydrogels for biomedical sciences. J. Mater. Chem. B 2021, 9, 1919–1940.
- Tang, L.; Tang, F.; Li, M.; Li, L. Facile synthesis of Ag@AgCl-contained cellulose hydrogels and their application. Colloids Surface A 2018, 553, 618–623.
- Zou, P.; Yao, J.; Cui, Y.N.; Zhao, T.; Che, J.; Yang, M.; Li, Z.; Gao, C. Advances in cellulose-based hydrogels for biomedical engineering: A review summary. Gels 2022, 8, 364.
- Rahman, A.; Wang, W.; Govindaraj, D.; Kang, S.; Vikesland, P.J. Recent advances in environmental science and engineering applications of cellulose nanocomposites. Crit. Rev. Environ. Sci. Technol. 2023, 53, 650–675.
- Hasanin, M.S.; Mostafa, A.M.; Mwafy, E.A.; Darwesh, O.M. Eco-friendly cellulose nano fibers via first reported Egyptian Humicola fuscoatra Egyptia X4: Isolation and characterization. Environ. Nanotechnol. Monit. Manag. 2018, 10, 409–418.
- Qi, Y.E.; Guo, Y.Z.; Liza, A.A.; Yang, G.H.; Sipponen, M.H.; Guo, J.Q.; Li, H.M. Nanocellulose: A review on preparation routes and applications in functional materials. Cellulose 2023, 30, 4115–4147.
- Zhang, M.; Du, H.; Liu, K.; Nie, S.; Xu, T.; Zhang, X.; Si, C. Fabrication and applications of cellulose-based nanogenerators. Adv. Compos. Hybrid Mater. 2021, 4, 865–884.
- Erfanian, E.; Moaref, R.; Ajdary, R.; Tam, K.C.; Rojas, O.J.; Kamkar, M.; Sundararaj, U. Electrochemically synthesized graphene/TEMPO-oxidized cellulose nanofibrils hydrogels: Highly conductive green inks for 3D printing of robust structured EMI shielding aerogels. Carbon 2023, 210, 118037.
- Li, B.; Chen, Y.; Wu, W.; Cao, X.; Luo, Z. Copolymer-grafted cellulose nanocrystal induced nanocomposite hydrogels with enhanced strength, high elasticity and adhesiveness for flexible strain and pressure sensors. Carbohydr. Polym. 2023, 317, 121092.
- Taheri, N.; Abdolmaleki, A.; Fashandi, H. Pyridinium-based ionic liquid/water mixture intended for efficient dissolution of cellulose, chitosan and chitin: The pivotal contribution of water. Carbohydr. Polym. 2018, 195, 413–419.
- Wong, L.C.; Leh, C.P.; Goh, C.F. Designing cellulose hydrogels from non-woody biomass. Carbohydr. Polym. 2021, 264, 118036.
- Ostlund, A.; Lundberg, D.; Nordstierna, L.; Holmberg, K.; Nyden, M. Dissolution and Gelation of Cellulose in TBAF/DMSO Solutions: The Roles of Fluoride Ions and Water. Biomacromolecules 2009, 10, 2401–2407.
- Zhang, H.; Wu, J.; Zhang, J.; He, J.S. 1-Allyl-3-methylimidazolium chloride room temperature ionic liquid: A new and powerful nonderivatizing solvent for cellulose. Macromolecules 2005, 38, 8272–8277.
- Kang, H.; Liu, R.; Huang, Y. Cellulose-based gels. Macromol. Chem. Phys. 2016, 217, 1322–1334.
- Shi, H.; Deng, Y.; Shi, Y. Cellulose-based stimuli-responsive anisotropic hydrogel for sensor applications. ACS Appl. Nano Mater. 2023, 6, 11524–11530.
- Guilherme, M.R.; Aouada, F.A.; Fajardo, A.R.; Martins, A.F.; Paulino, A.T.; Davi, M.F.T.; Rubira, A.F.; Muniz, E.C. Superabsorbent hydrogels based on polysaccharides for application in agriculture as soil conditioner and nutrient carrier: A review. Eur. Polym. J. 2015, 72, 365–385.
