Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Marta Laranjo.
Antimicrobial resistance is considered a complex problem and a global health concern for both humans and animals. Around 2.8 million human cases of infections by antimicrobial-resistant bacteria and 700,000 deaths are reported annually, and this number could reach 10 million by 2050 if antimicrobial-resistant (AMR) is not reduced.
- antimicrobial resistance
- multidrug-resistant bacteria
- human
1. Antimicrobial Activity
Antimicrobials are natural, semi-synthetic, or synthetic compounds capable of killing bacteria or preventing bacterial growth. These are used in the treatment of bacterial infections in humans and animals, or as feed additives or synthetic growth promoters in animals and aquaculture [33][1].
Antimicrobial activity may be divided into five main mechanisms, which are summarised in Figure 1 [44,45][2][3].
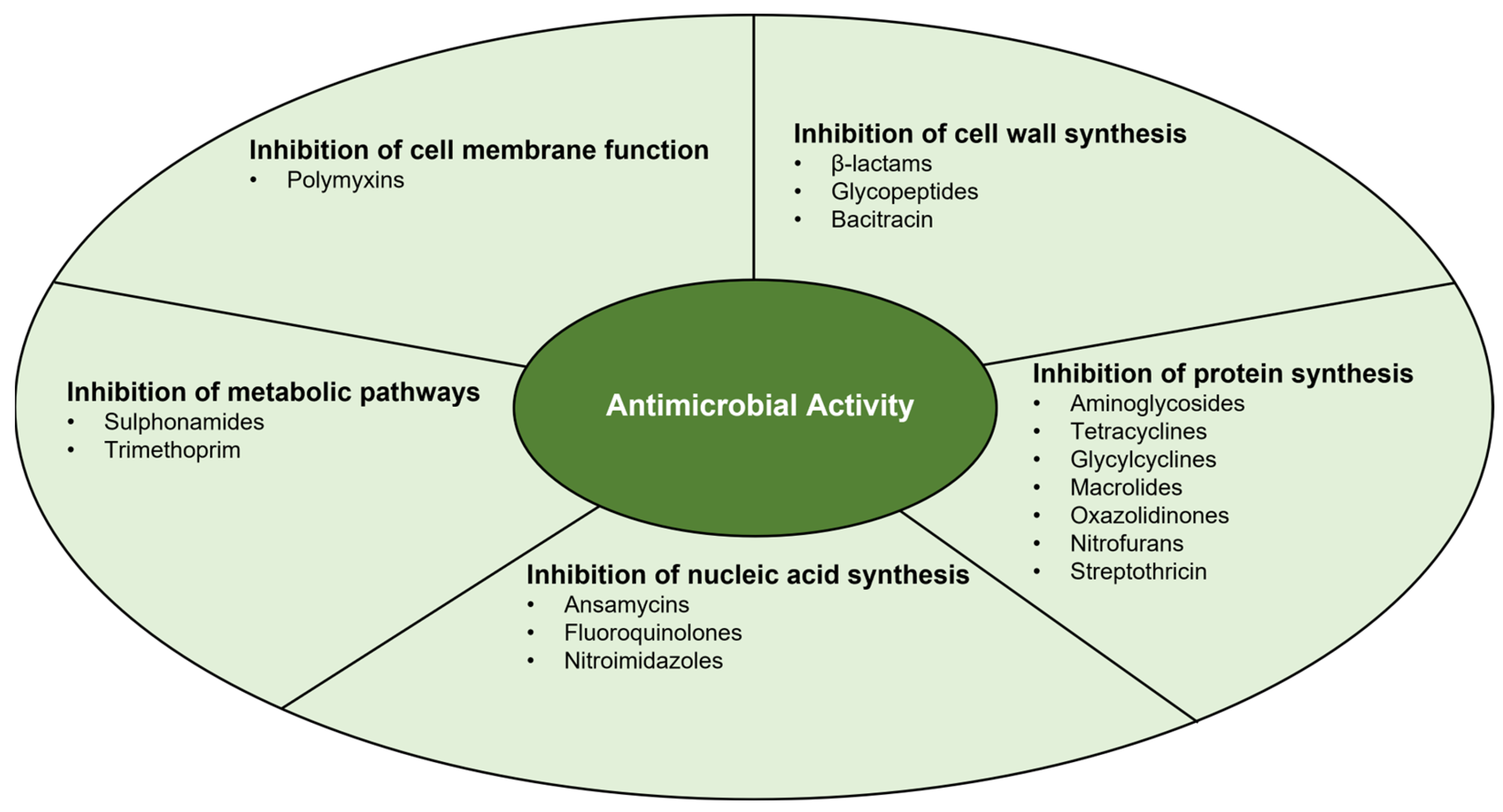
Figure 1.
