Myrtenal is a perspective monoterpenoid with therapeutic potential in various fields of medicine. Its chemical modifications often lead to new or more pronounced biological effects. As an example, the conjugation of myrtenal with the established pharmacophore adamantane enables the augmentation of several of its pivotal properties. Myrtenal–adamantane derivatives exhibited a variety of beneficial characteristics, such as antimicrobial, antifungal, antiviral, anticancer, anxiolytic, and neuroprotective properties, which are worth examining in more detail and at length.
- monoterpene
- pharmacophore
- chemical modification
- antiviral
- anticancer
- anxiolytic
- neuroprotective activity
1. Introduction
Terpenes constitute the largest cluster of secondary plant metabolites, encompassing a plethora of over 50,000 distinct substances, each characterized by a diverse array of biological attributes [14][1]. The most widespread terpenes are monoterpenes consisting of two isoprene fragments [15][2]. Monoterpenes that incorporate heteroatoms, such as oxygen, are categorized as monoterpenoids. As natural products, monoterpenes and monoterpenoids are the subject of increased attention from the world scientific community in the search for new pharmacological agents in various branches of medicine and pharmacy [16,17,18,19,20,21,22,23,24,25,26][3][4][5][6][7][8][9][10][11][12][13]. They have many biological properties, including antifungal, antibacterial, antioxidant, anticancer, antispasmodic, hypotensive, vasodilating effects, etc.
2. Therapeutic Potential of Myrtenal
(−)-Myrtenal, (1R)-2-pinen-10-al, (1R)-6,6-dimethylbicyclo[3.1.1]hept-2-en-2-carboxaldehyde (Figure 31), is a bicyclic monoterpenoid of natural origin.
2.1. Antidiabetic Potential
Myrtenal exhibited an antihyperglycemic effect in rats with an experimental streptozotocin-induced diabetes mellitus model. The compound lowered plasma glucose levels, improved plasma insulin levels, upregulated various glucose transporters, and subsequently improved glucose uptake in the liver and skeletal muscle. In the study by Rathinam and Pari (2016) [125][34], oral administration of myrtenal (80 mg/kg b.wt.) for 28 days produced a number of effects in rats with induced diabetes. It reduced plasma glucose and glycated hemoglobin A1c (HbA1c); increased levels of insulin and hemoglobin; regained body weight; and normalized activity of hexokinase, glucose-6-phosphatase, fructose-1,6-bisphosphatase, glucose-6-phosphate dehydrogenase and liver enzymes AST, ALT, and ALP. Myrtenal also increased glycogen content in the liver and muscles; recovered the hepatocytes; and improved pancreatic insulin levels and lipid profile values (total cholesterol, triglycerides, phospholipids, low-density lipoprotein, very-low-density lipoprotein, atherogenic index) (Figure 42).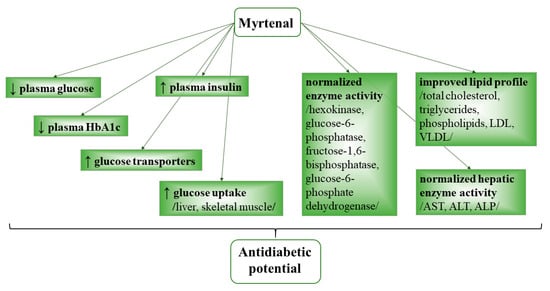
3.2. Antitumor Potential
2.2. Antitumor Potential
Myrtenal has been shown to have antioxidant and antitumor activity in peroral administration at a dose of 230 mg/kg b.wt. in corn oil for 28 days. These effects were achieved through a variety of mechanisms of action, including stabilizing endogenous antioxidant protection, influencing apoptotic and proapoptotic signaling pathways, inhibiting the expression of TNF- and reducing tumor growth, and regulating the activity of several lysosomal and mitochondrial enzymes [126,127,128,129][35][36][37][38]. The monoterpenoid was administered for 28 days in the experimental protocols of Babu et al., and Venkatachalam’s research group applied it for 15 weeks. Myrtenal was found to inhibit V-type ATPase on the surface of tumor cells in an in vitro study in melanoma cell lines, leading to their death and also to the suppression of melanoma metastasis in experimental mice at a dose of 15 mg/kg b.wt. (i.p. application for 21 days) [130][39]. The monoterpenoid demonstrated the ability to inactivate free radicals, resulting in inhibition of colon carcinogenesis and suppression of tumor progression after 30 weeks of intragastric administration at a dose of 230 mg/kg b.wt. [131,132][40][41].3.3. Analgesic Potential
