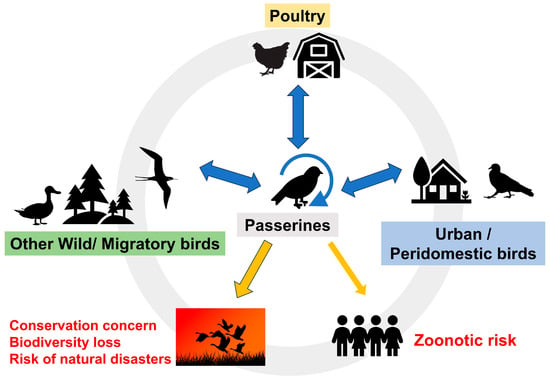
Interest in emerging viruses is growing because some can cause serious or lethal disease in humans and animals. The number of cloacal virome studies is also growing, however, these usually focus on poultry and other domestic birds, These studies reveal a wide variety of viruses, although the pathogenic significance of most newly discovered viruses is uncertain. Analysis of viruses detected in wild birds is complex and often biased towards waterfowl because of the obvious interest in avian influenza or other zoonotic viruses. Less is known about the viruses present in the order Passeriformes, which comprises approximately 60% of extant bird species. This review aims to compile the most significant contributions, from traditional and metagenomic studies, on the viruses that affect passerines. It highlights most passerine species have never been sampled. Some viruses, especially Flaviviridae, Orthomyxoviridae, Poxviridae and Togaviridae, and arguably others, are considered emerging because of increased incidence or avian mortality/morbidity, spread to new geographical areas or hosts and their zoonotic risk. However, many of these viruses have only recently been described in passerines using metagenomics and their role in the ecosystem is unknown.
- biodiversity
- DNA viruses
- emergence
- metagenomic studies
- passeriformes
- RNAviruses
- spillover
- zoonoses
1. Introduction
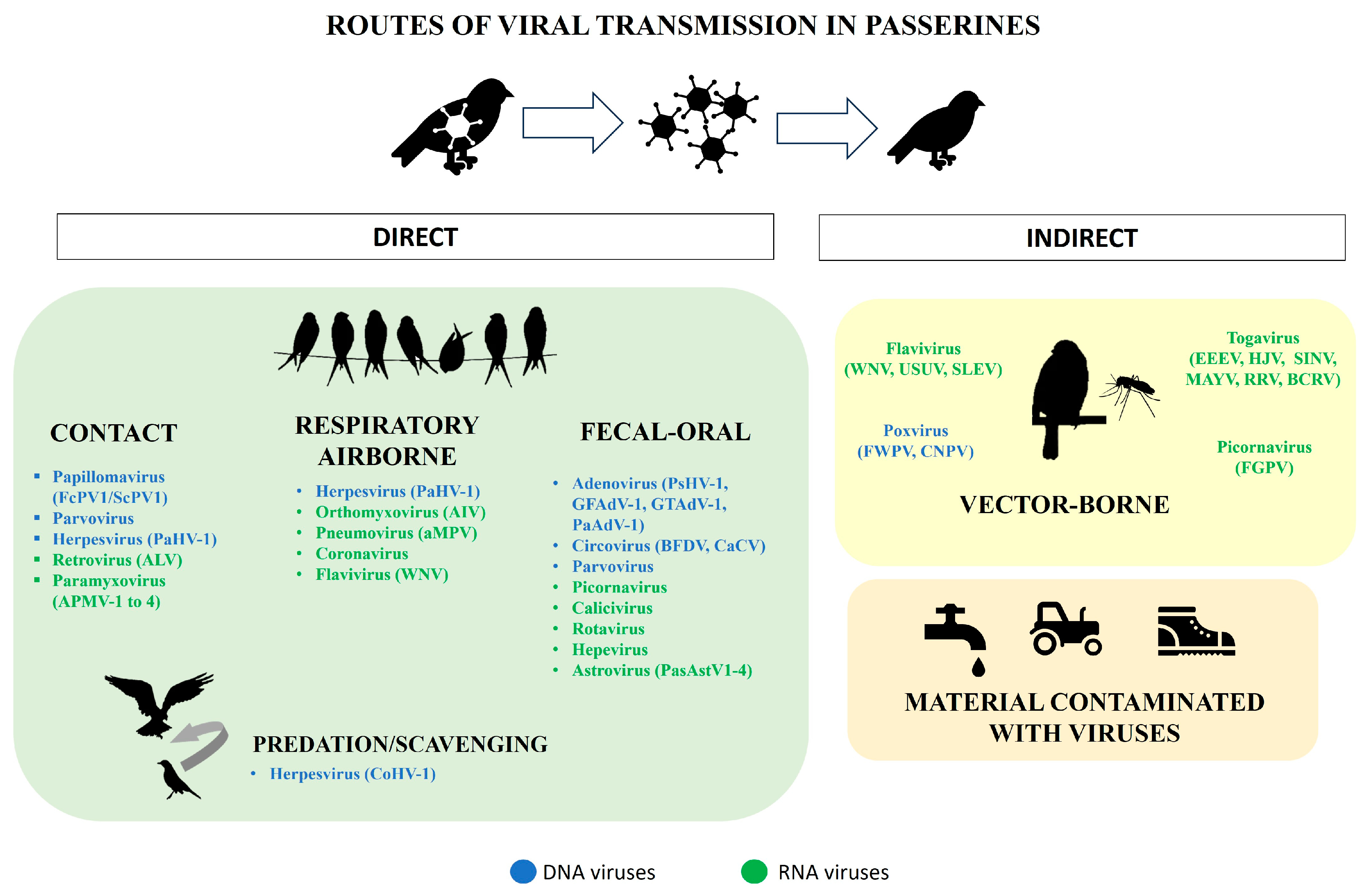
2. Families of Viruses Affecting Passeriformes
| Viral Family | Genus | Viral Taxa | Abbreviations | Nomenclature | Bird Family | Clinical Signs | Mortality Rate | References |
|---|---|---|---|---|---|---|---|---|
| dsDNA VIRUSES | ||||||||
| Herpesviridae | Iltovirus | Gallid herpesvirus 1 | GaHV-1 | Iltovirus gallidalpha1 | Turdidae, Estrildidae, Sturnidae | Subclinical infection/ Respiratory signs |
High | [7,26,34,7][25]35,[26]37][[27][28] |
| Psittacid herpesvirus 1 | PsHV-1 | Iltovirus psittacidalpha1 | Sturnidae | |||||
| passerid herpesvirus 1 | not recognized | Fringillidae | ||||||
| Mardivirus | Columbid herpesvirus 1 | CoHV-1 | Mardivirus columbidalpha1 | Corvidae, Turdidae | Subclinical infection | Probably not | [40][29] | |
| Poxviridae | Avipoxvirus | Fowlpox virus (Lineage A1) | FWPV | same | Fringillidae, Passeridae | Cutaneous lesions/Upper respiratory and digestive tract lesions | High | [42,43,53,54][30][44,3145,46,][3247,][3348,][3449,]35][36][3750,][3851,[][52,39][40][41][42] |
| Pigeonpox virus (Lineage A2) | PGPV | same | Fringillidae | High/Medium | ||||
| Accipitriformespox virus (Lineage A7) | same | Lanidae | High/Medium | |||||
| Canarypox virus (Lineage B1) | CNPV | same | Fringillidae, Paridae, Corvidae (and 11 other families) | High/Medium | ||||
| Starlingpox virus (Lineage B2) | same | Sturnidae, Passeridae | High/Medium | |||||
