Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Lindsay Dong and Version 2 by Lindsay Dong.
The use of plant extracts (e.g., essential oils and their active compounds) represents an interesting alternative to chemical additives and preservatives applied to delay the alteration and oxidation of foods during their storage. Essential oils (EO) are nowadays considered valuable sources of food preservatives as they provide a healthier alternative to synthetic chemicals while serving the same purpose without affecting food quality parameters. The natural antimicrobial molecules found in medicinal plants represent a possible solution against drug-resistant bacteria, which represent a global health problem, especially for foodborne infections.
- natural antimicrobials
- food preservatives
- biocontrol agents
- food safety
1. Introduction
Medicinal plants are a valuable source of new antibacterial, antifungal, and antioxidant compounds due to the large biological and structural diversity of their constituents [1][2][3]. Botanical species and their derivatives, including essential oils (EOs), extracts, and bioactive compounds (BACs), have been discovered to be key contributors to the pharmaceutical, agricultural, and food industries.
Traditionally, medicinal plants are used to treat diseases, but they are also well suited for the food industry as natural antimicrobial preservatives [4][5][6][7]. Chemicals (benzoates, propionates, sorbates, nitrates, nitrites, etc.) commonly used as food additives are reported to be the cause of health problems such as allergies, asthma, liver damage, and cancer [8][9][10][11][12]. These concerns reinforce the interest in using natural antimicrobials in food formulations.
Alcohols, ethers or oxides, aldehydes, ketones, esters, amines, amides, phenols, heterocycles, terpenes (an oxygenated derivative of terpenoids), and polyphenols represent the chemical classes to which belong the constituents of EOs and plant extracts. Some terpene compounds identified in EOs are limonene, β-caryophyllene, β-pinene, α-pinene, α-terpinene, sabinene, β-myrcene, γ-terpinene, cinnamyl alcohol, δ-3-carene, and p-cymene. Limonene is mainly distributed in citrus EOs: orange [13], grapefruit [14], and pomelo [15]. Thymol, carvacrol, and eugenol, deriving from Thymus [16], Origanum [17], and Ocimum [18], represent the main phenolic terpenes associated with EOs obtained from medical and aromatic plants (Figure 1).
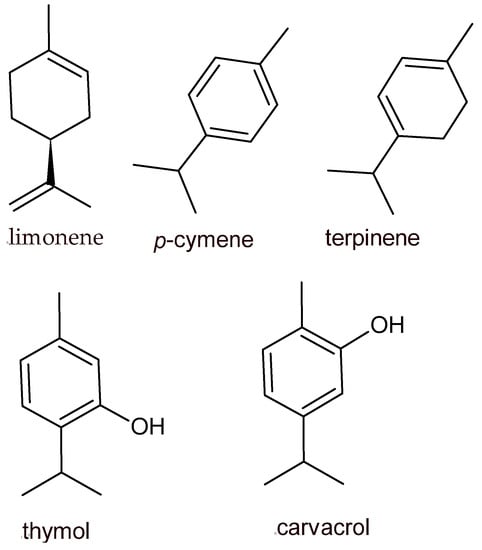
Figure 1. Chemical structures of the most applicable EO components in foods.
A large variety of these compounds are recognized to have strong and effective antimicrobial activity [19][20][21][22]. EOs from the Lamiaceae family aromatic plants (Table 1) (i.e., oregano and thyme) and their constituents (like carvacrol and thymol) have been described in the literature as potential preservatives with significant effects on food shelf-life [23][24][25][26][27][28][29].
The lipophilic nature of EOs is directly related to their antibacterial activity. EOs destabilize the membrane potential of bacterial cells by disrupting the permeability of the plasma membrane [30]. Degradation of membrane lipid fractions by the EO component thymol leads to destabilization of membrane permeability [31]. EOs affect quorum sensing signaling and can inhibit cell-to-cell communication, e.g., biofilm formation in bacterial cells [32]. Bouyahya et al. (2019) [33] reported the antimicrobial activity of Origanum compactum EO in the context of disrupting cell membrane stability and integrity and increasing membrane permeability, leading to leakage of cellular material (DNA and RNA). Moreover, the effect of EOs on inhibiting biofilm formation in bacterial communities triggers the breakdown of their sensing communication [34].
Several EOs, including those of thyme, marjoram, oregano, basil, ginger, lemongrass, and clove, were shown to be highly effective in inhibiting spoilage bacteria in meat [35], dairy products [36][37][38][39][40], and beverages [41]. It is generally accepted that plants are an important source of antimicrobial metabolites, including flavonoids, phenolic compounds, tannins, terpenoids, saponins, and alkaloids. Other than being endowed with antibacterial and antioxidant properties, BACs may help to enhance the food’s sensory and organoleptic properties and its acceptability, including its shelf life [42].
Many EOs and plant extracts have the GRAS (generally recognized as safe) status obtained by the Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA), (Figure 2). Therefore, they can be applied in the food industry, meeting in this way the consumer requirement for natural food preservatives [4].
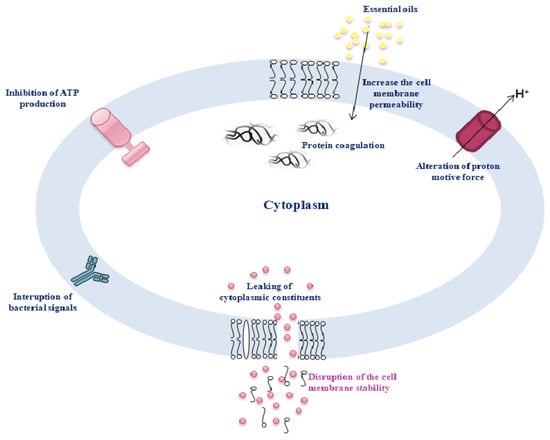
Figure 2. The antimicrobial mechanisms of action of essential oils.
However, despite EOs positive effect, their practical use as effective antimicrobial agents in the food sector is still a challenge due to their volatile characteristics, hydrophobicity, and low stability. Nowadays, new technologies and delivery strategies such as nanoencapsulation, encapsulation in active packaging, or polymer-based coatings have efficiently addressed these issues and improved the efficacy and gradual release of EOs [43]. Eventually, EO application can be limited by the sensory impact it imparts when applied at the required active concentrations. Therefore, a possible solution to overcome this aspect is their combination with other natural strategies.