2.3. Analgesic Potential
Pinene, whose derivative is M, showed analgesic potential in various models of induced pain [135][42]. On the other hand, the hydroxyl derivative myrtenol suppressed nociceptive and inflammatory responses in experimental conditions by inhibiting cell migration and neuromediation in pain pathways [136][43]. Intraperitoneal administration of this alcohol in experimental mice reduced the number of spasms in the acetic acid writhing test. For the first time, a recent investigation demonstrated myrtenal’s analgesic potential in laboratory mice. After a single, 7-day, and 14-day i.p. administration (30 mg/kg), the analgesic effect of M was established in two pain models: the acetic acid writhing test (antipyretic type analgesia) and the hot plate test (narcotic-type analgesia) [134][44].3.4. Anti-Inflammatory Potential
2.4. Anti-Inflammatory Potential
The anti-inflammatory activity of Myrtus communis, L. extracts was established in two chronic inflammation mice models: a xylene-induced ear edema and a cotton pellet test [137][45]. In rats with induced rheumatoid arthritis, myrtenal isolated from Liquidambra formosana L. exhibited anti-inflammatory properties [138][46]. They were manifested by lowering the plasma levels of interleukin-1β (IL-1β) and tumor necrosis factor α (TNF-α), and also by suppressing the activation of the nucleotide-binding, oligomerization domain (NOD)-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome, which was confirmed in vitro.3.5. CNS-Affecting Potential
2.5. CNS-Affecting Potential
Subsequent research into the myrtenal effects on the CNS in experiments on laboratory rodents showed potentiation of the classical sedative–hypnotic action of the drugs. These results are due to the interaction of myrtenal (20 and 30 mg/kg i.p. in a single dose) with the GABA receptor, since the introduction of the benzodiazepine antagonist Flumazenil (0.5 mg/kg) is followed by a sharp recovery in the condition of the experimental animals [134][44]. The central mechanism for action of myrtenal and its influence on GABA-ergic neurotransmission is related to the established anxiolytic properties of the substance.3.6. Neuroprotective Potential
2.6. Neuroprotective Potential
The neuroprotective potential of myrtenal was investigated in an injury model in which experimental mice were exposed to radiofrequency electromagnetic radiation during the gestational and neonatal periods. Akefe et al. (2023) administered myrtenal orally at doses of 100 and 200 mg/kg for 28 days, observing improvement in memory processes and in some biochemical parameters [140][47]. The substance improved short-term memory and spatial orientation. Additionally, it restored the activity of antioxidant enzymes, thereby rectifying the oxidative–inflammatory status within the brains of the experimental mice. The restoration of cholinergic neurotransmission and the levels of dopamine, noradrenaline, and serotonin manifested myrtenal’s neuromodulatory properties.3. Therapeutic Potential of Myrtenal Derivatives
3.1. Antitumor Potential
Zielińska-Błajet and Feder-Kubis (2020) provided an overview of diverse therapeutic effects on selected aliphatic, monocyclic, and bicyclic monoterpenes such as geraniol, thymol, myrtenal, pinene, camphor, borneol, and their modified structures [89][17]. A recent study with fourteen newly synthesized perillaldehyde and myrtenal-based benzohydrazides revealed the antiproliferative potential for two of them [145][48]. Increasingly more literature data are available regarding new modifications of these natural compounds, including their biological effects and medical applications. However, information regarding the biological and pharmacological properties of monoterpene derivatives remains limited to several review articles, published in the last ten years [91,122,146,147,148,149,150,151,152][19][32][49][50][51][52][53][54][55].