| Lineage B3 poxvirus | not recognized | Corvidae (and eight other mostly Passeriformes families) | High | |||||
| Adenoviridae | Atadenovirus | European robin adenovirus | not recognized | Meliphagidae, Fringillidae, Ploeceidae, Estrildidae, Turdidae, Ptilonorhynchidae, Sturnidae, Zosteropidae, Maluridae, Petroicidae | Subclinical infection/ Not known |
Probably not | [57,58][43][44] | |
| European greenfinch adenovirus | not recognized | |||||||
| Eurasian siskin adenovirus | not recognized | |||||||
| Eurasian bullfinch adenovirus | not recognized | |||||||
| common chaffinch adenovirus | not recognized | |||||||
| passerine adenovirus 1 | not recognized | |||||||
| white plumed honeyeater adenovirus 1, -2 | not recognized | |||||||
| vitelline masked weaver adenovirus strains 37869 and 38132 | not recognized | |||||||
| Aviadenovirus | great tit adenovirus strain 47292 | not recognized | Fringillidae, Estrildidae, Paridae, Ploceidae, Sylvidae, Falcunculidae, Petroicidae | Subclinical infection/ Not known |
Probably not | |||
| european greenfinch adenovirus | not recognized | |||||||
| goldfinch adenovirus | not recognized | |||||||
| vitelline masked weaver adenovirus strain 39658 | not recognized | |||||||
| Siadenovirus | double-barred finch adenovirus | not recognized | Estrildidae, Paridae, Sylvidae, Thraupidae, Meliphagidae, Pardalotidae, Rhipiduridae, Sturnidae | Subclinical infection/ Probable renal lesions |
Probably not | [56,60][45][46] | ||
| eurasian blackcap adenovirus | not recognized | |||||||
| gouldian finch adenovirus 1 | GFAdV-1 | not recognized | ||||||
| great tit adenovirus strain 47292 | GTAdV-1 | Great tit siadenovirus A | ||||||
| zebra finch adenovirus strain 47535 | not recognized | |||||||
| Papillomaviridae | Etapapillomavirus | Fringilla coelebs Papillomavirus 1 | FcPV1 | Etapapillomavirus 1 | Fringillidae | Cutaneous lesions | Low | [66,4767,][4869,][4970,]71,72][[50][51][52] |
| Serinus canaria papillomavirus 1 | ScPV1 | not recognized | Fringillidae | Not known | Not known | [68][53] | ||
| ssDNA VIRUSES | ||||||||
| Circoviridae | Circovirus | Beak and feather disease virus | BFDV | Circovirus parrot | Artamidae, Corvidae, Estrildidae, Fringillidae, Petoicidae, Sturnidae, Thamnophilidae | Feather lesions | Probably not | [76,77,78][54][55][56] |
| Circovirus canary | CaCV | same | Feather diseases and immunosupression/ Subclinical infection |
Yes/Not known | [25,79,80,81,82][57][58][59][60][61] | |||
| Circovirus finch | FiCV | same | ||||||
| Circovirus raven | RaCV | same | ||||||
| Circovirus starling | StCV | same | ||||||
| Circovirus zebrafinch | ZfiCV | same | ||||||
| Cyclovirus | Robinz virus RP_736 | RobinzV736 | Cyclovirus cervienka | Petroicidae | Not known | Not known | [17] | |
| Robinz virus RP_1170 | RobinzV1170 | Cyclovirus liepsnele | ||||||