Main mechanisms of antimicrobial activity.
1.1. Inhibition of Cell Wall Synthesis
The bacterial cell wall is composed of peptidoglycan that generates mechanical support and allows the bacteria to survive under extreme situations (e.g., osmotic pressure changes) [44,46][2][4]. Peptidoglycan is a polymer formed by chains of glycans, formed by disaccharide subunits of N-acetylglucosamine and acetylmuramic acid, cross-linked by pentapeptide chains [44][2]. This component can be found in both Gram-negative and Gram-positive bacteria. In Gram-negative bacteria, the cell wall comprises 1 or 2 layers of peptidoglycan, while in Gram-positive bacteria, 10–40 layers are present [44,46][2][4].
There are different antimicrobials whose mechanisms of action inhibit cell wall synthesis: β-lactams, glycopeptides, and bacitracin, which is a polypeptide antibiotic [44,46,47][2][4][5]. Beta-lactams bind to transpeptidases (also called PBPs—penicillin-binding proteins), inhibiting the formation of peptide bonds between tetrapeptides that crosslink glycan chains, inactivating the PBPs, which results in the lysis of microorganisms [44,46][2][4]. Glycopeptides block cell wall synthesis by binding to the D-ala-D-ala terminus of the tetrapeptide chain, which also results in the inhibition of PBPs [44][2]. Bacitracin inactivates the membrane carrier, bactoprenol, responsible for the transport of peptidoglycan building blocks from the cytoplasm to the cell wall [47][5].
1.2. Inhibition of Protein Synthesis
Protein synthesis involves mRNA, tRNA, ribosomes, and other cytoplasmic factors and consists of three steps: initiation, elongation, and termination. The bacterial ribosome has two subunits, the 50S and 30S, each composed of rRNA and proteins [46][4].
There are several classes of antimicrobials that act to inhibit protein synthesis by binding to the 30S subunit (aminoglycosides, tetracyclines, and glycylcyclines) or the 50S subunit (macrolides, chloramphenicol, oxazolidinones, lincosamides, and streptogramin) [44,48,49,50][2][6][7][8]. Aminoglycosides act by binding with high affinity to the 16S rRNA of the 30S subunit. Thus, codons are misread when aminoacyl tRNA is delivered, resulting in erroneous protein synthesis. Consequently, the wrong amino acids are compiled into a polypeptide that is released, leading to apoptosis [44][2]. Tetracyclines, on the other hand, act through passive diffusion in the cell membrane by porin channels and reversibly bind to the 30S subunit, resulting in blocking the binding of the tRNA to the mRNA-ribosome complex [44][2]. Glycylcyclines are an antimicrobial class developed to overcome the mechanisms of resistance to tetracycline (ribosomal protection and efflux pumps). They bind to the 30S subunit with five times more affinity, inhibiting protein synthesis. On the other hand, glycylcyclines are not recognized by the tetracycline efflux transporter, exhibiting significant antibacterial activity [50][8].
Macrolides and oxazolidinones bind to the 23S rRNA of the 50S subunit and inhibit the process of translocation or transpeptidation of protein synthesis, inducing a premature separation of incomplete peptide chains. Chloramphenicol crosses the cell membrane and reversibly binds to the L16 protein of the 50S subunit, thus inhibiting the formation of peptide bonds and preventing the elongation of peptide chains [44][2].