Gonda and Szakonyi (2018) reported the synthesis of 1,2,4- and 1,3,4-oxadiazole derivatives of (–)-myrtenal [157][56]. All compounds were tested in vitro for antiproliferative activity against four human malignant cell lines using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay [158][57]. One of them inhibited tumor growth, with IC50 values comparable to those of the reference cisplatin, but showed lower antiproliferative activity against the triple-negative breast cancer cell line (MDA-MB-231) compared to other cell lines used in gynecology. The remaining compounds exhibited a relatively diminished level of activity against ovarian cancer (cell line A2780).3.2. Anxiolytic Potential
Kapitsa et al. (2012) described new nitrogen-containing compounds with an adamantane–myrtenal structure and then investigated the anxiolytic activity of the resulting products in male Balb/C mice by using the elevated plus maze test [88][16].4.3. Antiviral Potential
3.3. Antiviral Potential
Teplov et al. (2013) tested in vitro the same conjugate of 2-amino adamantane and (–)-myrtenal for antiviral activity against influenza virus A/California/07/09 (H1N1)pdm09 and found that the introduction of a myrtenal fragment led to an increase in the antiviral activity of the adamantylamine derivatives against the adamantylamine-resistant virus [159][58]. The selectivity for most of the synthesized amines surpassed that for Rimantadine and Amantadine.4.4. Antifungal Potential
3.4. Antifungal Potential
Compound 10, which is a myrtenol-containing analogue of azole antifungals, demonstrated promising antifungal activity against both fluconazole-susceptible and fluconazole-resistant strains, including fluconazole-resistant clinical isolates of Candida parapsilosis and Candida glabrata, with excellent minimum inhibitory concentration in submicrogram and nanogram range. The compound was up to 100 times more active than fluconazole [160][59].4.5. Analgesic Potential
3.5. Analgesic Potential
A high analgesic effect with an active dose of 20 mg/kg was shown for myrtenal-derived diazaadamantanone [161][60]. The compound has a low acute oral toxicity with LD50 of more than 1000 mg/kg and does not cause damage to the gastric mucosa. Similar analgesic activity was demonstrated for diazaadamantane–myrtenal conjugate [162][61]. Having in mind evidences for analgesic potential of myrtenal established in previous studies, it can be concluded that this analgesic potential can be improved and extended via synthesis of some diazaadamantanone analogues of myrtenal.4.6. Memory-Improving Potential
3.6. Memory-Improving Potential
The conjugates of amino adamantane with myrtenal, in the form of hydrochlorides, studied by Kapitsa (2012) [88][16] and Teplov (2013) [159][58], showed the potential to influence memory processes in intact rats after 11 days of repeated intraperitoneal administration at a dose of 1 mg/kg [163][62]. The two compounds of amino adamantane with myrtenal investigated in this study exhibited antiacetylcholinesterase activity. Additionally, they demonstrated the capability to influence the levels of norepinephrine and serotonin in the cerebral cortex and hippocampus of the experimental animals. Notably, one of these substances also displayed antidepressant potential, which can be attributed to the induced increase in brain monoamines concentrations, the reduced levels of which are associated with depressive conditions.4. Conclusions
As a natural compound with a wide range in biological activities, myrtenal can have future implementation in medical practice. The chemical modification of this monoterpenoid with some pharmacophores will allow the enhancement of its essential properties, thereby augmenting its therapeutic potential.References
- Ghosh, S.; Roy, K.; Pal, C. Terpenoids against Infectious Diseases; Roy, D., Ed.; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2019; 270p, ISBN 3 978-0-8153-7066-6.
- De Alvarenga, J.F.R.; Genaro, B.; Costa, B.L.; Purgatto, E.; Manach, C.; Fiamoncini, J. Monoterpenes: Current knowledge on food source, metabolism, and health effects. Crit. Rev. Food Sci. Nutr. 2021, 63, 1352–1389.
- Shen, Y.; Sun, Z.; Guo, X. Citral inhibits lipopolysaccharide-induced acute lung injury by activating PPAR-γ. Eur. J. Pharmacol. 2015, 747, 45–51.