| Robinz virus RP_493 | RobinzV493 | Cyclovirus pettirosso | ||||||
| Robinz virus RP_620 | RobinzV620 | Cyclovirus prihor | ||||||
| Robinz virus RP_526 | RobinzV526 | Cyclovirus punarinta | ||||||
| Robinz virus RP_584 | RobinzV584 | Cyclovirus rudzik | ||||||
| Robinz virus RP_259 | RobinzV259 | Cyclovirus totoi | ||||||
| Probably new genus | Gyrovirus 11 | not recognized | Thamnophilidae | Not known | Not known | [89][62] | ||
| Genomoviridae | Gemycirculavirus | new species | not recognized | Petroicidae, Aegithalidae, Corvidae, Emberizidae, Fringillidae, Muscicapidae, Paridae, Sylviidae, Turdidae, Zosteropidae | Not known | Not known | [88][63] | |
| Chickadee associated gemycircularvirus 1 | Gemycircularvirus chickad1 | Paridae | [85][64] | |||||
| Finch associated genomovirus 5 | Gemycircularvirus haeme1 | Fringillidae | [86][65] | |||||
| Finch associated genomovirus 6 | Gemycircularvirus haeme2 | [86][65] | ||||||
| Gemykibivirus | Blackbird associated gemykibivirus 1 | Gemykibivirus blabi1 | Turdidae | [87][66] | ||||
| Black robin associated gemykibivirus 1 | Gemykibivirus blaro1 | Petroicidae | ||||||
| Finch associated genomovirus 3 | Gemykibivirus haeme1 | Fringillidae | [86][65] | |||||
| Finch associated genomovirus 2 | Gemykibivirus haeme3 | |||||||
| Finch associated genomovirus 4 | Gemykibivirus haeme4 | |||||||
| Finch associated genomovirus 1 | Gemykibivirus haeme5 | |||||||
| Gemykroznavirus | Finch associated genomovirus 8 | Gemykronzavirus haeme1 | Fringillidae | |||||
| Gemygorvirus | Starling associated gemygorvirus 1 | Gemygorvirus stara1 | Sturnidae | [87][66] | ||||
| Parvoviridae | Aveparvovirus | Aveparvovirus passeriform1 | PfPV | same | Thraupidae | Not known | Not known | [95][67] |
| ssRNA VIRUSES | ||||||||
| Orthomyxoviridae | Alphainfluenzavirus 1 | Highly Pathogenic Avian Influenza H5N1 | FLUAV/(H5N1) | Alphainfluenzavirus influenzae | Passeridae, Turdidae, Ploceidae, Corvidae, Hirundidae, Icteridae (and many other families) | Mild/Severe disease | High | [101,102,103,104,105,106,107,108,110,116][68][69][70][71][72][73][74][75][76][77] |
| Paramyxoviridae | Orthoavulavirus | Avian paramyxovirus 1 (NDV, Newcastle Disease virus) | APMV-1 | Orthoavulavirus javaense | Passeridae, Motacillidae, Passarellidae | Not known/Subclinical disease | High/Not known | [113,123,124,126,127][78][79][80][81][82] |
| Metaavulavirus | Avian paramyxovirus 2 | APMV-2 | Metaavulavirus yucaipaense | Corvidae, Motacillidae, Troglodytidae, Muscicapidae, Emberizidae, Estrildidae | Mild/Severe disease | [128,83129,130,][84131,][85132,]133,134][[86][87][88][89] | ||
| Paraavulavirus | Avian paramyxovirus 3 | APMV-3 | Paraavulavirus wisconsinense | [135,136][90][91] | ||||
| Paraavulavirus | Avian paramyxovirus 4 | APMV-4 | Paraavulavirus hongkongense | [138][92] | ||||