- Lu, Q.; Zhang, S.; Xiong, M.; Lin, F.; Tang, L.; Huang, B.; Chen, Y. One-pot construction of cellulose-gelatin supramolecular hydrogels with high strength and pH-responsive properties. Carbohydr. Polym. 2018, 196, 225–232.
- Li, J.; Chen, S.; Wu, Z.; Han, Z.; Qu, X.; Jin, M.; Jia, Y.; Zhou, Z.; Wang, H. Bacterial cellulose hydrogel-based wearable thermo-electrochemical cells for continuous body heat harvest. Nano Energy 2023, 112, 108482.
- Zhao, J.; Qiu, P.; Wang, Y.; Wang, Y.; Zhou, J.; Zhang, B.; Zhang, L.; Gou, D. Chitosan-based hydrogel wound dressing: From mechanism to applications, a review. Int. J. Biol. Macromol. 2023, 244, 125250.
- Peers, S.; Montembault, A.; Ladaviere, C. Chitosan hydrogels for sustained drug delivery. J. Control. Release 2020, 326, 150–163.
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C 2013, 33, 1819–1841.
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Sakib, M.N.; Rashid, T.U. Chitosan based bioactive materials in tissue engineering applications—A review. Bioact. Mater. 2020, 5, 164–183.
- Troy, E.; Tilbury, M.A.; Power, A.M.; Wall, J.G. Nature-based biomaterials and their application in biomedicine. Polymers 2021, 13, 3321.
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan-A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014.
- Kim, Y.; Zharkinbekov, Z.; Raziyeva, K.; Tabyldiyeva, L.; Berikova, K.; Zhumagul, D.; Temirkhanova, K.; Saparov, A. Chitosan-based biomaterials for tissue regeneration. Pharmaceutics 2023, 15, 807.
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99.
- Thirupathi, K.; Raorane, C.J.; Ramkumar, V.; Ulagesan, S.; Santhamoorthy, M.; Raj, V.; Krishnakumar, G.S.; Phan, T.T.V.; Kim, S.C. Update on chitosan-based hydrogels: Preparation, characterization, and its antimicrobial and antibiofilm applications. Gels 2023, 9, 35.
- Tian, B.; Liu, J. Smart stimuli-responsive chitosan hydrogel for drug delivery: A review. Int. J. Biol. Macromol. 2023, 235, 123902.
- Xu, C.H.; Zhan, W.; Tang, X.Z.; Mo, F.; Fu, L.H.; Lin, B.F. Self-healing chitosan/vanillin hydrogels based on Schiff-base bond/hydrogen bond hybrid linkages. Polym. Test. 2018, 66, 155–163.
- Lee, J.S.; Nah, H.; Moon, H.J.; Lee, S.J.; Heo, D.N.; Kwon, I.K. Controllable delivery system: A temperature and pH-responsive injectable hydrogel from succinylated chitosan. Appl. Surf. Sci. 2020, 528, 146812.
- Lee, J.S.; Kim, H.S.; Nah, H.; Lee, S.J.; Moon, H.J.; Bang, J.B.; Lee, J.B.; Do, S.H.; Kwon, I.K.; Heo, D.N. The Effectiveness of Compartmentalized Bone Graft Sponges Made Using Complementary Bone Graft Materials and Succinylated Chitosan Hydrogels. Biomedicines 2021, 9, 1765.
- Sukpaita, T.; Chirachanchai, S.; Pimkhaokham, A.; Ampornaramveth, R.S. Chitosan-Based Scaffold for Mineralized Tissues Regeneration. Mar. Drugs 2021, 19, 551.
- Liu, Q.; Dong, Z.; Ding, Z.; Hu, Z.; Yu, D.; Hu, Y.; Abidi, N.; Li, W. Electroresponsive Homogeneous Polyelectrolyte Complex Hydrogels from Naturally Derived Polysaccharides. ACS Sustain. Chem. Eng. 2018, 6, 7052–7063.