Additionally, nitrofurans are bacteriostatic antimicrobials whose multiple mechanisms of action are not fully understood [51][9]. They inhibit the synthesis of proteins, DNA, and RNA [52][10]. Moreover, their wide mechanisms of action may explain the lack of acquired bacterial resistance to nitrofurans [51,52][9][10].
Very recently, streptothricin F has been revisited as a bactericidal antimicrobial effective against highly drug-resistant Gram-negative bacteria, namely carbapenem-resistant Enterobacterales (CRE), Acinetobacter baumannii, and Brucella abortus, as well as Mycobacterium tuberculosis. Streptothricin is a natural product mixture, currently referred to as nourseothricin. Its therapeutic use was abandoned due to its induced reversible kidney toxicity; however, new cytotoxic studies have shown that streptothricin F exhibits at least 10-fold lower toxicity than streptothricin D and nourseothricin, both in vitro and in vivo. Moreover, streptothricin F has an alternative and unique mechanism of action, interacting with the 30S subunit of the 70S ribosome [53][11].
1.3. Inhibition of Nucleic Acid Synthesis
Examples of antimicrobials that inhibit nucleic acid synthesis are ansamycins (e.g., rifamycin and rifampicin), fluoroquinolones, and nitroimidazoles (e.g., metronidazole) [44,46,54][2][4][12].
Ansamycins bind to the β-subunit of RNA polymerase, blocking RNA elongation and inhibiting RNA synthesis [44][2]. Fluoroquinolones act by inhibiting DNA gyrase and other topoisomerases, interfering with DNA replication [44,46][2][4]. Nitroimidazoles inhibit nucleic acid synthesis by forming nitroso radicals, which disrupt DNA. This class of antimicrobials is only effective against anaerobic bacteria, whose ferredoxin reduces them to active radicals [54][12].
1.4. Inhibition of Metabolic Pathways
Nitrogenous bases (purines and pyrimidines), formed through the folic acid pathway, are necessary for the synthesis of nucleic acids. This process is initiated with para-aminobenzoic acid (PABA), which is catalysed in dihydroflolic acid and subsequently in tetrahydrofolic acid, which is later used to synthesize nitrogenous bases [46][4].
Antimicrobials that inhibit folic acid synthesis are sulphonamides and trimethoprim [44][2]. Sulphonamides are structural analogues of PABA, competitively inhibiting the enzymatic conversion that leads to the production of dihydroflolic acid [44][2]. As for trimethoprim, it reversely inhibits the formation of tetrahydrofolic acid [44][2]. Used separately, trimethoprim and sulphonamides are bacteriostatic; however, combined, they seem to have a bactericidal effect [44][2].
1.5. Inhibition of Cell Membrane Function
Only a small class of antimicrobials act by inhibiting cell membrane function, the polymyxins. This class of lipopeptides consists of lipophilic detergent-type antimicrobials, which lyse cell membranes by destroying the lipopolysaccharide (LPS) layer [44][2].
2. Antimicrobial Resistance
Bacterial antimicrobial resistance may be natural or acquired. Natural resistance is either innate when constitutively expressed, or mediated if triggered by an antibiotic treatment. On the other hand, acquired resistance occurs through DNA mutation or via the transfer of genetic material between bacteria [44][2].
Bacteria can acquire antimicrobial resistance through genetic mutation, namely spontaneous mutation, hypermutation, and adaptive mutation. Spontaneous mutation can be driven by several factors, mainly errors in DNA replication, such as transitions, transversions, insertions and deletions, which are transmitted to the progeny. Hypermutation plays a crucial role in the evolution of antimicrobial resistance. Hypermutation is regulated by the SOS-inducible DNA polymerase IV. This mutation occurs in bacteria called hypermutators, as they have a greater affinity to undergo spontaneous mutations due to defects or repairs in DNA, or errors in the avoidance system. Therefore, hypermutators can quickly adapt to antimicrobials. Finally, adaptive mutation arises in non-diving bacteria, upon non-lethal selective pressure, such as nutrient conditions, or sub-inhibitory antimicrobial concentrations. This type of mutation is transient and can be reverted to the original condition in the absence of the pressure factor [45][3].