| Pneumoviridae | Metapneumovirus | Avian metapneumovirus | AMPV | Metapneumovirus avis | Passeridae, Sturnidae | Not known | Not known | [141,142][93][94] |
| Bornaviridae | Orthobornavirus | Estrildid finch bornavirus 1 | EsBV-1 | Orthobornavirus estrildidae | Estrildidae, Corvidae, Fringillidae | Not known | Not known | [146,147,148,[96][97149][95]][98] |
| Canary bornavirus 1 | CnBV-1 | Orthobornavirus serini | ||||||
| Canary bornavirus 2 | CnBV-2 | |||||||
| Canary bornavirus 3 | CnBV-3 | |||||||
| Flaviviridae | Orthoflavivirus 1 | Japanese encephalitis virus | JEV | Orthoflavivirus japonicum | Passeridae, Corvidae, Fringillidae, Turdidae (and >23 more families) | Not known | High | [183][99] |
| Saint Louis encephalitis virus | SLEV | Orthoflavivirus louisense | [191,192,193,194][100][101][102][103] | |||||
| Murray Valley Encephalitis virus | MVEV | Orthoflavivirus murrayense | [186][104] | |||||
| Usutu virus | USUV | Orthoflavivirus usutuense | Severe disease | [174,175,176][105][106][107] | ||||
| West Nile virus | WNV | Orthoflavivirus nilense | Mild/Severe disease | [158,159,160,161,162,169][108][109][110][111][112][113] | ||||
| Togaviridae | Alphavirus 1 | Eastern equine encephalitis virus | EEEV | same | Turdidae, Hirundidae, Icteridae, Petroicidae, Estrildidae, Monarchidae | Neurological disease | Yes | [206,207,208][114][115][116] |
| Highlands J virus | HJV | same | Mild disease | [210][117] | ||||
| Sindbis virus | SINV | same | Severe disease | [215,[118216]][119] | ||||
| Ross River virus | RRV | same | Not known | [219,220][120][121] | ||||
| Mayaro virus | MAYV | same | [225,226,227,228][122][123][124][125] | |||||
| Buggy Creek virus | BUCGV | Fort Morgan virus | Neurological disease | [230,231,232][126][127][128] | ||||
| Deltacoronavirus 2 | Bulbul coronavirus HKU11 | BulCV_HKU11 | same | Turdidae, Estrildidae, Pycnonotidae, Passeridae, Zosteropidae, Fringillidae, Emeberizidae | Severe disease | Probably high | [239,240,241][129][130][131] | |
| Munia coronavirus HKU13 | MunCV_HKU13 | same | ||||||
| Thrush coronavirus | not recognized | |||||||
| White-eye coronavirus HKU16 | WECoV_HKU16 | same | ||||||
| Coronavirus HKU15 | PoCoV_HKU15 | same | ||||||
| Picornaviridae | Oscivirus | Oscivirus A1 and A2 (turdivirus 2 and 3) | OsV-A1/OsV-A2 | Oscivirus A | Turdidae, Muscicapidae | Severe disease | Not known | [247,248][132][133] |
| Passerivirus | Passerivirus A and B | PasV-A/PasV-B | same | Turdidae, Estrildidae | High | |||
| Poecivirus | Poecivirus A1 | PoeV-A | Poecivirus A | Paridae | Yes | [251,252][134][135] | ||
| Probably new genus | French Guiana Picornavirus | FGPV | not recognized | Thamnophilidae | Not known | Not known | [13] | |
| Astroviridae | Avastovirus | novel avastrovirus clades 4 and 5 | not recognized | Fringillidae, Monarchidae Parulidae, Passeridae | Subclinical infectionMild disease | Not known | [258,259,260][136][137][138] | |