- Salgado, P.R.R.; Da Fonsêca, D.V.; Braga, R.M.; De Melo, C.G.F.; Andrade, L.N.; De Almeida, R.N.; De Sousa, D.P. Comparative Anticonvulsant Study of Epoxycarvone Stereoisomers. Molecules 2015, 20, 19660–19673.
- Ribeiro-Filho, H.V.; de Silva, C.M.; de Siqueira, R.J.B.; Lahlou, S.; dos Santos, A.A.; Magalhães, P.J.C. Biphasic cardiovascular and respiratory effects induced by β-citronellol. Eur. J. Pharmacol. 2016, 775, 96–105.
- Camargo, S.B.; Simões, L.O.; de Medeiros, A.C.F.; de Jesus, M.A.; Fregoneze, J.B.; Evangelista, A.; Villarreal, C.F.; de Araújo, S.A.A.; Quintans, L.J.; Silva, D.F. Antihypertensive potential of linalool and linalool complexed with β-cyclodextrin: Effects of subchronic treatment on blood pressure and vascular reactivity. Biochem. Pharmacol. 2018, 151, 38–46.
- Nuutinen, T. Medicinal properties of terpenes found in Cannabis sativa and Humulus lupulus. Eur. J. Med. Chem. 2018, 157, 198–228.
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471.
- Iftikhar, F.; Khan, M.B.N.; Musharraf, S.G. Monoterpenes as therapeutic candidates to induce fetal hemoglobin synthesis and up-regulation of gamma-globin gene: An in vitro and in vivo investigation. Eur. J. Pharmacol. 2021, 891, 173700.
- Wojtunik-Kulesza, K.; Rudkowska, M.; Kasprzak-Drozd, K.; Oniszczuk, A.; Borowicz-Reutt, K. Activity of Selected Group of Monoterpenes in Alzheimer’s Disease Symptoms in Experimental Model Studies—A Non-Systematic Review. Int. J. Mol. Sci. 2021, 22, 7366.
- Paulino, B.N.; da Silva, G.N.S.; Araújo, F.F.; Néri-Numa, I.A.; Pastore, G.M.; Bicas, J.L.; Molina, G. Beyond natural aromas: The bioactive and technological potential of monoterpenes. Trends Food Sci. Technol. 2022, 128, 188–201.
- Piccialli, I.; Tedeschi, V.; Caputo, L.; D’errico, S.; Ciccone, R.; De Feo, V.; Secondo, A.; Pannaccione, A. Exploring the Therapeutic Potential of Phytochemicals in Alzheimer’s Disease: Focus on Polyphenols and Monoterpenes. Front. Pharmacol. 2022, 13, 876614.
- Yang, J.; Zhong, C.; Yu, J. Natural Monoterpenes as Potential Therapeutic Agents against Atherosclerosis. Int. J. Mol. Sci. 2023, 24, 2429.
- Shi, Y.-H.; Zhu, S.; Ge, Y.-W.; He, Y.-M.; Kazuma, K.; Wang, Z.; Yoshimatsu, K.; Komatsu, K. Monoterpene derivatives with anti-allergic activity from red peony root, the root of Paeonia lactiflora. Fitoterapia 2016, 108, 55–61.
- Salakhutdinov, N.F.; Volcho, K.P.; Yarovaya, O.I. Monoterpenes as a renewable source of biologically active compounds. Pure Appl. Chem. 2017, 89, 1105–1117.
- Kapitsa, I.G.; Suslov, E.V.; Teplov, G.V.; Korchagina, D.V.; Komarova, N.I.; Volcho, K.P.; Voronina, T.A.; Shevela, A.I.; Salakhutdinov, N.F. Synthesis and anxiolytic activity of 2-aminoadamantane derivatives containing monoterpene fragments. Pharm. Chem. J. 2012, 46, 263–265.
- Zielińska-Błajet, M.; Feder-Kubis, J. Monoterpenes and Their Derivatives—Recent Development in Biological and Medical Applications. Int. J. Mol. Sci. 2020, 21, 7078.