Antimicrobial resistance genes can be acquired by horizontal gene transfer between bacteria, either by conjugation, transformation, or transduction [21,55,56,57][13][14][15][16]. Conjugation (Figure 2) is the transient fusion between two bacteria, where the transfer of genetic material takes place from the donor to the recipient through conjugation pili. Transformation is the uptake of free genetic material, released by a donor bacterium, by a recipient bacterium. Finally, transduction is the transfer of resistant genes mediated by bacteriophages [21,55,57][13][14][16]. Among antimicrobial-resistant (AMR) gene transfer mechanisms, conjugation has been shown to play an important role in the transmission and dissemination of AMR in food [18][17].

Figure 2. Horizontal gene transfer through conjugation. (a) Microbial community with antimicrobial susceptible bacteria (green) and antimicrobial resistant bacteria (blue); (b) Fusion between resistant and susceptible bacteria, allowing the transfer of genetic information through conjugation pili; (c) Microbial community with antimicrobial resistant bacteria.
The persistent use of antimicrobials, as well as misuse and self-medication, leads to the abovementioned acquired AMR. Moreover, the appearance of MDR, bacteria resistant to three or more antimicrobial classes, is a critical public health problem [2,22,33,58][1][18][19][20]. The treatment of infections caused by MDR bacteria poses a relevant clinical challenge since the increase in AMR leads to higher rates of therapeutic failures, relapses, longer hospitalizations, and worse clinical outcomes [3][21].
The increase in AMR triggers the need for surveillance of bacteria resistant to antimicrobials, which has been carried out in public health and food safety laboratories through Whole Genome Sequencing (WGS), enabling the description of the full AMR profile [21,59][13][22].
Bacterial resistance processes are divided into four biochemical mechanisms, which are highlighted in Figure 3 [44,45][2][3].
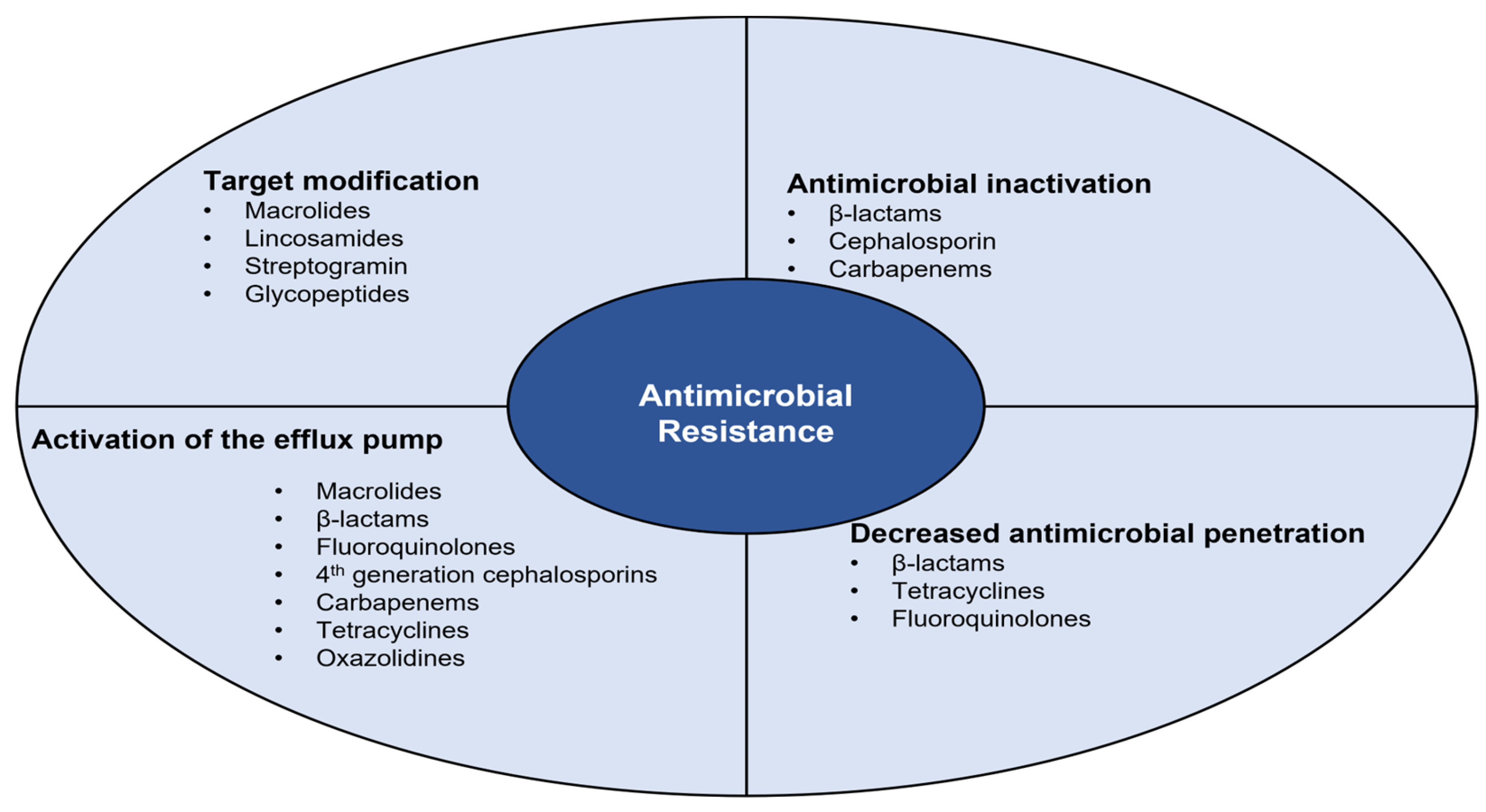
Figure 3.
Biochemical mechanisms involved in the different bacterial resistance processes.