| Caliciviridae | Probably new genera | new calicivirus | not recognized | Prunellidae, Turdidae, Emberizidae, Fringillidae, Muscicapidae, Petroicidae | Subclinical infection | Not known | [7,15,275,276][7][15][139][140] | |
| Hepeviridae | Probably new genera | new hepe-like viruses | not recognized | Thamnophilidae, Turdidae, Zosteropidae | Not known | Not known | [7,13,15][7][13][15] | |
| dsRNA VIRUSES | ||||||||
| Reoviridae | Orthoreovirus 2 | new orthoreovirus | not recognized | Emberizidae, Turdidae, Fringillidae | Not known | High mortality | [283,284][141][142] | |
| RNA REVERSE TRANSCRIBING VIRUS | ||||||||
| Retroviridae | Alpharetrovirus | Avian leukosis virus | ALV | same | Emberizidae, Paridae, Corvidae, Muscicapidae, Thraupidae, Phylloscopidae | Subclinical infection | Possibly | [291,292,293][143][144][145] |
References
- Burrell, C.J.; Howard, C.R.; Murphy, F.A. (Eds.) Chapter 15—Emerging Virus Diseases. In Fenner and White’s Medical Virology, 5th ed.; Academic Press: London, UK, 2017; pp. 217–225. ISBN 978-0-12-375156-0.
- Daly, J.M. Zoonosis, Emerging and Re-Emerging Viral Diseases. In Encyclopedia of Virology; Bamford, D., Zuckerman, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 569–576. ISBN 978-0-12-814516-6.
- Ertunc, F. Emerging Plant Viruses. In Emerging and Reemerging Viral Pathogens; Ennaji, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1041–1062. ISBN 978-0-12-819400-3.
- World Health Organization. A Brief Guide to Emerging Infectious Diseases and Zoonoses; WHO Regional Office for South-East Asia: New Delhi, India, 2014; ISBN 978-92-9022-458-7.
- World Organization for Animal Health. Terrestrial Code Online Access. Available online: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access/ (accessed on 19 June 2023).
- Yadav, M.P.; Singh, R.K.; Malik, Y.S. Emerging and Transboundary Animal Viral Diseases: Perspectives and Preparedness. In Emerging and Transboundary Animal Viruses; Malik, Y.S., Singh, R.K., Yadav, M.P., Eds.; Livestock Diseases and Management; Springer: Singapore, 2020; pp. 1–25. ISBN 9789811504020.
- French, R.K.; Filion, A.; Niebuhr, C.N.; Holmes, E.C. Metatranscriptomic Comparison of Viromes in Endemic and Introduced Passerines in New Zealand. Viruses 2022, 14, 1364.
- Manne, L.L.; Brooks, T.M.; Pimm, S.L. Relative Risk of Extinction of Passerine Birds on Continents and Islands. Nature 1999, 399, 258–261.
- Jiménez-Clavero, M.Á. Animal Viral Diseases and Global Change: Bluetongue and West Nile Fever as Paradigms. Front. Genet. 2012, 3, 105.
- Canuti, M.; Munro, H.J.; Robertson, G.J.; Kroyer, A.N.K.; Roul, S.; Ojkic, D.; Whitney, H.G.; Lang, A.S. New Insight into Avian Papillomavirus Ecology and Evolution from Characterization of Novel Wild Bird Papillomaviruses. Front. Microbiol. 2019, 10, 701.