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The development of collagen based composite scaffolds for bone regeneration. Bioact. Mater. 2018, 3, 129–138.
- Valentino, C.; Vigani, B.; Zucca, G.; Ruggeri, M.; Boselli, C.; Cornaglia, A.I.; Malavasi, L.; Sandri, G.; Rossi, S. Formulation development of collagen/chitosan-based porous scaffolds for skin wounds repair and regeneration. Int. J. Biol. Macromol. 2023, 242, 125000.
- Gajbhiye, S.; Wairkar, S. Collagen fabricated delivery systems for wound healing: A new roadmap. Biomater. Adv. 2022, 142, 213152.
- Copes, F.; Pien, N.; Van Vlierberghe, S.; Boccafoschi, F.; Mantovani, D. Collagen-Based Tissue Engineering Strategies for Vascular Medicine. Front. Bioeng. Biotechnol. 2019, 7, 166.
- Montalbano, G.; Toumpaniari, S.; Popov, A.; Duan, P.; Chen, J.; Dalgarno, K.; Scott, W.E., 3rd; Ferreira, A.M. Synthesis of bioinspired collagen/alginate/fibrin based hydrogels for soft tissue engineering. Mater. Sci. Eng. C-Mater. 2018, 91, 236–246.
- Miranda-Nieves, D.; Chaikof, E.L. Collagen and Elastin Biomaterials for the Fabrication of Engineered Living Tissues. ACS Biomater. Sci. Eng. 2017, 3, 694–711.
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200.
- Yuan, Y.; Fan, D.; Shen, S.; Ma, X. An M2 macrophage-polarized anti-inflammatory hydrogel combined with mild heat stimulation for regulating chronic inflammation and impaired angiogenesis of diabetic wounds. Chem. Eng. J. 2022, 433, 133859.
- Akilbekova, D.; Shaimerdenova, M.; Adilov, S.; Berillo, D. Biocompatible scaffolds based on natural polymers for regenerative medicine. Int. J. Biol. Macromol. 2018, 114, 324–333.
- Guillen-Carvajal, K.; Valdez-Salas, B.; Beltran-Partida, E.; Salomon-Carlos, J.; Cheng, N. Chitosan, Gelatin, and Collagen Hydrogels for Bone Regeneration. Polymers 2023, 15, 2762.
- Bohidar, H.B.; Jena, S.S. Kinetics of sol-gel transition in thermoreversible gelation of gelatin. J. Chem. Phys. 1993, 98, 8970–8977.
- Smith, M.L.; Heitfeld, K.; Slone, C.; Vaia, R.A. Autonomic Hydrogels through Postfunctionalization of Gelatin. Chem. Mater. 2012, 24, 3074–3080.
- Mad-Ali, S.; Benjakul, S.; Prodpran, T.; Maqsood, S. Characteristics and gelling properties of gelatin from goat skin as affected by drying methods. J. Food Sci. Technol. 2017, 54, 1646–1654.
- Scherzer, T.; Beckert, A.; Langguth, H.; Rummel, S.; Mehnert, R. Electron beam curing of methacrylated gelatin. 1. Dependence of the degree of crosslinking on the irradiation dose. J. Appl. Polym. Sci. 1997, 63, 1303–1312.
- Kurian, A.G.; Singh, R.K.; Patel, K.D.; Lee, J.H.; Kim, H.W. Multifunctional GelMA platforms with nanomaterials for advanced tissue therapeutics. Bioact. Mater. 2022, 8, 267–295.
- Bupphathong, S.; Quiroz, C.; Huang, W.; Chung, P.F.; Tao, H.Y.; Lin, C.H. Gelatin Methacrylate Hydrogel for Tissue Engineering Applications—A Review on Material Modifications. Pharmaceuticals 2022, 15, 171.
- Tan, J.; Luo, Y.; Guo, Y.; Zhou, Y.; Liao, X.; Li, D.; Lai, X.; Liu, Y. Development of alginate-based hydrogels: Crosslinking strategies and biomedical applications. Int. J. Biol. Macromol. 2023, 239, 124275.