2.1. Antimicrobial Inactivation
Inactivation of the antimicrobial molecule occurs through the action of enzymes produced by resistant bacteria, such as β-lactamases and aminoglycoside-modifying enzymes [44,45,60][2][3][23]. Enzymes act on the antimicrobial molecule through hydrolysis, group transfer, or redox process. Hydrolysis is the process of destruction of the β-lactam ring of penicillin, cephalosporins, and carbapenems by β-lactamase-producing bacteria. Acyltransferases, phosphotransferases, and thioltransferases are examples of enzymes involved in hydrolysis, causing the destruction of the β-lactam ring and inhibiting the antimicrobial molecule binding to PBPs [44,45,60][2][3][23]. Group transfer, namely phosphoryl, acetyl, or adenyl group transfer to the antimicrobial active molecule, is considered the most effective mechanism of antimicrobial inactivation. An example of group transfer is acetylation on aminoglycosides, where enzymes alter hydroxyl or amino groups covalently, rendering antimicrobials inactive [44][2]. Finally, the redox process is the least studied mechanism, where antimicrobials are inactivated by oxidation or reduction [45][3].
2.2. Decreased Antimicrobial Penetration
Decreased antimicrobial penetration occurs through decreased cell wall permeability [44,45][2][3]. Gram-negative bacteria are intrinsically less permeable to certain antimicrobials than Gram-positive bacteria due to the large layer of LPS in the outer membrane of the cell wall that creates a permeability shield [44][2]. Hydrophilic molecules may penetrate the Gram-negative cell wall through porin proteins [45][3]. However, high-molecular-weight hydrophilic molecules, such as vancomycin, cannot pass through porins and are thus ineffective against Gram-negative bacteria [44][2].
Some bacteria are able to downregulate the expression of porins or even replace them with non-selective channels, decreasing the cell wall permeability and becoming thus resistant to some antimicrobials [44][2]. Hydrophilic molecules, such as β-lactams, tetracyclines, and some fluoroquinolones, are greatly affected by changes in the permeability of the outer membrane [44][2].