- Chan, J.F.-W.; To, K.K.-W.; Chen, H.; Yuen, K.-Y. Cross-Species Transmission and Emergence of Novel Viruses from Birds. Curr. Opin. Virol. 2015, 10, 63–69.
- Wang, C.C.; Prather, K.A.; Sznitman, J.; Jimenez, J.L.; Lakdawala, S.S.; Tufekci, Z.; Marr, L.C. Airborne Transmission of Respiratory Viruses. Science 2021, 373, eabd9149.
- Truchado, D.A.; Llanos-Garrido, A.; Oropesa-Olmedo, D.A.; Cerrada, B.; Cea, P.; Moens, M.A.J.; Gomez-Lucia, E.; Doménech, A.; Milá, B.; Pérez-Tris, J.; et al. Comparative Metagenomics of Palearctic and Neotropical Avian Cloacal Viromes Reveal Geographic Bias in Virus Discovery. Microorganisms 2020, 8, 1869.
- Dai, Z.; Wang, H.; Wu, H.; Zhang, Q.; Ji, L.; Wang, X.; Shen, Q.; Yang, S.; Ma, X.; Shan, T.; et al. Parvovirus Dark Matter in the Cloaca of Wild Birds. GigaScience 2023, 12, giad001.
- Shan, T.; Yang, S.; Wang, H.; Wang, H.; Zhang, J.; Gong, G.; Xiao, Y.; Yang, J.; Wang, X.; Lu, J.; et al. Virome in the Cloaca of Wild and Breeding Birds Revealed a Diversity of Significant Viruses. Microbiome 2022, 10, 60.
- Chang, W.-S.; Eden, J.-S.; Hall, J.; Shi, M.; Rose, K.; Holmes, E.C. Metatranscriptomic Analysis of Virus Diversity in Urban Wild Birds with Paretic Disease. J. Virol. 2020, 94, e00606–e00620.
- Custer, J.M.; White, R.; Taylor, H.; Schmidlin, K.; Fontenele, R.S.; Stainton, D.; Kraberger, S.; Briskie, J.V.; Varsani, A. Diverse Single-Stranded DNA Viruses Identified in New Zealand (Aotearoa) South Island Robin (Petroica australis) Fecal Samples. Virology 2022, 565, 38–51.
- Woolhouse, M.; Scott, F.; Hudson, Z.; Howey, R.; Chase-Topping, M. Human Viruses: Discovery and Emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 2864–2871.
- Clements, J.; Schulenberg, T.; Iliff, M.; Billerman, S.; Fredericks, T.; Gerbracht, J.; Woods, C. Checklist of Birds of the World. Available online: https://www.birds.cornell.edu/clementschecklist/download/ (accessed on 19 July 2023).
- Callaghan, C.T.; Nakagawa, S.; Cornwell, W.K. Global Abundance Estimates for 9700 Bird Species. Proc. Natl. Acad. Sci. USA 2021, 118, e2023170118.
- Gibb, R.; Redding, D.W.; Chin, K.Q.; Donnelly, C.A.; Blackburn, T.M.; Newbold, T.; Jones, K.E. Zoonotic Host Diversity Increases in Human-Dominated Ecosystems. Nature 2020, 584, 398–402.
- Boseret, G.; Losson, B.; Mainil, J.G.; Thiry, E.; Saegerman, C. Zoonoses in Pet Birds: Review and Perspectives. Vet. Res. 2013, 44, 36.
- Ayala, A.J.; Yabsley, M.J.; Hernandez, S.M. A Review of Pathogen Transmission at the Backyard Chicken–Wild Bird Interface. Front. Vet. Sci. 2020, 7, 539925.
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Adriaenssens, E.M.; Alfenas-Zerbini, P.; Davison, A.J.; Dempsey, D.M.; Dutilh, B.E.; García, M.L.; et al. Changes to Virus Taxonomy and to the International Code of Virus Classification and Nomenclature Ratified by the International Committee on Taxonomy of Viruses (2021). Arch. Virol. 2021, 166, 2633–2648.
- Tomaszewski, E.K.; Gravendyck, M.; Kaleta, E.F.; Phalen, D.N. Genetic Characterization of a Herpesvirus Isolate from a Superb Starling (Lamprotornis superbus) as a Psittacid Herpesvirus Genotype 1. Avian Dis. 2004, 48, 212–214.