- Silva, R.; Singh, R.; Sarker, B.; Papageorgiou, D.G.; Juhasz-Bortuzzo, J.A.; Roether, J.A.; Cicha, I.; Kaschta, J.; Schubert, D.W.; Chrissafis, K.; et al. Hydrogel matrices based on elastin and alginate for tissue engineering applications. Int. J. Biol. Macromol. 2018, 114, 614–625.
- Li, Y.; Xu, Z.; Wang, J.; Pei, X.; Chen, J.; Wan, Q. Alginate-based biomaterial-mediated regulation of macrophages in bone tissue engineering. Int. J. Biol. Macromol. 2023, 230, 123246.
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126.
- Tonnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630.
- Chandel, A.K.S.; Shimizu, A.; Hasegawa, K.; Ito, T. Advancement of Biomaterial-Based Postoperative Adhesion Barriers. Macromol. Biosci. 2021, 21, 2000395.
- Ohta, S.; Toda, T.; Inagaki, F.; Omichi, K.; Shimizu, A.; Kokudo, N.; Hasegawa, K.; Ito, T. The Prevention of Hepatectomy-Induced Adhesions by Bilayer Sponge Composed of Ultrapure Alginate. J. Surg. Res. 2019, 242, 286–295.
- Chandel, A.K.S.; Ohta, S.; Taniguchi, M.; Yoshida, H.; Tanaka, D.; Omichi, K.; Shimizu, A.; Isaji, M.; Hasegawa, K.; Ito, T. Balance of antiperitoneal adhesion, hemostasis, and operability of compressed bilayer ultrapure alginate sponges. Biomater. Adv. 2022, 137, 212825.
- Li, G.; Zhang, G.; Sun, R.; Wong, C.P. Mechanical strengthened alginate/polyacrylamide hydrogel crosslinked by barium and ferric dual ions. J. Mater. Sci. 2017, 52, 8538–8545.
- Wang, Q.Q.; Liu, Y.; Zhang, C.J.; Zhang, C.; Zhu, P. Alginate/gelatin blended hydrogel fibers cross-linked by Ca2+ and oxidized starch: Preparation and properties. Mater. Sci. Eng. C-Mater. 2019, 99, 1469–1476.
- Kim, M.; Cha, C. Graft Architecture Guided Simultaneous Control of Degradation and Mechanical Properties of In Situ Forming and Fast Dissolving Polyaspartamide Hydrogels. Biomacromolecules 2020, 21, 3693–3703.
- Lee, K.Y.; Bouhadir, K.H.; Mooney, D.J. Controlled degradation of hydrogels using multi-functional cross-linking molecules. Biomaterials 2004, 25, 2461–2466.
- Chalanqui, M.J.; Pentlavalli, S.; McCrudden, C.; Chambers, P.; Ziminska, M.; Dunne, N.; McCarthy, H.O. Influence of alginate backbone on efficacy of thermo-responsive alginate-g-P (NIPAAm) hydrogel as a vehicle for sustained and controlled gene delivery. Mater. Sci. Eng. C-Mater. 2019, 95, 409–421.
- Shi, G.; Ding, Y.; Zhang, X.; Wu, L.; He, F.; Ni, C. Drug release behavior of poly (lactic-glycolic acid) grafting from sodium alginate (ALG-g-PLGA) prepared by direct polycondensation. J. Biomater. Sci.-Polym. Ed. 2015, 26, 1152–1162.
- Anitha, A.; Fletcher, N.L.; Houston, Z.H.; Thurecht, K.J.; Grondahl, L. Evaluation of the in vivo fate of ultrapure alginate in a BALB/c mouse model. Carbohydr. Polym. 2021, 262, 117947.
- Hay, I.D.; Rehman, Z.U.; Ghafoor, A.; Rehm, B.H.A. Bacterial biosynthesis of alginates. J. Chem. Technol. Biotechnol. 2010, 85, 752–759.
- Alshamkhani, A.; Duncan, R. Radioiodination of alginate via covalently-bound tyrosinamide allows monitoring of tis fate in vivo. J. Bioact. Compat. Polym. 1995, 10, 4–13.