Finally, LABs can produce different classes of antimicrobial peptides called bacteriocins. These molecules are antimicrobial peptides produced by specific bacterial species to protect themselves from other bacteria by inhibiting or killing them without harming themselves. The bacteriocins action mechanism is mainly associated with the permeabilization of the cell membrane, but it is reported that they can also act on protein metabolism, DNA and RNA, and quorum sensing [44][45]. The application of bacteriocins in the food sector has increased in recent years. They have attracted noticeable attention for their potential application as natural and safe food preservatives since they are easily digestible in the gastrointestinal tract. Nisin represents the most known and studied bacteriocin; it is produced by Lactococcus lactis, and its use was authorized by the Food Drug and Administration (FDA) in more than 50 countries [46]. Three ways of bacteriocin applications in foods are reported: inoculation of bacteriocin-producing LABs directly in the food products (starter or protective cultures), application as a food additive, and incorporation in food packaging of the purified or partially purified bacteriocin.
2. Use of Essential Oils and Biocontrol in Minimally Processed Fruit and Vegetables
Over recent years, the market for minimally processed fruits and vegetables has increased steadily. Their popularity is due to their convenience, reduced waste production, and high nutritional content. In fact, these foods represent a source of valuable compounds such as vitamins, minerals, fibers, and antioxidants and may help prevent chronic diseases. Nevertheless, fruits and vegetables are a suitable matrix for microbial proliferation [47][48]. In fact, these foods can be easily spoiled due to the high nutrient and water content and the numerous processing steps that fresh produce undergoes (i.e., peeling, cutting, or slicing), which can promote microbial proliferation by releasing nutrients and transporting the microbial population present on the surfaces of vegetables and fruits into the cut ones [49][50].
Currently, the use of chemical sanitizers in the washing step, as well as the maintenance of refrigerated temperatures during processing and storage, are the only stages capable of reducing and controlling the microbial population in minimally processed vegetables [51]. However, the use of chemical sanitizers, especially chlorine-based ones, is not always effective, can result in the production of toxic molecules, and is not well accepted by consumers [52]. Because of these drawbacks, researchers are looking to alternative sanitizers (i.e., ozone, hydrogen peroxide, and peroxyacetic acid), physical treatments (UV light, ultrasound, and gamma rays), and natural food additives. As a result of their antibacterial and antioxidant properties as well as their GRAS classification, EOs have the potential to extend the shelf life of several foods, including fresh-cut fruits and vegetables. EOs can be used in various stages of food and vegetable processing, including the washing step, directly on the product, or in the packaging. In fact, the incorporation of EOs and their components in active food packaging is of high relevance. Active packaging is a solid matrix from which EOs are gradually released during food storage. Generally, various methods, including direct incorporation, coating, and surface modification, are applied to incorporate EOs into active packaging [53]. Antimicrobial active packaging is designed to enhance product safety and shelf life by inhibiting or reducing microbial growth in packaged foods [54][55][56]. In this context, it is fundamental to use suitable solvents or polymeric carriers, as EOs are always used in diluted form, which should be food-grade and not interfere with the antimicrobial and antioxidant activities of EO constituents [57].
Other researchers found that dipping sliced apples in a solution containing dissolved hexanal (250 ppm) or the combinations hexanal/2-(E)-hexenal and citral/2-(E)-hexenal (125 ppm of each compound) increased quality parameters and extended the shelf life by reducing the proliferation of naturally occurring yeast [47]. In addition, sliced apples treated with hexanal/2-(E)-hexenal, and citral better retained both color and textural properties. The combination with an active, modified atmosphere (7% O2 and 0% CO2) enhanced the safety and shelf life of minimally processed apples washed with a solution containing a mixture of hexanal/2-(E)-hexenal. Other researchers discovered that active packaging containing thymol and eugenol inhibited the growth of mesophilic bacteria and yeasts and consequently extended the shelf life of table grapes stored in a modified atmosphere (MAP) compared to the control group [55].
The use of EOs has great potential, even in minimally processed vegetables. In fact, herbal EOs and their constituents were studied as alternative natural disinfectants for reducing the presence of spoiling and pathogenic bacteria. Due to the strong antimicrobial activity in vitro of oregano (Origanum vulgare) and thyme (Thymus vulgaris) EOs and their main constituents (carvacrol and thymol), these compounds are the most suitable to be used in minimally processed vegetables [30][44][58][59][60].
For example, the inclusion of oregano and thyme EOs at a concentration of 250 ppm in the washing solution of minimally processed lamb’s lettuce resulted in a product shelf life comparable to the one obtained by using chlorine (120 ppm) in the washing step [59]. In fact, the use of EOs resulted in a preliminary decrease in the microbial population. In addition, the color and turgidity of the lettuce were not affected throughout the storage period while the sensorial properties were not negatively affected [59].
When used in the washing solution, oregano oil (Origanum onites) was able to inactivate Salmonella typhimurium inoculated on iceberg lettuce. The effectiveness of oregano EO treatment (75 ppm) was comparable to that of chlorine treatment at 50 ppm [61].
When oregano EO was applied to ready to eat lettuce and carrots, the initial decontamination effect was comparable to that of chlorine [62]. Furthermore, oregano EO did not negatively affect the color, texture, or water activity of the samples. However, sensory acceptance of the product treated with oregano EO was only observed for carrots. Volatile antimicrobial compounds such as borneol, carvacrol, cinnamaldehyde, eugenol, menthol, thymol, and vanillin were able to inhibit the growth of Bacillus cereus inoculated in carrot juice. Direct application of EOs in the food system has some drawbacks due to their strong odor, chemical reactivity, hydrophobicity, low solubility, and potential negative interaction with the food matrix, leading to alteration of organoleptic properties. Several technological approaches have been tested for EO delivery in food. EO encapsulation is a novel and advanced delivery system. It provides enhanced antimicrobial efficacy as well as control over the release of EO flavors into the food system [63].