2.3. Activation of the Efflux Pump
The efflux system consists of energy-dependent membrane transport systems that pump a wide range of molecules [60][23]. In this transport system, there are efflux pumps, which are transport proteins that are located mostly in the bacterial cytoplasmic membrane [45,60][3][23]. These proteins transport nutrients and excrete cellular toxic compounds through the proton matrix force [45][3].
Efflux pumps can be specific to a particular antimicrobial or multi-resistant efflux pumps capable of excreting various antimicrobials [44,45,60][2][3][23]. The main families of efflux pumps are ATP-binding cassettes (ABC), small multidrug resistance (SMR), multidrug and toxic component extrusion (MATE), resistance-nodulation cell division (RND), and large facilitator superfamily (MFS) [44,60][2][23]. This mechanism confers resistance to macrolides, β-lactams, fluoroquinolones, 4th generation cephalosporins, carbapenems, tetracyclines, and oxazolidines [44,45][2][3].
4.4. Target Modification
2.4. Target Modification
The modification of the antimicrobial target is one of the most common resistance mechanisms. For β-lactams, changes may occur either in the composition or the amount of PBPs. Thus, the amount of antimicrobial that can bind to the target is affected by the change in the number of PBPs, while a structural modification decreases or completely prevents the binding of the molecules [44,60][2][23]. Another method is the production of alternative proteins that adopt the role of the bacterium’s native protein, resulting in antimicrobial resistance [45][3]. Moreover, modification of ribosomes or the peptidoglycan precursor can also occur. Ribosome modification consists of ribosome methylation, commonly mediated by erm gene products, which can be constitutive or inducible. This modification results in resistance to macrolides, lincosamides, and streptogramin B [60][23]. Regarding the modification of the peptidoglycan precursor, in the case of resistance to glycopeptides, it occurs through an amino acid substitution. The change occurs at the end of the D-alanyl-D-alanine dipeptide that is found at the terminals of the tetrapeptide [60][23].
References
- Thapa, S.P.; Shrestha, S.; Anal, A.K. Addressing the antibiotic resistance and improving the food safety in food supply chain (farm-to-fork) in Southeast Asia. Food Control 2020, 108, 106809.
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.M.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766.
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; Sulaiman, A.M.; Chee, J.Y.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial resistance: Prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur. J. Pharm. Sci. 2022, 170, 106103.
- Upmanyu, N.; Malviya, V.N. Antibiotics: Mechanisms of action and modern challenges. In Microorganisms for Sustainable Environment and Health; Elsevier: Amsterdam, The Netherlands, 2020; pp. 367–382.
- Singh, S.P.; Qureshi, A.; Hassan, W. Mechanisms of action by antimicrobial agents: A review. McGill J. Med. 2021, 19.
- Lin, J.; Zhou, D.; Steitz, T.A.; Polikanov, Y.S.; Gagnon, M.G. Ribosome-Targeting Antibiotics: Modes of Action, Mechanisms of Resistance, and Implications for Drug Design. Annu. Rev. Biochem. 2018, 87, 451–478.
- Johnston, N.; Mukhtar, T.; Wright, G. Streptogramin Antibiotics: Mode of Action and Resistance. Curr. Drug Targets 2002, 3, 335–344.
- Zuckerman, J.M.; Qamar, F.; Bono, B.R. Review of Macrolides (Azithromycin, Clarithromycin), Ketolids (Telithromycin) and Glycylcyclines (Tigecycline). Med. Clin. N. Am. 2011, 95, 761–791.
- Wijma, R.A.; Huttner, A.; Koch, B.C.P.; Mouton, J.W.; Muller, A.E. Review of the pharmacokinetic properties of nitrofurantoin and nitroxoline. J. Antimicrob. Chemother. 2018, 73, 2916–2926.
- Vallée, M.; Harding, C.; Hall, J.; Aldridge, P.D.; Tan, A. Exploring the in situ evolution of nitrofurantoin resistance in clinically derived uropathogenic Escherichia coli isolates. J. Antimicrob. Chemother. 2022, 78, 373–379.