- Silva, E.A.P.; Santos, D.M.; de Carvalho, F.O.; Menezes, I.A.C.; Barreto, A.S.; Souza, D.S.; Quintans-Júnior, L.J.; Santos, M.R.V. Monoterpenes and their derivatives as agents for cardiovascular disease management: A systematic review and meta-analysis. Phytomedicine 2021, 88, 153451.
- Bergman, M.E.; Franks, A.E.; Phillips, M.A. Biosynthesis, natural distribution, and biological activities of acyclic monoterpenes and their derivatives. Phytochem. Rev. 2023, 22, 361–384.
- Khomenko, T.M.; Zarubaev, V.V.; Orshanskaya, I.R.; Kadyrova, R.A.; Sannikova, V.A.; Korchagina, D.V.; Volcho, K.P.; Salakhutdinov, N.F. Anti-influenza activity of monoterpene-containing substituted coumarins. Bioorg. Med. Chem. Lett. 2017, 27, 2920–2925.
- Khomenko, T.M.; Shtro, A.A.; Galochkina, A.V.; Nikolaeva, Y.V.; Garshinina, A.V.; Borisevich, S.S.; Korchagina, D.V.; Volcho, K.P.; Salakhutdinov, N.F. New Inhibitors of Respiratory Syncytial Virus (RSV) Replication Based on Monoterpene-Substituted Arylcoumarins. Molecules 2023, 28, 2673.
- Khomenko, T.M.; Zakharenko, A.L.; Chepanova, A.A.; Ilina, E.S.; Zakharova, O.D.; Kaledin, V.I.; Nikolin, V.P.; Popova, N.A.; Korchagina, D.V.; Reynisson, J.; et al. Promising New Inhibitors of Tyrosyl-DNA Phosphodiesterase I (Tdp 1) Combining 4-Arylcoumarin and Monoterpenoid Moieties as Components of Complex Antitumor Therapy. Int. J. Mol. Sci. 2020, 21, 126.
- Khomenko, T.M.; Zakharenko, A.L.; Kornienko, T.E.; Chepanova, A.A.; Dyrkheeva, N.S.; Artemova, A.O.; Korchagina, D.V.; Achara, C.; Curtis, A.; Reynisson, J.; et al. New 5-Hydroxycoumarin-Based Tyrosyl-DNA Phosphodiesterase I Inhibitors Sensitize Tumor Cell Line to Topotecan. Int. J. Mol. Sci. 2023, 24, 9155.
- Munkuev, A.A.; Dyrkheeva, N.S.; Kornienko, T.E.; Ilina, E.S.; Ivankin, D.I.; Suslov, E.V.; Korchagina, D.V.; Gatilov, Y.V.; Zakharenko, A.L.; Malakhova, A.A.; et al. Adamantane-Monoterpenoid Conjugates Linked via Heterocyclic Linkers Enhance the Cytotoxic Effect of Topotecan. Molecules 2022, 27, 3374.
- Ivankin, D.I.; Kornienko, T.E.; Mikhailova, M.A.; Dyrkheeva, N.S.; Zakharenko, A.L.; Achara, C.; Reynisson, J.; Golyshev, V.M.; Luzina, O.A.; Volcho, K.P.; et al. Novel TDP1 Inhibitors: Disubstituted Thiazolidine-2,4-Diones Containing Monoterpene Moieties. Int. J. Mol. Sci. 2023, 24, 3834.
- Cardoso, D.S.P.; Kincses, A.; Nové, M.; Spengler, G.; Mulhovo, S.; Aires-De-Sousa, J.; dos Santos, D.J.V.A.; Ferreira, M.-U. Alkylated monoterpene indole alkaloid derivatives as potent P-glycoprotein inhibitors in resistant cancer cells. Eur. J. Med. Chem. 2021, 210, 112985.
- Paterna, A.; Borralho, P.M.; Gomes, S.E.; Mulhovo, S.; Rodrigues, C.M.; Ferreira, M.-J.U. Monoterpene indole alkaloid hydrazone derivatives with apoptosis inducing activity in human HCT116 colon and HepG2 liver carcinoma cells. Bioorg. Med. Chem. Lett. 2015, 25, 3556–3559.