3. Application of Essential Oils as Meat and Dairy Preservatives
3.1. Antimicrobials as Meat Preservatives
Fresh meat and fresh meat produce are extremely perishable and prone to oxidative and microbial spoilage. These products are easily subject to oxidation, which reduces nutritional value, affecting lipids, proteins, myoglobin (pigments), texture, and flavor [64]. Plant extracts are a natural source of compounds with antioxidants and antimicrobial activity that can be used as an alternative to synthetic ones. EOs and their constituents have been successfully used in meat and meat products to prevent oxidation, degradation, and microbial proliferation [65][66][67]. Promising results can be achieved with EOs from the Lamiaceae family, including oregano, thyme, sage, and rosemary, which are commonly used as flavoring agents and prevent oxidative degradation. The antioxidant activity of EOs is well documented and can be attributed to phenolic compounds including eugenol, carvacrol, and thymol, which act as hydrogen donors and scavengers of free radicals [68][69]. Fasseas and colleagues (2007) [70] measured the antioxidant activity of pork and beef meats (raw and cooked) treated with sage and oregano EOs over a 12 day storage period at 4 °C. Compared to controls without added EOs, the EO-treated meat (raw and cooked) had higher TBA (thiobarbituric acid) scores and 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity. Meat contaminated with spoilage microorganisms shows color changes, the development of uncharacteristic and undesirable odors and tastes, and the formation of superficial slime [71][72]. Bacteria belonging to the genera Pseudomonas, Acinetobacter, Staphylococcus, Brochothrix, Moraxella, Micrococcus, and Flavobacterium, as well as LAB and genera belonging to the Enterobacteriaceae family, are reported to be involved in the spoilage of meat and meat derivatives [72][73]. Cell loads of foodborne pathogen L. monocytogenes deliberately inoculated on ham (3 log CFU/g) were decreased by 10 and 19% after supplementation of Cinnamon cassia and oregano EOs, respectively [74]. Cinnamon cassia EO was also effective in promoting the shelf life of ground lamb meat throughout refrigerated storage at 4 °C. Compared to control samples, supplementation of Cinnamon cassia EO (0.5%) reduced from the 4th to the 16th day the lactic acid bacteria spoilage population up to 1.9 log CFU/g and Enterobacteriaceae up to 1.1 CFU/g [75]. A reduced microbial spoilage population was also observed in minced meat with rosemary, thyme, and oregano EOs at concentrations between 1 and 1.5% (v/w). Compared to the control group, treated samples showed reduced loads of LAB, molds, and yeast [76]. Thyme EOs tested at two different concentrations (0.02 and 0.05 w/w) inhibited the growth of coliform in chicken hamburgers [77]. The use of EOs as antioxidants and preservatives for meat is dependent on the bioactivity and stability of their components. Most EO constituents lose physical stability when they interact with meat constituents [78], probably due to the binding ability of fats and proteins in meat to volatiles in EOs [78]. When encapsulated EOs are added to edible coatings, plastic films, or meat during marinating, their stability increases significantly. The possibility of using active packaging supplemented with natural antioxidants and antimicrobials has recently been investigated [79][80]. The stability, antioxidant, and antimicrobial properties of rosemary EO can be enforced by encapsulation with nanogel. Compared to samples treated with free rosemary EO, rosemary EO encapsulated in chitosan-benzoic acid-based nanogels showed greater efficacy in reducing the cell load of Salmonella typhimurium inoculated on beef chops during storage at 4 °C [63]. Chitosan-based coatings also improved the performance of oregano EO in reducing lipid oxidation in dry fermented sausages in comparison to control samples after 7 months of storage [81]. LABs can be added for two different purposes: as starting materials for producing fermented meat products or as a bioprotective culture used only to compete with naturally occurring microflora and produce antimicrobial peptides [82]. The constant release of antimicrobial peptides from the packaging to the product surface could help to maintain the bacteriocin concentration at the optimal level to carry out their bioprotective effect [83]. In various studies, films containing the bacteriocin nisin have been successfully used to preserve meat products [82]. Pullulan film containing Nisin Z, which was used for the packaging of refrigerated vacuum-packed raw beef and deli ham, strongly inhibited various foodborne pathogens, including Salmonella spp., S. aureus, L. monocytogenes, and E. coli [84]. Furthermore, the efficacy of this nisin/pullulan film was improved by the addition of lauric alginate [84]. Reduced growth of S. aureus on chilled sliced beef was observed when packaged with an alginate-based palmitoylated film supplemented with nisin [85]. Nisin-internalized cellulose envelopes demonstrated anti-Listeria properties on chilled vacuum-packed frankfurters (sausages) [86][87] and vacuum-packed hot dogs wrapped with a plastic film enriched with nisin. A combination of nisin and Nisaplin® (a commercial product from DuPont) adsorbed in cellulose-based packaging paper was shown to inhibit S. aureus, L. innocua, and LAB on cooked ham [88].3.2. Essential Oils as Dairy Preservatives