- Bouhadir, K.H.; Lee, K.Y.; Alsberg, E.; Damm, K.L.; Anderson, K.W.; Mooney, D.J. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol. Prog. 2001, 17, 945–950.
- Leor, J.; Tuvia, S.; Guetta, V.; Manczur, F.; Castel, D.; Willenz, U.; Petneházy, Ö.; Landa, N.; Feinberg, M.S.; Konen, E.; et al. Intracoronary Injection of In Situ Forming Alginate Hydrogel Reverses Left Ventricular Remodeling After Myocardial Infarction in Swine. J. Am. Coll. Cardiol. 2009, 54, 1014–1023.
- Landa, N.; Miller, L.; Feinberg, M.S.; Holbova, R.; Shachar, M.; Freeman, I.; Cohen, S.; Leor, J. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation 2008, 117, 1388–1396.
- Follin, B.; Juhl, M.; Cohen, S.; Pedersen, A.E.; Gad, M.; Kastrup, J.; Ekblond, A. Human adipose-derived stromal cells in a clinically applicable injectable alginate hydrogel: Phenotypic and immunomodulatory evaluation. Cytotherapy 2015, 17, 1104–1118.
- Hong, S.H.; Kim, S.; Park, J.P.; Shin, M.; Kim, K.; Ryu, J.H.; Lee, H. Dynamic Bonds between Boronic Acid and Alginate: Hydrogels with Stretchable, Self-Healing, Stimuli-Responsive, Remoldable, and Adhesive Properties. Biomacromolecules 2018, 19, 2053–2061.
- Yang, J.Y.; Chen, Y.; Zhao, L.; Zhang, J.H.; Luo, H. Constructions and Properties of Physically Cross-Linked Hydrogels Based on Natural Polymers. Polym. Rev. 2022, 63, 574–612.
- Bao, Z.; Xian, C.; Yuan, Q.; Liu, G.; Wu, J. Natural polymer-based hydrogels with enhanced mechanical performances: Preparation, structure, and property. Adv. Healthc. Mater. 2019, 8, 1900670.
- Hu, R.; Zheng, H.; Cao, J.; Davoudi, Z.; Wang, Q. Self-Assembled Hyaluronic Acid Nanoparticles for pH-Sensitive Release of Doxorubicin: Synthesis and In Vitro Characterization. J. Biomed. Nanotechnol. 2017, 13, 1058–1068.
- Luo, X.; Liu, Y.; Zheng, C.; Huo, Q.; Liu, X. Development of novel hyaluronic acid/human-like collagen bio-composite membranes: A facile “surface modification-assembly” approach. Int. J. Biol. Macromol. 2021, 193, 378–386.
- Yang, H.; Song, L.; Zou, Y.; Sun, D.; Wang, L.; Yu, Z.; Guo, J. Role of Hyaluronic Acids and Potential as Regenerative Biomaterials in Wound Healing. ACS Appl. Bio Mater. 2021, 4, 311–324.
- Jensen, G.; Holloway, J.L.; Stabenfeldt, S.E. Hyaluronic Acid Biomaterials for Central Nervous System Regenerative Medicine. Cells 2020, 9, 2113.
- Hussain, Z.; Thu, H.E.; Katas, H.; Bukhari, S.N.A. Hyaluronic Acid-Based Biomaterials: A Versatile and Smart Approach to Tissue Regeneration and Treating Traumatic, Surgical, and Chronic Wounds. Polym. Rev. 2017, 57, 594–630.
- Gottschalk, J.; Assmann, M.; Kuballa, J.; Elling, L. Repetitive Synthesis of High-Molecular-Weight Hyaluronic Acid with Immobilized Enzyme Cascades. ChemSusChem 2022, 15, e202101071.
- Zhu, Q.; Jiang, M.; Liu, Q.; Yan, S.; Feng, L.; Lan, Y.; Shan, G.; Xue, W.; Guo, R. Enhanced healing activity of burn wound infection by a dextran-HA hydrogel enriched with sanguinarine. Biomater. Sci. 2018, 6, 2472–2486.