- Hardie, J.; Isaacs, R.; Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M. Methyl salicylate and (−)-(1R,5S)-myrtenal are plant-derived repellents for black bean aphid, Aphis fabae Scop. (Homoptera: Aphididae). J. Chem. Ecol. 1994, 20, 2847–2855.
- Negoi, A.; Parvulescu, V.I.; Tudorache, M. Peroxidase-based biocatalysis in a two-phase system for allylic oxidation of α-pinene. Catal. Today 2017, 306, 199–206.
- Ishida, T.; Toyota, M.; Asakawa, Y. Terpenoid biotransformation in mammals. V. Metabolism of (+)-citronellal, (±)-7-hydroxycitronellal, citral, (−)-perillaldehyde, (−)-myrtenal, cuminaldehyde, thujone, and (±)-carvone in rabbits. Xenobiotica 1989, 19, 843–855.
- Scheline, R. Handbook of Mammalian Metabolism of Plant Compounds; CRC Press: Boca Raton, FL, USA, 1991; 522p, Available online: https://books.google.bg/books?id=LwZDDwAAQBAJ&printsec=frontcover&hl=bg#v=onepage&q&f=false (accessed on 4 June 2019).
- Vegezzi, D. United States Patent 1980. Available online: http://www.google.fr/patents/US4190675?hl=fr&dq=myrt%C3%A9nal#v=onepage&q&f=false (accessed on 11 November 2017).
- Dragomanova, S.; Tancheva, L.; Georgieva, M. A review: Biological activity of myrtenal and some myrtenal-containing medicinal plant essential oils. Scr. Sci. Pharm. 2018, 31, 22–33.
- Rathinam, A.; Pari, L. Myrtenal ameliorates hyperglycemia by enhancing GLUT2 through Akt in the skeletal muscle and liver of diabetic rats. Chem. Biol. Interact. 2016, 256, 161–166.
- Babu, H.L.; Perumal, S.; Balasubramanian, M.P. Myrtenal, a natural monoterpene, down-regulates TNF-α expression and suppresses carcinogen-induced hepatocellular carcinoma in rats. Mol. Cell. Biochem. 2012, 369, 183–193.
- Babu, L.H.; Perumal, S.; Balasubramanian, M.P. Myrtenal attenuates diethylnitrosamine-induced hepatocellular carcinoma in rats by stabilizing intrinsic antioxidants and modulating apoptotic and anti-apoptotic cascades. Cell. Oncol. 2012, 35, 269–283.
- Babu, L.H.; Natarajan, N.; Thamaraiselvan, R.; Srinivasan, P.; Periyasamy, B.M. Myrtenal ameliorates diethylnitrosamine-induced hepatocarcinogenesis through the activation of tumor suppressor protein p53 and regulation of lysosomal and mitochondrial enzymes. Fundam. Clin. Pharmacol. 2013, 27, 443–454.
- Venkatachalam, S.; Boobathi, L.; Balasubramanian, M.P. Salubrious therapeutic efficacy of myrtenal on colon carcinoma induced by 1,2-dimethyl-hydrazine studied in experimental albino rats. Res. J. Pharmacol. Pharmacodyn. 2014, 6, 146–152. Available online: https://rjppd.org/AbstractView.aspx?PID=2014-6-3-17 (accessed on 30 October 2018).
- Martins, B.X.; Arruda, R.F.; Costa, G.A.; Jerdy, H.; de Souza, S.B.; Santos, J.M.; de Freitas, W.R.; Kanashiro, M.M.; de Carvalho, E.C.Q.; Sant’Anna, N.F.; et al. Myrtenal-induced V-ATPase inhibition—A toxicity mechanism behind tumor cell death and suppressed migration and invasion in melanoma. BBA-Gen. Subj. 2019, 1863, 1–12.
- Lokeshkumar, B.; Sathishkumar, V.; Nandakumar, N.; Rengarajan, T.; Madankumar, A.; Balasubramanian, M.P. Anti-Oxidative Effect of Myrtenal in Prevention and Treatment of Colon Cancer Induced by 1, 2-Dimethyl Hydrazine (DMH) in Experimental Animals. Biomol. Ther. 2015, 23, 471–478.