Plants and spices have been used in cheese production since ancient times, often linked to local traditions. Traditionally, spices and herbs or their extracts were rubbed directly on the cheese. In fact, herbs and spices can be applied as antioxidant, antimicrobial, flavoring, enriching, and functionalizing ingredients, which may improve the appearance and appeal of the product [89]. Although many dairy products undergo heat treatment during their manufacture, they are perishable and can be easily contaminated by microorganisms, resulting in food spoilage, consumer health risks, and a shortened product shelf life. Furthermore, as they are rich in lipids, oxidative processes can lead to a loss of flavor, nutrients, and color, the development of off-flavors, and the accumulation of compounds that may be of concern to human health [90]. For that reason, there is growing interest in the application of plants, extracts, and EOs as natural preservatives in the dairy sector as an alternative to synthetic preservatives [91]. The antimicrobial activity of natural products is commonly assessed against the main pathogens and spoilage bacteria commonly found in the dairy sector, such as L. monocytogenes, S. aureus, E. coli, Salmonella spp., and Pseudomonas spp., as well as yeasts and molds, such as Penicillium and Aspergillus [89]. Natural products have also been proven in additional types of dairy products, including milk. Jemaa et al., 2017 [92] showed that EO of Thymus capitatus ameliorated pasteurization effectiveness in maintaining raw milk quality. Incorporation of EOs from basil, peppermint, and zataria in the formulation of probiotic yoghurt increased the inhibition of E. coli and L. monocytogenes compared to yoghurt without EOs. However, only the EOs of basil and peppermint showed good antioxidant and antiradical activity along with good sensory acceptability [93]. The application of Echinophora platyloba EO and lycopene was effective as natural preservatives for dairy products with high fat content, such as butter and cream. Pasteurized cream treated with a mixture of EO and lycopene (0.5% and 50 ppm, respectively) showed improved shelf life (both from a microbiological and chemical point of view) compared to control samples. Sensory evaluation results showed that all the samples have satisfactory overall acceptability, although the greatest sensorial features were detected in creams prepared with a combination of low concentrations of Echinophora EO and lycopene (0.1% and 20 ppm, respectively) [94]. Other authors extended the shelf life of butter using thyme and cumin EOs [95], while Ozkan et al. (2007) [96] explored the potential of Satureja cilicica EO as a natural flavoring and antioxidant in the same dairy product. Although many studies highlight the beneficial properties of herbal preservatives, other papers report that dairy fats, carbohydrates, or proteins may interact with them and reduce their functional properties. Consequently, higher amounts of EOs are necessary to attain the desired effect, with subsequent drawbacks related to sensorial impacts. To overcome this aspect, different approaches have been investigated. Nisin is permitted as a food additive for processed cheese by the FAO/WHO Codex Committee at a concentration of 12.5 mg/kg product, while the US FDA is permitted to use up to 250 mg/kg [97]. It is generally known that the effectiveness of nisin is dependent on the dairy products considered. In particular, neutral-pH dairy products made from whole milk are not suitable for nisin use [98].4. Conclusions
Essential oils and bioactive compounds can be applied as food preservatives due to their antimicrobial and antioxidant activities, which prevent spoiling processes and guarantee food safety. On the other hand, an additional strategy to increase the safety and shelf life of various types of foods, including minimally processed fruits and vegetables, vegetable beverages, meat, and dairy products, can be represented by the use of protective cultures, especially lactic acid bacteria, which are able to produce antimicrobial compounds such as bacteriocins in addition to organic acids and hydrogen peroxide. Recently, innovative new technologies and delivery strategies such as nanoencapsulation or polymer-based coatings have improved the efficacy and allowed the controlled release of natural antimicrobials. In addition, the use of EOs and bioactive plant molecules in combination with bioprotective cultures or bacteriocins can exert an additive or synergistic effect and reduce the applied concentration of EO. The combination of these antimicrobial agents represents an interesting strategy to increase the shelf life and safety of food due to their antimicrobial and antioxidant properties. The synergistic combinations of EOs and LAB metabolites such as bacteriocins or nisins allow the exploration of promising ways to overcome both the narrow range of antimicrobial action and the unpleasant sensory properties of foods.References
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. The Antimicrobial Efficacy of Plant Essential Oil Combinations and Interactions with Food Ingredients. Int. J. Food Microbiol. 2008, 124, 91–97.
- Gutierrez, J.; Rodriguez, G.; Barry-Ryan, C.; Bourke, P. Efficacy of Plant Essential Oils against Foodborne Pathogens and Spoilage Bacteria Associated with Ready-to-Eat Vegetables: Antimicrobial and Sensory Screening. J. Food Prot. 2008, 71, 1846–1854.
- Aqil, F.; Zahin, M.; Ahmad, I.; Owais, M.; Khan, M.S.A.; Bansal, S.S.; Farooq, S. Antifungal Activity of Medicinal Plant Extracts and Phytocompounds: A Review. In Combating Fungal Infections Problems and Remedy; Springer: Berlin/Heidelberg, Germany, 2010; pp. 449–484.
- Saeed, F.; Afzaal, M.; Tufail, T.; Ahmad, A. Use of Natural Antimicrobial Agents: A Safe Preservation Approach. In Active Antimicrobial Food Packaging; IntechOpen: London, UK, 2019.
- Davidson, P.M.; Critzer, F.J.; Matthew Taylor, T. Naturally Occurring Antimicrobials for Minimally Processed Foods. Annu. Rev. Food Sci. Technol. 2013, 4, 163–190.
- Moreira, M.R.; Ponce, A.G.; Del Valle, C.E.; Roura, S.I. Inhibitory Parameters of Essential Oils to Reduce a Foodborne Pathogen. LWT Food Sci. Technol. 2005, 38, 565–570.
- Weerakkody, N.S.; Caffin, N.; Turner, M.S.; Dykes, G.A. In Vitro Antimicrobial Activity of Less-Utilized Spice and Herb Extracts against Selected Food-Borne Bacteria. Food Control 2010, 21, 1408–1414.
- Adu, A.A.; Sudiana, I.K.; Martini, S. The Effect of Nitrite Food Preservatives Added to Se’i Meat on the Expression of Wild-Type P53 Protein. Open Chem. 2020, 18, 559–564.
- Karwowska, M.; Kononiuk, A. Nitrates/Nitrites in Food—Risk for Nitrosative Stress and Benefits. Antioxidants 2020, 9, 241.
- Song, P.; Wu, L.; Guan, W. Dietary Nitrates, Nitrites, and Nitrosamines Intake and the Risk of Gastric Cancer: A Meta-Analysis. Nutrients 2015, 7, 9872–9895.
- Crowe, W.; Elliott, C.T.; Green, B.D. A Review of the In Vivo Evidence Investigating the Role of Nitrite Exposure from Processed Meat Consumption in the Development of Colorectal Cancer. Nutrients 2019, 11, 2673.
- Schwarz, U.I.; Seemann, D.; Oertel, R.; Miehlke, S.; Kuhlisch, E.; Fromm, M.F.; Kim, R.B.; Bailey, D.G.; Kirch, W. Grapefruit Juice Ingestion Significantly Reduces Talinolol Bioavailability. Clin. Pharmacol. Ther. 2005, 77, 291–301.