- Wang, X.; Zhang, J.; Cui, W.; Fang, Y.; Li, L.; Ji, S.; Mao, D.; Ke, T.; Yao, X.; Ding, D.; et al. Composite Hydrogel Modified by IGF-1C Domain Improves Stem Cell Therapy for Limb Ischemia. ACS Appl. Mater. Interfaces 2018, 10, 4481–4493.
- Hosseinzadeh, B.; Ahmadi, M. Degradable hydrogels: Design mechanisms and versatile applications. Mater. Today Sustain. 2023, 23, 100468.
- Chung, E.J.; Choi, J.S.; Shin, J.; Cho, H.N.; Kim, S.; Park, J.Y.; Lee, Y.S.; Kim, Y.I.; Wu, H.G.; Cho, S.W.; et al. Prevention of irradiation-induced damage to salivary glands by local delivery of adipose-derived stem cells via hyaluronic acid-based hydrogels. J. Ind. Eng. Chem. 2020, 90, 47–57.
- Kadokawa, J.; Shoji, T.; Yamamoto, K. Preparation of supramolecular network materials by means of amylose helical assemblies. Polymer 2018, 140, 73–79.
- Qamruzzaman, M.; Ahmed, F.; Mondal, M.I.H. An Overview on Starch-Based Sustainable Hydrogels: Potential Applications and Aspects. J. Polym. Environ. 2021, 30, 19–50.
- Cui, C.; Jia, Y.; Sun, Q.; Yu, M.; Ji, N.; Dai, L.; Wang, Y.; Qin, Y.; Xiong, L. Recent advances in the preparation, characterization, and food application of starch-based hydrogels. Carbohydr. Polym. 2022, 291, 119624.
- Chen, X.; Ren, Y.; Cai, Y.; Huang, X.; Zhou, L.; Ai, Y.; Jiang, B. Interactions between exogenous free fatty acids and maize starches varying in amylose content at high heating temperatures. Food Hydrocoll. 2023, 143, 108855.
- Guo, P.; Li, Y.; An, J.; Shen, S.; Dou, H. Study on structure-function of starch by asymmetrical flow field-flow fractionation coupled with multiple detectors: A review. Carbohydr. Polym. 2019, 226, 115330.
- Biduski, B.; da Silva, W.M.F.; Colussi, R.; El Halal, S.L.D.; Lim, L.T.; Dias, A.R.G.; Zavareze, E.D. Starch hydrogels: The influence of the amylose content and gelatinization method. Int. J. Biol. Macromol. 2018, 113, 443–449.
- Olad, A.; Doustdar, F.; Gharekhani, H. Starch-based semi-IPN hydrogel nanocomposite integrated with clinoptilolite: Preparation and swelling kinetic study. Carbohydr. Polym. 2018, 200, 516–528.
- Maniglia, B.C.; Lima, D.C.; Matta, M.D.; Le-Bail, P.; Le-Bail, A.; Augusto, P.E.D. Hydrogels based on ozonated cassava starch: Effect of ozone processing and gelatinization conditions on enhancing 3D-printing applications. Int. J. Biol. Macromol. 2019, 138, 1087–1097.
- Mendes, J.F.; Paschoalin, R.T.; Carmona, V.B.; Sena Neto, A.R.; Marques, A.C.P.; Marconcini, M.; Mattoso, L.H.C.; Medeiros, E.S.; Oliveira, J.E. Biodegradable polymer blends based on corn starch and thermoplastic chitosan processed by extrusion. Carbohydr. Polym. 2016, 137, 452–458.
- Moghadam, M.; Dorraji, M.S.S.; Dodangeh, F.; Ashjari, H.R.; Mousavi, S.N.; Rasoulifard, M.H. Design of a new light curable starch-based hydrogel drug delivery system to improve the release rate of quercetin as a poorly water-soluble drug. Eur. J. Pharm. Sci. 2022, 174, 106191.