- Lokeshkumar, B.; Sathishkumar, V.; Nandakumar, N.; Rengarajan, T.; Madankumar, A.; Balasubramanian, M.P. Chemopreventive effect of myrtenal on bacterial enzyme activity and the development of 1,2-dimethyl hydrazine-induced aberrant crypt foci in Wistar Rats. J. Food Drug Anal. 2016, 24, 206–213.
- Guimarães, A.G.; Quintans, J.S.; Quintans-Júnior, L.J. Monoterpenes with Analgesic Activity—A Systematic Review. Phytother. Res. 2013, 27, 1–15.
- Silva, R.O.; Salvadori, M.S.; Sousa, F.B.M.; Santos, M.S.; Carvalho, N.S.; Sousa, D.P.; Gomes, B.S.; Oliveira, F.A.; Barbosa, A.L.R.; Freitas, R.M.; et al. Evaluation of the anti-inflammatory and antinociceptive effects of myrtenol, a plant-derived monoterpene alcohol, in mice. Flavour Fragr. J. 2014, 29, 184–192.
- Dragomanova, S. Pharmacological, Toxicological and Neurobiological Studies of Myrtenal—A Bicyclic Monoterpenoid of Natural Origin. Ph.D. Thesis, Institute of Neurobiology at Bulgarian Academy of Sciences, Sofia, Bulgaria, 2020. Available online: https://ras.nacid.bg/api/reg/FilesStorage?key=6b37588e-6c9b-4708-bb22-e5872dca6bd3&mimeType=application/pdf&fileName=Authoreferate%20S%20Dragomanova.pdf&dbId=1 (accessed on 15 September 2020).
- Hosseinzadeh, H.; Khoshdel, M.; Ghorbani, M. Antinociceptive, Anti-inflammatory Effects and Acute Toxicity of Aqueous and Ethanolic Extracts of Myrtus communis L. Aerial Parts in Mice. J. Acupunct. Meridian Stud. 2011, 4, 242–247.
- Li, W.-X.; Qian, P.; Guo, Y.-T.; Gu, L.; Jurat, J.; Bai, Y.; Zhang, D.-F. Myrtenal and β-caryophyllene oxide screened from Liquidambaris Fructus suppress NLRP3 inflammasome components in rheumatoid arthritis. BMC Complement. Med. Ther. 2021, 21, 242.
- Akefe, I.; Nyan, E.; Adegoke, V.; Lamidi, I.; Ameh, M.; Chidiebere, U.; Ubah, S.; Ajayi, I. Myrtenal improves memory deficits in mice exposed to radiofrequency-electromagnetic radiation during gestational and neonatal development via enhancing oxido-inflammatory, and neurotransmitter functions. Heliyon 2023, 9, e15321.
- Garberová, M.; Potočňák, I.; Tvrdoňová, M.; Bago-Pilátová, M.; Bekešová, S.; Kudličková, Z.; Samoľová, E.; Kešeľáková, A.; Elečko, J.; Vilková, M. Spectral, structural, and pharmacological studies of perillaldehyde and myrtenal based benzohydrazides. J. Mol. Struct. 2023, 1271, 134112.
- Koziol, A.; Stryjewska, A.; Librowski, T.; Salat, K.; Gawel, M.; Moniczewski, A.; Lochynski, S. An Overview of the Pharmacological Properties and Potential Applications of Natural Monoterpenes. Mini-Reviews Med. Chem. 2014, 14, 1156–1168.
- Barreto, R.S.S.; Albuquerque-Júnior, R.L.C.; Araújo, A.A.S.; Almeida, J.R.G.S.; Santos, M.R.V.; Barreto, A.S.; DeSantana, J.M.; Siqueira-Lima, P.S.; Quintans, J.S.S.; Quintans-Júnior, L.J. A Systematic Review of the Wound-Healing Effects of Monoterpenes and Iridoid Derivatives. Molecules 2014, 19, 846–862.
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414.