- Geraci, A.; Di Stefano, V.; Di Martino, E.; Schillaci, D.; Schicchi, R. Essential Oil Components of Orange Peels and Antimicrobial Activity. Nat. Prod. Res. 2017, 31, 653–659.
- Deng, W.; Liu, K.; Cao, S.; Sun, J.; Zhong, B.; Chun, J. Chemical Composition, Antimicrobial, Antioxidant, and Antiproliferative Properties of Grapefruit Essential Oil Prepared by Molecular Distillation. Molecules 2020, 25, 217.
- Ou, M.C.; Liu, Y.H.; Sun, Y.W.; Chan, C.F. The Composition, Antioxidant and Antibacterial Activities of Cold-Pressed and Distilled Essential Oils of Citrus paradisi and Citrus grandis (L.) Osbeck. Evid. Based Complement. Altern. Med. 2015, 2015, 804091.
- Satyal, P.; Murray, B.L.; McFeeters, R.L.; Setzer, W.N. Essential Oil Characterization of Thymus vulgaris from Various Geographical Locations. Foods 2016, 5, 70.
- Teixeira, B.; Marques, A.; Ramos, C.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. Chemical Composition and Bioactivity of Different Oregano (Origanum Vulgare) Extracts and Essential Oil. J. Sci. Food Agric. 2013, 93, 2707–2714.
- Sharma, P.; Upadhyaya, K. Characteristic Features and Comparative Analysis of Essential Oil Composition of Selected Genus of Ocimum sanctum L. through GC-MS. J. Vector Borne Dis. 2023, 60, 94–100.
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429–438.
- Babovic, N.; Djilas, S.; Jadranin, M.; Vajs, V.; Ivanovic, J.; Petrovic, S.; Zizovic, I. Supercritical Carbon Dioxide Extraction of Antioxidant Fractions from Selected Lamiaceae Herbs and Their Antioxidant Capacity. Innov. Food Sci. Emerg. Technol. 2010, 11, 98–107.
- Radaelli, M.; da Silva, B.P.; Weidlich, L.; Hoehne, L.; Flach, A.; da Costa, L.A.M.A.; Ethur, E.M. Antimicrobial Activities of Six Essential Oils Commonly Used as Condiments in Brazil against Clostridium Perfringens. Braz. J. Microbiol. 2016, 47, 424–430.
- Ksouri, S.; Djebir, S.; Bentorki, A.A.; Gouri, A.; Hadef, Y.; Benakhla, A. Antifungal Activity of Essential Oils Extract from Origanum floribundum Munby, Rosmarinus officinalis L. and Thymus ciliatus Desf. against Candida albicans Isolated from Bovine Clinical Mastitis. J. Mycol. Med. 2017, 27, 245–249.
- Boskovic, M.; Djordjevic, J.; Glisic, M.; Ciric, J.; Janjic, J.; Zdravkovic, N.; Krnjaic, D.; Baltic, M.Z. The Effect of Oregano (Origanum vulgare) Essential Oil on Four Salmonella Serovars and Shelf Life of Refrigerated Pork Meat Packaged under Vacuum and Modified Atmosphere. J. Food Process. Preserv. 2020, 44, e14311.
- Naghdibadi, H.; Abdollahi, M.; Mehrafarin, A.; Ghorbanpour, M.; Tolyat, S.; Qaderi, A.; Ghiaci Yekta, M. An Overview on Two Valuable Natural and Bioactive Compounds, Thymol and Carvacrol, in Medicinal Plants. J. Med. Plants 2017, 16, 1–32.
- Javidanpour, S.; Dianat, M.; Badavi, M.; Mard, S.A. The Cardioprotective Effect of Rosmarinic Acid on Acute Myocardial Infarction and Genes Involved in Ca2+homeostasis. Free Radic. Res. 2017, 51, 911–923.
- Memar, M.Y.; Raei, P.; Alizadeh, N.; Aghdam, M.A.; Kafil, H.S. Carvacrol and Thymol: Strong Antimicrobial Agents against Resistant Isolates. Rev. Med. Microbiol. 2017, 28, 63–68.
- Wei, Y.; Pu, X.; Zhao, L. Preclinical Studies for the Combination of Paclitaxel and Curcumin in Cancer Therapy. Oncol. Rep. 2017, 37, 3159–3166.
- Fachini-Queiroz, F.C.; Kummer, R.; Estevão-Silva, C.F.; Carvalho, M.D.D.B.; Cunha, J.M.; Grespan, R.; Bersani-Amado, C.A.; Cuman, R.K.N. Effects of Thymol and Carvacrol, Constituents of Thymus vulgaris L. Essential Oil, on the Inflammatory Response. Evid.-Based Complement. Altern. Med. 2012, 2012, 657026.
- Xu, J.; Zhou, F.; Ji, B.P.; Pei, R.S.; Xu, N. The Antibacterial Mechanism of Carvacrol and Thymol against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179.
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253.
- Sokolik, C.G.; Ben-Shabat-Binyamini, R.; Gedanken, A.; Lellouche, J.P. Proteinaceous Microspheres as a Delivery System for Carvacrol and Thymol in Antibacterial Applications. Ultrason. Sonochem. 2018, 41, 288–296.
- El-Tarabily, K.A.; El-Saadony, M.T.; Alagawany, M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Elwan, H.A.M.; Elnesr, S.S.; Abd El-Hack, M.E. Using Essential Oils to Overcome Bacterial Biofilm Formation and Their Antimicrobial Resistance. Saudi J. Biol. Sci. 2021, 28, 5145–5156.
- Bouyahya, A.; Abrini, J.; Dakka, N.; Bakri, Y. Essential Oils of Origanum Compactum Increase Membrane Permeability, Disturb Cell Membrane Integrity, and Suppress Quorum-Sensing Phenotype in Bacteria. J. Pharm. Anal. 2019, 9, 301–311.
- Bouyahya, A.; Dakka, N.; Et-Touys, A.; Abrini, J.; Bakri, Y. Medicinal Plant Products Targeting Quorum Sensing for Combating Bacterial Infections. Asian Pac. J. Trop. Med. 2017, 10, 729–743.