- Morgan, C.E.; Kang, Y.-S.; Green, A.B.; Smith, K.P.; Dowgiallo, M.G.; Miller, B.C.; Chiaraviglio, L.; Truelson, K.A.; Zulauf, K.E.; Rodriguez, S.; et al. Streptothricin F is a bactericidal antibiotic effective against highly drug-resistant gram-negative bacteria that interacts with the 30S subunit of the 70S ribosome. PLoS Biol. 2023, 21, e3002091.
- Edwards, D.I. Nitroimidazole drugs-action and resistance mechanisms I. Mechanisms of action. J. Antimicrob. Chemother. 1993, 31, 9–20.
- Collineau, L.; Boerlin, P.; Carson, C.A.; Chapman, B.; Fazil, A.; Hetman, B.; McEwen, S.A.; Jane Parmley, E.; Reid-Smith, R.J.; Taboada, E.N.; et al. Integrating whole-genome sequencing data into quantitative risk assessment of foodborne antimicrobial resistance: A review of opportunities and challenges. Front. Microbiol. 2019, 10, 1107.
- De Lucia, A.; Card, R.M.; Duggett, N.; Smith, R.P.; Davies, R.; Cawthraw, S.A.; Anjum, M.F.; Rambaldi, M.; Ostanello, F.; Martelli, F. Reduction in antimicrobial resistance prevalence in Escherichia coli from a pig farm following withdrawal of group antimicrobial treatment. Vet. Microbiol. 2021, 258, 109125.
- Mellor, K.C.; Petrovska, L.; Thomson, N.R.; Harris, K.; Reid, S.W.J.; Mather, A.E. Antimicrobial resistance diversity suggestive of distinct salmonella typhimurium sources or selective pressures in food-production animals. Front. Microbiol. 2019, 10, 708.
- Tsigalou, C.; Konstantinidis, T.; Stavropoulou, E.; Bezirtzoglou, E.E.; Tsakris, A. Potential Elimination of Human Gut Resistome by Exploiting the Benefits of Functional Foods. Front. Microbiol. 2020, 11, 50.
- Mouiche, M.M.M.; Moffo, F.; Akoachere, J.F.T.K.; Okah-Nnane, N.H.; Mapiefou, N.P.; Ndze, V.N.; Wade, A.; Djuikwo-Teukeng, F.F.; Toghoua, D.G.T.; Zambou, H.R.; et al. Antimicrobial resistance from a one health perspective in Cameroon: A systematic review and meta-analysis. BMC Public Health 2019, 19, 1135.
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Front. Microbiol. 2019, 10, 539.
- Pérez-Rodríguez, F.; Mercanoglu Taban, B. A State-of-Art Review on Multi-Drug Resistant Pathogens in Foods of Animal Origin: Risk Factors and Mitigation Strategies. Front. Microbiol. 2019, 10, 2091.
- Rafailidis, P.I.; Kofteridis, D. Proposed amendments regarding the definitions of multidrug-resistant and extensively drug-resistant bacteria. Expert Rev. Anti-Infect. Ther. 2021, 20, 139–146.
- Gargiullo, L.; Del Chierico, F.; D’Argenio, P.; Putignani, L. Gut microbiota modulation for multidrug-resistant organism decolonization: Present and future perspectives. Front. Microbiol. 2019, 10, 1704.
- Hickman, R.A.; Leangapichart, T.; Lunha, K.; Jiwakanon, J.; Angkititrakul, S.; Magnusson, U.; Sunde, M.; Järhult, J.D. Exploring the Antibiotic Resistance Burden in Livestock, Livestock Handlers and Their Non-Livestock Handling Contacts: A One Health Perspective. Front. Microbiol. 2021, 12, 651461.
- Lai, C.K.C.; Ng, R.W.Y.; Leung, S.S.Y.; Hui, M.; Ip, M. Overcoming the rising incidence and evolving mechanisms of antibiotic resistance by novel drug delivery approaches—An overview. Adv. Drug Deliv. Rev. 2022, 181, 114078.
More