- Žlabravec, Z.; Slavec, B.; Vrezec, A.; Kuhar, U.; Zorman Rojs, O.; Golob, Z.; Račnik, J. Detection of Herpesviruses in Wild Bird Casualties in Slovenia. Front. Vet. Sci. 2022, 9, 822212.
- Žlabravec, Z.; Trilar, T.; Slavec, B.; Krapež, U.; Vrezec, A.; Rojs, O.Z.; Račnik, J. Detection of Herpesviruses in Passerine Birds Captured during Autumn Migration in Slovenia. J. Wildl. Dis. 2021, 57, 368–375.
- Paulman, A.; Lichtensteiger, C.A.; Kohrt, L.J. Outbreak of Herpesviral Conjunctivitis and Respiratory Disease in Gouldian Finches. Vet. Pathol. 2006, 43, 963–970.
- Woźniakowski, G.J.; Samorek-Salamonowicz, E.; Szymański, P.; Wencel, P.; Houszka, M. Phylogenetic Analysis of Columbid Herpesvirus-1 in Rock Pigeons, Birds of Prey and Non-Raptorial Birds in Poland. BMC Vet. Res. 2013, 9, 52.
- Lawson, B.; Lachish, S.; Colvile, K.M.; Durrant, C.; Peck, K.M.; Toms, M.P.; Sheldon, B.C.; Cunningham, A.A. Emergence of a Novel Avian Pox Disease in British Tit Species. PLoS ONE 2012, 7, e40176.
- Williams, R.A.J.; Benitez, L. Chapter 5: Avian Poxvirus. In Ecology of Wild Bird Diseases; Fereidouni, S., Ed.; CRC Press: Boca Raton, FL, USA, in press; pp. 154–176. ISBN 978-0-8153-7945-4.
- Manuel Medina, F.; Adolfo Ramírez, G.; Hernández, A. Avian Pox in White-Tailed Laurel-Pigeons from the Canary Islands. J. Wildl. Dis. 2004, 40, 351–355.
- McDonald, S.E.; Lowenstine, L.J.; Ardans, A.A. Avian Pox in Blue-Fronted Amazon Parrots. J. Am. Vet. Med. Assoc. 1981, 179, 1218–1222.
- Van Riper, C., III; van Riper, S.G.; Hansen, W.R. Epizootiology and Effect of Avian Pox on Hawaiian Forest Birds. Auk 2002, 119, 929–942.
- Davidson, W.R.; Kellogg, F.E.; Doster, G.L. An Epornitic of Avian Pox in Wild Bobwhite Quail. J. Wildl. Dis. 1980, 16, 293–298.
- Tripathy, D.N.; Schnitzlein, W.M.; Morris, P.J.; Janssen, D.L.; Zuba, J.K.; Massey, G.; Atkinson, C.T. Characterization of Poxviruses from Forest Birds in Hawaii. J. Wildl. Dis. 2000, 36, 225–230.
- Tripathy, D.N.; Reed, W.M. Pox. In Diseases of Poultry; Swayne, D., Ed.; Wiley-Blackwell: Ames, IA, USA, 2013; pp. 333–349.
- Yeo, G.; Wang, Y.; Chong, S.M.; Humaidi, M.; Lim, X.F.; Mailepessov, D.; Chan, S.; How, C.B.; Lin, Y.N.; Huangfu, T.; et al. Characterization of Fowlpox Virus in Chickens and Bird-Biting Mosquitoes: A Molecular Approach to Investigating Avipoxvirus Transmission. J. Gen. Virol. 2019, 100, 838–850.
- Williams, R.A.J.; Truchado, D.A.; Benitez, L. A Review on the Prevalence of Poxvirus Disease in Free-Living and Captive Wild Birds. Microbiol. Res. 2021, 12, 403–418.
- Gyuranecz, M.; Foster, J.T.; Dán, Á.; Ip, H.S.; Egstad, K.F.; Parker, P.G.; Higashiguchi, J.M.; Skinner, M.A.; Höfle, U.; Kreizinger, Z.; et al. Worldwide Phylogenetic Relationship of Avian Poxviruses. J. Virol. 2013, 87, 4938–4951.