- Barbosa, L.N.; Rall, V.L.M.; Fernandes, A.A.H.; Ushimaru, P.I.; da Silva Probst, I.; Fernandes, A., Jr. Essential Oils Against Foodborne Pathogens and Spoilage Bacteria in Minced Meat. Foodborne Pathog. Dis. 2009, 6, 725.
- Bukvicki, D.; Giweli, A.; Stojkovic, D.; Vujisic, L.; Tesevic, V.; Nikolic, M.; Sokovic, M.; Marin, P.D. Short Communication: Cheese Supplemented with Thymus algeriensis Oil, a Potential Natural Food Preservative. J. Dairy Sci. 2018, 101, 3859–3865.
- Sessou, P.; Farougou, S.; Ahounou, S.; Hounnankpon, Y.; Azokpota, P.; Youssao, I.; Sohounhloue, D. Comparative Study of Antifungal Activities of Six Selected Essential Oils against Fungal Isolates from Cheese Wagashi in Benin. Pak. J. Biol. Sci. 2013, 16, 1751–1757.
- Kulabas, S.S.; Ipek, H.; Tufekci, A.R.; Arslan, S.; Demirtas, I.; Ekren, R.; Sezerman, U.; Tumer, T.B. Ameliorative Potential of Lavandula Stoechas in Metabolic Syndrome via Multitarget Interactions. J. Ethnopharmacol. 2018, 223, 88–98.
- Ekpenyong, C.E.; Akpan, E.E. Use of Cymbopogon Citratus Essential Oil in Food Preservation: Recent Advances and Future Perspectives. Crit. Rev. Food Sci. Nutr. 2017, 57, 2541–2559.
- Nardoni, S.; D’Ascenzi, C.; Caracciolo, I.; Mannaioni, G.; Amerigo Papini, R.; Pistelli, L.; Najar, B.; Mancianti, F. Activity of Selected Essential Oils on Spoiling Fungi Cultured from Marzolino Cheese. Ann. Agric. Environ. Med. 2018, 25, 280–284.
- Pinto, L.; Cefola, M.; Bonifacio, M.A.; Cometa, S.; Bocchino, C.; Pace, B.; De Giglio, E.; Palumbo, M.; Sada, A.; Logrieco, A.F.; et al. Effect of Red Thyme Oil (Thymus vulgaris L.) Vapours on Fungal Decay, Quality Parameters and Shelf-Life of Oranges during Cold Storage. Food Chem. 2021, 336, 127590.
- Juneja, V.K.; Dwivedi, H.P.; Yan, X. Novel Natural Food Antimicrobials. Annu. Rev. Food Sci. Technol. 2012, 3, 381–403.
- Maurya, A.; Prasad, J.; Das, S.; Dwivedy, A.K. Essential Oils and Their Application in Food Safety. Front. Sustain. Food Syst. 2021, 5, 653420.
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Gardini, F.; Lanciotti, R. Innovative Strategies Based on the Use of Bio-Control Agents to Improve the Safety, Shelf-Life and Quality of Minimally Processed Fruits and Vegetables. Trends Food Sci. Technol. 2015, 46, 302–310.
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins-a Viable Alternative to Antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105.
- Castellano, P.; Ibarreche, M.P.; Massani, M.B.; Fontana, C.; Vignolo, G.M. Strategies for Pathogen Biocontrol Using Lactic Acid Bacteria and Their Metabolites: A Focus on Meat Ecosystems and Industrial Environments. Microorganisms 2017, 5, 38.
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Tabanelli, G.; Montanari, C.; Tappi, S.; Rocculi, P.; Gardini, F.; Lanciotti, R. Efficacy of Natural Antimicrobials to Prolong the Shelf-Life of Minimally Processed Apples Packaged in Modified Atmosphere. Food Control 2014, 46, 403–411.
- De Azeredo, G.A.; Stamford, T.L.M.; Nunes, P.C.; Gomes Neto, N.J.; De Oliveira, M.E.G.; De Souza, E.L. Combined Application of Essential Oils from Origanum vulgare L. and Rosmarinus officinalis L. to Inhibit Bacteria and Autochthonous Microflora Associated with Minimally Processed Vegetables. Food Res. Int. 2011, 44, 1541–1548.
- Abadias, M.; Alegre, I.; Usall, J.; Torres, R.; Viñas, I. Evaluation of Alternative Sanitizers to Chlorine Disinfection for Reducing Foodborne Pathogens in Fresh-Cut Apple. Postharvest Biol. Technol. 2011, 3, 289–297.
- Rojas-Graü, M.A.; Raybaudi-Massilia, R.M.; Soliva-Fortuny, R.C.; Avena-Bustillos, R.J.; McHugh, T.H.; Martín-Belloso, O. Apple Puree-Alginate Edible Coating as Carrier of Antimicrobial Agents to Prolong Shelf-Life of Fresh-Cut Apples. Postharvest Biol. Technol. 2007, 45, 254–264.
- Singh, N.; Singh, R.K.; Bhunia, A.K.; Stroshine, R.L. Efficacy of Chlorine Dioxide, Ozone, and Thyme Essential Oil or a Sequential Washing in Killing Escherichia coli O157:H7 on Lettuce and Baby Carrots. LWT Food Sci. Technol. 2002, 35, 720–729.
- Bhargava, K.; Conti, D.S.; da Rocha, S.R.P.; Zhang, Y. Application of an Oregano Oil Nanoemulsion to the Control of Foodborne Bacteria on Fresh Lettuce. Food Microbiol. 2015, 47, 69–73.
- Mukurumbira, A.R.; Shellie, R.A.; Keast, R.; Palombo, E.A.; Jadhav, S.R. Encapsulation of Essential Oils and Their Application in Antimicrobial Active Packaging. Food Control 2022, 136, 108883.
- Sirocchi, V.; Devlieghere, F.; Peelman, N.; Sagratini, G.; Maggi, F.; Vittori, S.; Ragaert, P. Effect of Rosmarinus officinalis L. Essential Oil Combined with Different Packaging Conditions to Extend the Shelf Life of Refrigerated Beef Meat. Food Chem. 2017, 221, 1069–1076.