- Verma, D.; Sharma, S.K. Recent advances in guar gum based drug delivery systems and their administrative routes. Int. J. Biol. Macromol. 2021, 181, 653–671.
- Zarbab, A.; Sajjad, A.; Rasul, A.; Jabeen, F.; Iqbal, M.J. Synthesis and characterization of Guar gum based biopolymeric hydrogels as carrier materials for controlled delivery of methotrexate to treat colon cancer. Saudi J. Biol. Sci. 2023, 30, 103731.
- Li, N.; Liu, C.J.; Chen, W. Facile Access to Guar Gum Based Supramolecular Hydrogels with Rapid Self-Healing Ability and Multistimuli Responsive Gel-Sol Transitions. J. Agric. Food Chem. 2019, 67, 746–752.
- Prabaharan, M.; Sathya Seeli, D. 10–Progress of guar gum-based biomaterials as drug delivery carriers. In Natural Biopolymers in Drug Delivery and Tissue Engineering; Jayakumar, R., Murali, V.P., Eds.; Woodhead Publishing Series in Biomaterials: Cambridge, UK, 2023; pp. 2239–2255.
- Pak, S.; Chen, F. Functional Enhancement of Guar Gum−Based Hydrogel by Polydopamine and Nanocellulose. Foods 2023, 12, 1304.
- Zhang, H.; Sun, X.Y.; Wang, J.; Zhang, Y.L.; Dong, M.N.; Bu, T.; Li, L.H.; Liu, Y.N.; Wang, L. Multifunctional Injectable Hydrogel Dressings for Effectively Accelerating Wound Healing: Enhancing Biomineralization Strategy. Adv. Funct. Mater. 2021, 31, 2100093.
- Rao, Z.L.; Liu, S.M.; Wu, R.Y.; Wang, G.L.; Sun, Z.X.; Bai, L.J.; Wang, W.X.; Chen, H.; Yang, H.W.; Wei, D.L.; et al. Fabrication of dual network self-healing alginate/guar gum hydrogels based on polydopamine-type microcapsules from mesoporous silica nanoparticles. Int. J. Biol. Macromol. 2019, 129, 916–926.
- Sharma, S.; Afgan, S.; Deepak; Kumar, A.; Kumar, R. L-Alanine induced thermally stable self-healing guar gum hydrogel as potential drug vehicle for sustained release of hydrophilic drug. Mater. Sci. Eng. C-Mater. 2019, 99, 1384–1391.
- Madhavikutty, A.S.; Chandel, A.K.S.; Tsai, C.C.; Inagaki, N.F.; Ohta, S.; Ito, T. pH responsive cationic guar gum-borate self-healing hydrogels for muco-adhesion. Sci. Technol. Adv. Mater. 2023, 24, 2175586.
- Wang, Z.K.; Xu, Z.W.; Yang, X.; Li, M.; Yip, R.C.S.; Li, Y.Y.; Chen, H. Current application and modification strategy of marine polysaccharides in tissue regeneration: A review. Biomater. Adv. 2023, 154, 213580.
- Moghaddam, R.H.; Dadfarnia, S.; Shabani, A.M.H.; Moghaddam, Z.H.; Tavakol, M. Electron beam irradiation synthesis of porous and non-porous pectin based hydrogels for a tetracycline drug delivery system. Mater. Sci. Eng. C-Mater. 2019, 102, 391–404.
- Sun, R.; Åhlén, M.; Tai, C.W.; Bajnóczi, É.; de Kleijne, F.; Ferraz, N.; Persson, I.; Stromme, M.; Cheung, O. Highly Porous Amorphous Calcium Phosphate for Drug Delivery and Bio-Medical Applications. Nanomaterials 2020, 10, 20.
- Yazdi, M.K.; Taghizadeh, A.; Taghizadeh, M.; Stadler, F.J.; Farokhi, M.; Mottaghitalab, F.; Zarrintaj, P.; Ramsey, J.D.; Seidi, F.; Saeb, M.R.; et al. Agarose-based biomaterials for advanced drug delivery. J. Control. Release 2020, 326, 523–543.
