Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Guanchen Liu and Version 2 by Sirius Huang.
To achieve optimal growth and productivity of poultry, ensuring their health and well-being is essential. Studies have illuminated the functional roles of certain amino acids, highlighting their unique contributions to various physiological processes and the synthesis of metabolically important molecules. Methionine and arginine are two notable examples of such amino acids.
- arginine
- methionine
- intestinal health
- functional amino acids
- poultry
1. Introduction
The global poultry industry holds immense significance in meeting the continuously increasing demand for high-quality protein-rich food, driven by the continuous rise in the global population [1]. To achieve optimal growth and productivity of the birds, ensuring their health and well-being is essential.
The intestines are responsible for the digestion and absorption of essential nutrients from the feed consumed by poultry [2]. Furthermore, the intestinal tract is immensely important in the immune system of the animals. It serves as the physical barrier and also hosts abundant organized lymphoid tissue and immune effector cells, which collectively provide protection against pathogens and toxins [3][4][5][3,4,5]. Nevertheless, the intestines possess the most extensive exposed surface in the body [6]. The constant exposure to a wide range of potentially harmful substances makes them susceptible to many diseases, such as coccidiosis and necrotic enteritis, which have a significant impact on the poultry industry [7][8][7,8]. Maintaining a healthy intestinal tract has been a focus of research for decades, and this area is now gaining more attention due to the growing awareness of animal welfare, food safety, and the increasing public scrutiny towards the use of antibiotic growth promoters [9].
2. Methionine and Arginine in Intestinal Health
2.1. Methionine and Arginine in Intestinal Development and Repair
The intestinal epithelium, which comprises a single layer of epithelial cells and a protein-rich mucus layer, has one of the highest turnover rates in the body [10][56]. The renewal of epithelial cells in the intestinal epithelium involves a highly coordinated process of cellular proliferation, differentiation, migration, and apoptosis. This constant cell turnover relies on ongoing protein synthesis, which is largely mediated through the activation of mTOR pathway [11][57]. In addition to serving as essential building blocks of proteins, Met and Arg have been shown to activate mTORC1 [12][13][58,59]. Met activates the mTOR signaling pathway by promoting the methylation of phosphatase 2A through the action of SAM. Furthermore, previous studies showed that SAM could bind to the S-adenosylmethionine sensor upstream of mTORC1 (SAMTOR), counteracting its inhibitory effects on mTORC1 activity [13][14][59,60]. On the other hand, Arg activates the mTOR pathway through inhibiting the activity of tuberous sclerosis complex 2, a protein that normally suppresses the mTOR pathway [15][61]. The mTOR pathway is essential for maintaining intestinal epithelial cell proliferation during both homeostasis and regeneration, as research showed that a disruption in this signaling pathway would lead to intestinal epithelial cell defects and hinder the intestinal regeneration [16][17][62,63]. The Wnt/β-catenin signaling pathway is also well established for its role in maintaining intestinal structure and homeostasis as it regulates the self-renewal and differentiation of the intestinal stem cells [18][19][20][64,65,66]. Interestingly, studies have also shown that both Met and Arg can activate the Wnt/β-catenin pathway to enhance the intestinal epithelial development [21][22][32,67]. Met is required for the sequestration of glycogen synthase kinase 3, which is an essential step in activating the Wnt signaling pathway [23][68]. The modulating effects of Arg on the Wnt/β-catenin signaling pathway can be attributed to the synthesis of NO, which is a known activator of this pathway [24][69]. Intriguingly, activation of the Wnt/β-catenin signaling pathway is closely regulated by the methylation of the Arg residues in Ras GTPase-activating protein-binding protein 1 (G3BP1) with SAM as the methyl donor. This linkage underscores the collaborative regulatory roles of Met and Arg in this pathway [23][25][68,70]. The activation roles of Met and Arg in both pathways contribute to the regenerative capacity and development of the intestine. Beyond their roles in pathway activation, Met and Arg are indispensable for polyamine synthesis. Arg serves as the precursor for polyamine synthesis, while SAM, a product of Met metabolism, functions as the methyl donor in this process [26][71]. Polyamines are