- Valero, D.; Valverde, J.M.; Martínez-Romero, D.; Guillén, F.; Castillo, S.; Serrano, M. The Combination of Modified Atmosphere Packaging with Eugenol or Thymol to Maintain Quality, Safety and Functional Properties of Table Grapes. Postharvest Biol. Technol. 2006, 41, 317–327.
- Balaguer, M.P.; Lopez-Carballo, G.; Catala, R.; Gavara, R.; Hernandez-Munoz, P. Antifungal Properties of Gliadin Films Incorporating Cinnamaldehyde and Application in Active Food Packaging of Bread and Cheese Spread Foodstuffs. Int. J. Food Microbiol. 2013, 166, 369–377.
- Chiriac, A.P.; Rusu, A.G.; Nita, L.E.; Chiriac, V.M.; Neamtu, I.; Sandu, A. Polymeric Carriers Designed for Encapsulation of Essential Oils with Biological Activity. Pharmaceutics 2021, 13, 631.
- Lanciotti, R.; Gianotti, A.; Patrignani, F.; Belletti, N.; Guerzoni, M.E.; Gardini, F. Use of Natural Aroma Compounds to Improve Shelf-Life and Safety of Minimally Processed Fruits. Trends Food Sci. Technol. 2004, 15, 201–208.
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Tappi, S.; Rocculi, P.; Gardini, F.; Lanciotti, R. Natural Antimicrobials to Prolong the Shelf-Life of Minimally Processed Lamb’s Lettuce. Postharvest Biol. Technol. 2015, 103, 35–44.
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Tabanelli, G.; Montanari, C.; Gardini, F.; Lanciotti, R. Lactic Acid Bacteria and Natural Antimicrobials to Improve the Safety and Shelf-Life of Minimally Processed Sliced Apples and Lamb’s Lettuce. Food Microbiol. 2015, 47, 74–84.
- Gündüz, G.T.; Gönül, Ş.A.; Karapinar, M. Efficacy of Oregano Oil in the Inactivation of Salmonella Typhimurium on Lettuce. Food Control 2010, 21, 513–517.
- Gutierrez, J.; Bourke, P.; Lonchamp, J.; Barry-Ryan, C. Impact of Plant Essential Oils on Microbiological, Organoleptic and Quality Markers of Minimally Processed Vegetables. Innov. Food Sci. Emerg. Technol. 2009, 10, 195–202.
- Hadian, M.; Rajaei, A.; Mohsenifar, A.; Tabatabaei, M. Encapsulation of Rosmarinus officinalis Essential Oils in Chitosan-Benzoic Acid Nanogel with Enhanced Antibacterial Activity in Beef Cutlet against Salmonella Typhimurium during Refrigerated Storage. LWT 2017, 84, 394–401.
- Devatkal, S.K.; Thorat, P.; Manjunatha, M. Effect of Vacuum Packaging and Pomegranate Peel Extract on Quality Aspects of Ground Goat Meat and Nuggets. J. Food Sci. Technol. 2014, 51, 2685–2691.
- Alirezalu, K.; Pateiro, M.; Yaghoubi, M.; Alirezalu, A.; Peighambardoust, S.H.; Lorenzo, J.M. Phytochemical Constituents, Advanced Extraction Technologies and Techno-Functional Properties of Selected Mediterranean Plants for Use in Meat Products. A Comprehensive Review. Trends Food Sci. Technol. 2020, 100, 292–306.
- Aminzare, M.; Hashemi, M.; Ansarian, E.; Bimkar, M.; Azar, H.H.; Mehrasbi, M.R.; Daneshamooz, S.; Raeisi, M.; Jannat, B.; Afshari, A. Using Natural Antioxidants in Meat and Meat Products as Preservatives: A Review. Adv. Anim. Vet. Sci. 2019, 7, 417–426.
- Bukvički, D.; Stojković, D.; Soković, M.; Vannini, L.; Montanari, C.; Pejin, B.; Savić, A.; Veljić, M.; Grujić, S.; Marin, P.D. Satureja Horvatii Essential Oil: In Vitro Antimicrobial and Antiradical Properties and in Situ Control of Listeria monocytogenes in Pork Meat. Meat Sci. 2014, 96, 1355–1360.
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12.
- Seydim, A.C.; Sarikus, G. Antimicrobial Activity of Whey Protein Based Edible Films Incorporated with Oregano, Rosemary and Garlic Essential Oils. Food Res. Int. 2006, 39, 639–644.
- Fasseas, M.K.; Mountzouris, K.C.; Tarantilis, P.A.; Polissiou, M.; Zervas, G. Antioxidant Activity in Meat Treated with Oregano and Sage Essential Oils. Food Chem. 2008, 106, 1188–1194.
- Casaburi, A.; Piombino, P.; Nychas, G.J.; Villani, F.; Ercolini, D. Bacterial Populations and the Volatilome Associated to Meat Spoilage. Food Microbiol. 2015, 45, 83–102.
- Doulgeraki, A.I.; Ercolini, D.; Villani, F.; Nychas, G.J.E. Spoilage Microbiota Associated to the Storage of Raw Meat in Different Conditions. Int. J. Food Microbiol. 2012, 157, 130–141.
- Pennacchia, C.; Ercolini, D.; Villani, F. Spoilage-Related Microbiota Associated with Chilled Beef Stored in Air or Vacuum Pack. Food Microbiol. 2011, 28, 84–93.
- Dussault, D.; Vu, K.D.; Lacroix, M. In Vitro Evaluation of Antimicrobial Activities of Various Commercial Essential Oils, Oleoresin and Pure Compounds against Food Pathogens and Application in Ham. Meat Sci. 2014, 96, 514–520.
- Hussain, Z.; Li, X.; Zhang, D.; Hou, C.; Ijaz, M.; Bai, Y.; Xiao, X.; Zheng, X. Influence of Adding Cinnamon Bark Oil on Meat Quality of Ground Lamb during Storage at 4 °C. Meat Sci. 2021, 171, 108269.