- Arnott, S.; Fulmer, A.; Scott, W.E.; Dea, I.C.M.; Moorhouse, R.; Rees, D.A. The agarose double helix and its function in agarose gel structure. J. Mol. Biol. 1974, 90, 269–284.
- Zhang, N.; Wang, J.L.; Ye, J.; Zhao, P.; Xiao, M.T. Oxyalkylation modification as a promising method for preparing low-melting-point agarose. Int. J. Biol. Macromol. 2018, 117, 696–703.
- Forget, A.; Pique, R.A.; Ahmadi, V.; Lüdeke, S.; Shastri, V.P. Mechanically Tailored Agarose Hydrogels through Molecular Alloying with β-Sheet Polysaccharides. Macromol. Rapid Commum. 2015, 36, 196–203.
- Beaumont, M.; Tran, R.; Vera, G.; Niedrist, D.; Rousset, A.; Pierre, R.; Shastri, V.P.; Forget, A. Hydrogel-Forming Algae Polysaccharides: From Seaweed to Biomedical Applications. Biomacromolecules 2021, 22, 1027–1052.
- Kim, C.; Jeong, D.; Kim, S.; Kim, Y.; Jung, S. Cyclodextrin functionalized agarose gel with low gelling temperature for controlled drug delivery systems. Carbohydr. Polym. 2019, 222, 115011.
- Zhao, J.; Marczynski, M.; Henkel, M.; Lieleg, O. Agarose-based hydrogels with tunable, charge-selective permeability properties. J. Appl. Polym. Sci. 2023, 140, e54303.
- Wang, Y.C.; Wang, F.; Wang, R.Y.; Tian, C.; Hua, X.T.; Zhao, P.; Xia, Q.Y. Human-derived cytokine functionalized sericin/agarose composite gel material with cell proliferation-promoting activity fabricated using genetically engineered silk for medical application. Mater. Design 2022, 224, 111362.
- Karimi, T.; Mottaghitalab, F.; Keshvari, H.; Farokhi, M. Carboxymethyl chitosan/sodium carboxymethyl cellulose/agarose hydrogel dressings containing silk fibroin/polydopamine nanoparticles for antibiotic delivery. J. Drug Deliv. Sci. Technol. 2023, 80, 104134.
- Heinze, T.; Liebert, T.; Heublein, B.; Hornig, S. Functional polymers based on dextran. In Polysaccharides II; Klemm, D., Ed.; Springer International Publishing: Cham, Switzerland, 2006; pp. 199–291.
- Hu, Q.; Lu, Y.; Luo, Y. Recent advances in dextran-based drug delivery systems: From fabrication strategies to applications. Carbohydr. Polym. 2021, 264, 117999.
- Varshosaz, J. Dextran conjugates in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 509–523.
- Jeanes, A.; Haynes, W.C.; Wilham, C.A.; Rankin, J.C.; Melvin, E.H.; Austin, M.J.; Cluskey, J.E.; Fisher, B.E.; Tsuchiya, H.M.; Rist, C.E. Characterization and Classification of Dextrans from Ninety-six Strains of Bacteria1b. J. Am. Chem. Soc. 1954, 76, 5041–5052.
- Huang, S.; Huang, G. Preparation and drug delivery of dextran-drug complex. Drug Deliv. 2019, 26, 252–261.
- Ding, X.; Zang, M.; Zhang, Y.; Chen, Y.; Du, J.; Yan, A.; Gu, J.; Li, Y.; Wei, S.; Xu, J.; et al. A bioresponsive diselenide-functionalized hydrogel with cascade catalytic activities for enhanced local starvation- and hypoxia-activated melanoma therapy. Acta Biomater. 2023, 167, 182–194.
- Shen, J.; Jiao, W.; Chen, Z.; Wang, C.; Song, X.; Ma, L.; Tang, Z.; Yan, W.; Xie, H.; Yuan, B.; et al. Injectable multifunctional chitosan/dextran-based hydrogel accelerates wound healing in combined radiation and burn injury. Carbohydr. Polym. 2023, 316, 121024.
More