essential for intestinal epithelial renewal and repair, ensuring its important roles in cell proliferation, development, and migration [27][28][72,73]. Studies have demonstrated that providing dividing cells in the crypts with polyamines can stimulate mucosal growth and facilitate the repair of damaged mucosa [29][30][31][74,75,76]. The proposed mechanism underlying the stimulation effect of polyamines in mucosal growth involves their ability to regulate expression of various genes encoding growth promoting and inhibiting factors [26][32][71,77]. Polyamines are also shown to be vital for the expression of tight junction and adhesion junction proteins which maintain the intestinal barrier function [33][34][78,79]. Overall, Met and Arg are essential for the development and repair of the intestinal epithelium. They contribute to this process by activating pathways essential for intestinal regeneration and by participating in polyamine synthesis.2.2. Antioxidant Effects of Methionine and Arginine on Intestinal Health
2.2.1. Oxidative Stress
The integrity of the intestinal barrier can be disrupted by various factors, among which oxidative stress is a significant contributor. Reactive oxygen species (ROS) are byproducts generated during normal metabolic processes [35][36][80,81]. Under normal conditions, the production of ROS is balanced by the antioxidant system [37][82]. However, an excessive production of ROS or a decline in antioxidant defenses can disrupt this equilibrium, leading to the accumulation of ROS. The highly reactive ROS will react with the cellular components causing cellular damage, dysfunction, and apoptosis, which ultimately leads to impaired organ functions and the development of oxidative stress [35][80]. Several factors during poultry production can cause oxidative stress to the birds, such as nutritional factors like nutrient imbalances and feed toxins, environmental factors like heat stress and stocking density stress, as well as pathological factors [38][39][40][83,84,85]. Oxidative stress can damage the structure and function of tight junctions, resulting in compromised intestinal barrier functions and increased permeability [41][86]. Oxidative stress is also known to damage the intestinal epithelial cells directly, leading to further disruption of the barrier function and inflammation. The recruited macrophage and heterophils intensify the production of ROS, stimulating a positive feedback loop that exacerbates oxidative stress and inflammation [42][43][87,88]. Oxidative stress is also reported to cause morphometric changes in the intestinal tract by reducing the villi height and lowering the villus: crypt ratio, interfering with nutrient absorption [44][45][89,90].2.2.2. Antioxidant Effects of Methionine and Arginine
Methionine is reported to exhibit potent antioxidant capacity through two major mechanisms [46][47][91,92]. Firstly, it produces antioxidant metabolites by undergoing metabolic processes. SAM, as one of the metabolites in the pathway, is not only an important methyl donor, but also a key metabolite modifying antioxidant enzymes, such as superoxide dismutase (SOD) and catalase (CAT) [46][47][91,92]. Such modification effects were confirmed by different research groups observing increased activities of antioxidant enzymes in birds fed Met supplemented diets [48][49][50][51][52][93,94,95,96,97]. Cysteine, another sulfur-containing amino acid, also exhibits antioxidant ability and is further metabolized to GSH, a well-known intrinsic antioxidant. Previous studies have also demonstrated increased GSH or improved GSH: glutathione disulfide (GSSG) ratio in broilers fed Met supplemented diets [39][53][54][84,98,99]. Secondly, Met residues in proteins can directly scavenge ROS. As a sulfur-containing amino acid, Met residues on the surface of proteins are readily oxidized. By scavenging ROS and being oxidized into methionine sulfoxide, Met can protect other critical components from oxidation, thus maintaining their integrity and function [25][55][17,70]. Furthermore, methionine sulfoxide can be reduced by methionine sulfoxide reductases (MSR) back to Met to restore its antioxidant capacity [56][57][58][20,100,101]. The antioxidant capacity of methionine and its metabolites is presented in Figure 1.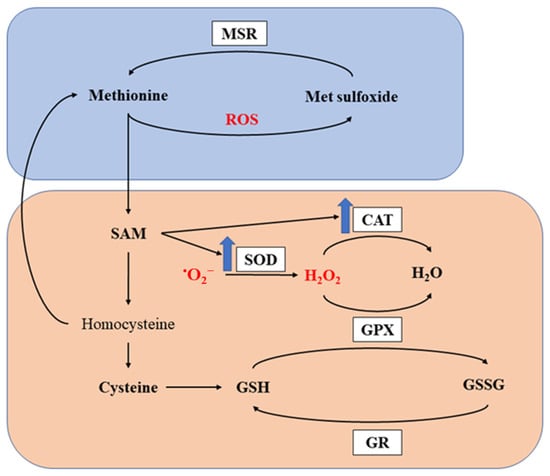
Figure 1. Antioxidant capacity of methionine. MSR, methionine sulfoxide reductase; SOD, superoxide dismutase; CAT, catalase; GPX, glutathione peroxidase; GR, glutathione reductase; ROS, reactive oxygen species; SAM, S-adenosylmethionine; GSH, glutathione; GSSG, glutathione disulfide. The orange and boxed represents the first and second mechanism for methionine to exert its antioxidant capacity. The blue arrows indicate upregulation of the enzymes.