- Amariei, S.; Poroch- Serițan, M.; Gutt, G.; Oroian, M.; Ciornei, E. Rosemary, thyme and oregano essential oils influence on physicochemical properties and microbiological stability of minced meat. J. Microbiol. Biotechnol. Food Sci. 2016, 2017, 670–676.
- Sariçoban, C.; Yilmaz, M.T. Effect of Thyme/Cumin Essential Oils and Butylated Hydroxyl Anisole/Butylated Hydroxyl Toluene on Physicochemical Properties and Oxidative/Microbial Stability of Chicken Patties. Poult. Sci. 2014, 93, 456–463.
- Sultanbawa, Y. Plant Antimicrobials in Food Applications: Minireview. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Mendez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2011; Volume 2, pp. 1084–1093.
- Šimat, V.; Čagalj, M.; Skroza, D.; Gardini, F.; Tabanelli, G.; Montanari, C.; Hassoun, A.; Ozogul, F. Sustainable Sources for Antioxidant and Antimicrobial Compounds Used in Meat and Seafood Products. Adv. Food Nutr. Res. 2021, 97, 55–118.
- Domínguez, R.; Barba, F.J.; Gómez, B.; Putnik, P.; Bursać Kovačević, D.; Pateiro, M.; Santos, E.M.; Lorenzo, J.M. Active Packaging Films with Natural Antioxidants to Be Used in Meat Industry: A Review. Food Res. Int. 2018, 113, 93–101.
- Krkić, N.; Šojić, B.; Lazić, V.; Petrović, L.; Mandić, A.; Sedej, I.; Tomović, V. Lipid Oxidative Changes in Chitosan-Oregano Coated Traditional Dry Fermented Sausage Petrovská Klobása. Meat Sci. 2013, 93, 767–770.
- Woraprayote, W.; Malila, Y.; Sorapukdee, S.; Swetwiwathana, A.; Benjakul, S.; Visessanguan, W. Bacteriocins from Lactic Acid Bacteria and Their Applications in Meat and Meat Products. Meat Sci. 2016, 120, 118–132.
- Quintavalla, S.; Vicini, L. Antimicrobial Food Packaging in Meat Industry. Meat Sci. 2002, 62, 373–380.
- Pattanayaiying, R.; H-Kittikun, A.; Cutter, C.N. Incorporation of Nisin Z and Lauric Arginate into Pullulan Films to Inhibit Foodborne Pathogens Associated with Fresh and Ready-to-Eat Muscle Foods. Int. J. Food Microbiol. 2015, 207, 77–82.
- Millette, M.; Le Tien, C.; Smoragiewicz, W.; Lacroix, M. Inhibition of Staphylococcus Aureus on Beef by Nisin-Containing Modified Alginate Films and Beads. Food Control 2007, 18, 878–884.
- Luchansky, J.B.; Call, J.E. Evaluation of Nisin-Coated Cellulose Casings for the Control of Listeria monocytogenes Inoculated onto the Surface of Commercially Prepared Frankfurters. J. Food Prot. 2004, 67, 1017–1021.
- Franklin, N.B.; Cooksey, K.D.; Getty, K.J.K. Inhibition of Listeria monocytogenes on the Surface of Individually Packaged Hot Dogs with a Packaging Film Coating Containing Nisin. J. Food Prot. 2004, 67, 480–485.
- Scannell, A.G.M.; Hill, C.; Ross, R.P.; Marx, S.; Hartmeier, W.; Arendt, E.K. Development of Bioactive Food Packaging Materials Using Immobilised Bacteriocins Lacticin 3147 and Nisaplin. Int. J. Food Microbiol. 2000, 60, 241–249.
- Ritota, M.; Manzi, P. Natural Preservatives from Plant in Cheese Making. Animals 2020, 10, 749.
- Gad, A.S.; Sayd, A.F.; Gad, A.S.; Sayd, A.F. Antioxidant Properties of Rosemary and Its Potential Uses as Natural Antioxidant in Dairy Products—A Review. Food Nutr. Sci. 2015, 6, 179–193.
- Mishra, A.P.; Devkota, H.P.; Nigam, M.; Adetunji, C.O.; Srivastava, N.; Saklani, S.; Shukla, I.; Azmi, L.; Shariati, M.A.; Melo Coutinho, H.D.; et al. Combination of Essential Oils in Dairy Products: A Review of Their Functions and Potential Benefits. LWT 2020, 133, 110116.
- Ben Jemaa, M.; Falleh, H.; Saada, M.; Oueslati, M.; Snoussi, M.; Ksouri, R. Thymus Capitatus Essential Oil Ameliorates Pasteurization Efficiency. J. Food Sci. Technol. 2018, 55, 3446–3452.
- Azizkhani, M.; Tooryan, F. Antimicrobial Activities of Probiotic Yogurts Flavored with Peppermint, Basil, and Zataria against Escherichia coli and Listeria monocytogenes. J. Food Qual. Hazards Control 2016, 3, 79–86.
- Ehsani, A.; Hashemi, M.; Jazani, N.H.; Aliakbarlu, J.; Shokri, S.; Naghibi, S.S.; Dvm, M.H. Effect of Echinophora Platyloba DC. Essential Oil and Lycopene on the Stability of Pasteurized Cream Obtained from Cow Milk. Vet. Res. Forum 2016, 7, 139.
- Farag, R.S.; Ali, M.N.; Taha, S.H. Use of Some Essential Oils as Natural Preservatives for Butter. J. Am. Oil Chem. Soc. 1990, 67, 188–191.
- Ozkan, G.; Simsek, B.; Kuleasan, H. Antioxidant Activities of Satureja Cilicica Essential Oil in Butter and in Vitro. J. Food Eng. 2007, 79, 1391–1396.
- Sobrino-López, A.; Martín-Belloso, O. Use of Nisin and Other Bacteriocins for Preservation of Dairy Products. Int. Dairy J. 2008, 18, 329–343.
- Gharsallaoui, A.; Oulahal, N.; Joly, C.; Degraeve, P. Nisin as a Food Preservative: Part 1: Physicochemical Properties, Antimicrobial Activity, and Main Uses. Crit. Rev. Food Sci. Nutr. 2015, 56, 1262–1274.
More