- Wojtunik-Kulesza, K.A.; Kasprzak, K.; Oniszczuk, T.; Oniszczuk, A. Natural Monoterpenes: Much More than Only a Scent. Chem. Biodivers. 2019, 16, e1900434.
- Dheer, D.; Singh, D.; Kumar, G.; Karnatak, M.; Chandra, S.; Prakash Verma, V.; Shankar, R. Thymol Chemistry: A Medicinal Toolbox. Curr. Bioact. Compd. 2019, 15, 454–474.
- Stephane, F.F.Y.; Jean Jules, B.K. Terpenoids as Important Bioactive Constituents of Essential Oils . In Essential Oils—Bioactive Compounds, New Perspectives and Applications; IntechOpen: London, UK, 2020.
- Coêlho, M.L.; Islam, M.T.; da Silva Oliveira, G.L.; de Alencar, M.V.O.B.; de Oliveira Santos, J.V.; dos Reis, A.C.; da Mata, A.M.O.F.; Paz, M.F.C.J.; Docea, A.O.; Calina, D.; et al. Cytotoxic and Antioxidant Properties of Natural Bioactive Monoterpenes Nerol, Estragole, and 3,7-Dimethyl-1-Octanol. Adv. Pharmacol. Pharm. Sci. 2022, 2022, 8002766.
- Gonda, T.; Szakonyi, Z. Stereoselective Synthesis and Application of Bi-and Trifunctional Monoterpene-based Compounds. Ph.D. Thesis, Institute of Pharmaceutical Chemistry, University of Szeged, Szeged, Hungary, 2018. Available online: http://doktori.bibl.u-szeged.hu/9832/1/Gonda20Timea20disszertacio.pdf (accessed on 10 July 2023).
- Gonda, T.; Bérdi, P.; Zupkó, I.; Fülöp, F.; Szakonyi, Z. Stereoselective Synthesis, Synthetic and Pharmacological Application of Monoterpene-Based 1,2,4- and 1,3,4-Oxadiazoles. Int. J. Mol. Sci. 2017, 19, 81.
- Teplov, G.; Suslov, E.; Zarubaev, V.; Shtro, A.; Karpinskaya, L.; Rogachev, A.; Korchagina, D.; Volcho, K.; Salakhutdinov, N.; Kiselev, O. Synthesis of New Compounds Combining Adamantanamine and Monoterpene Fragments and their Antiviral Activity Against Influenza Virus A (H1N1) pdm09. Lett. Drug Des. Discov. 2013, 10, 477–485.
- Li-Zhulanov, N.S.; Zaikova, N.P.; Sari, S.; Gülmez, D.; Sabuncuoğlu, S.; Ozadali-Sari, K.; Arikan-Akdagli, S.; Nefedov, A.A.; Rybalova, T.V.; Volcho, K.P.; et al. Rational Design of New Monoterpene-Containing Azoles and Their Antifungal Activity. Antibiotics 2023, 12, 818.
- Ponomarev, K.; Pavlova, A.; Suslov, E.; Ardashov, O.; Korchagina, D.; Nefedov, A.; Tolstikova, T.; Volcho, K.; Salakhutdinov, N. Synthesis and analgesic activity of new compounds combining azaadamantane and monoterpene moieties. Med. Chem. Res. 2015, 24, 4146–4156.
- Ponomarev, K.; Morozova, E.; Pavlova, A.; Suslov, E.; Korchagina, D.; Nefedov, A.; Tolstikova, T.; Volcho, K.; Salakhutdinov, N. Synthesis and Analgesic Activity of Amines Combining Diazaadamantane and Monoterpene Fragments. Med. Chem. 2017, 13, 773–779.
- Dragomanova, S.; Andonova, V.; Lazarova, M.; Munkuev, A.; Suslov, E.; Volcho, K.; Salakhutdinov, N.; Stefanova, M.; Gavrilova, P.; Uzunova, D.; et al. Memory-improving effects of myrtenal-adamantane conjugates. J. Chem. Technol. Metall. 2023, 58, 627–634. Available online: https://journal.uctm.edu/node/j2023-3/JCTM_2023_58_26_23-11_pp627-634.pdf (accessed on 20 August 2023).