- Le Loc’h, G.; Ducatez, M.F.; Camus-Bouclainville, C.; Guérin, J.-L.; Bertagnoli, S. Diversity of Avipoxviruses in Captive-Bred Houbara Bustard. Vet. Res. 2014, 45, 98.
- MacDonald, A.M.; Gibson, D.J.; Barta, J.R.; Poulson, R.; Brown, J.D.; Allison, A.B.; Nemeth, N.M. Bayesian Phylogenetic Analysis of Avipoxviruses from North American Wild Birds Demonstrates New Insights into Host Specificity and Interspecies Transmission. Avian Dis. 2019, 63, 427–432.
- Phalen, D.N.; Agius, J.; Vaz, F.F.; Eden, J.-S.; Setyo, L.C.; Donahoe, S. A Survey of a Mixed Species Aviary Provides New Insights into the Pathogenicity, Diversity, Evolution, Host Range, and Distribution of Psittacine and Passerine Adenoviruses. Avian Pathol. 2019, 48, 437–443.
- Rinder, M.; Schmitz, A.; Baas, N.; Korbel, R. Molecular Identification of Novel and Genetically Diverse Adenoviruses in Passeriform Birds. Virus Genes. 2020, 56, 316–324.
- Joseph, H.M.; Ballmann, M.Z.; Garner, M.M.; Hanley, C.S.; Berlinski, R.; Erdélyi, K.; Childress, A.L.; Fish, S.S.; Harrach, B.; Wellehan, J.F.X. A Novel Siadenovirus Detected in the Kidneys and Liver of Gouldian Finches (Erythura gouldiae). Vet. Microbiol. 2014, 172, 35–43.
- Vaz, F.F.; Raso, T.F.; Agius, J.E.; Hunt, T.; Leishman, A.; Eden, J.-S.; Phalen, D.N. Opportunistic Sampling of Wild Native and Invasive Birds Reveals a Rich Diversity of Adenoviruses in Australia. Virus Evol. 2020, 6, veaa024.
- Keymer, I.F.; Blackmore, D.K. Diseases of the Skin and Soft Parts of Wild Birds. Br. Birds 1964, 57, 175–179.
- Lina, P.H.; van Noord, M.J.; de Groot, F.G. Detection of Virus in Squamous Papillomas of the Wild Bird Species Fringilla coelebs. J. Natl. Cancer Inst. 1973, 50, 567–571.
- Dom, P.; Ducatelle, R.; Charlier, G.; de Groot, P. Papillomavirus-like Infections in Canaries (Serinus canarius). Avian Pathol. 1993, 22, 797–803.
- Literak, I.; Smid, B.; Dusbabek, F.; Halouzka, R.; Novotny, L. Co-Infection with Papillomavirus and Knemidokoptes Jamaicensis (Acari: Knemidokoptidae) in a Chaffinch (Fringilla coelebs) and a Case of Beak Papillomatosis in Another Chaffinch. Vet. Med. 2005, 50, 276–280.
- Osterhaus, A.D.; Ellens, D.J.; Horzinek, M.C. Identification and Characterization of a Papillomavirus from Birds (Fringillidae). Intervirology 1977, 8, 351–359.
- Prosperi, A.; Chiari, M.; Zanoni, M.; Gallina, L.; Casà, G.; Scagliarini, A.; Lavazza, A. Identification and Characterization of Fringilla coelebs Papillomavirus 1 (FcPV1) in Free-Living and Captive Birds in Italy. J. Wildl. Dis. 2016, 52, 756–758.
- Truchado, D.A.; Moens, M.A.J.; Callejas, S.; Pérez-Tris, J.; Benítez, L. Genomic Characterization of the First Oral Avian Papillomavirus in a Colony of Breeding Canaries (Serinus canaria). Vet. Res. Commun. 2018, 42, 111–120.
- Amery-Gale, J.; Marenda, M.S.; Owens, J.; Eden, P.A.; Browning, G.F.; Devlin, J.M. A High Prevalence of Beak and Feather Disease Virus in Non-Psittacine Australian Birds. J. Med. Microbiol. 2017, 66, 1005–1013.
- Circella, E.; Legretto, M.; Pugliese, N.; Caroli, A.; Bozzo, G.; Accogli, G.; Lavazza, A.; Camarda, A. Psittacine Beak and Feather Disease-like Illness in Gouldian Finches (Chloebia gouldiae). Avian Dis. 2014, 58, 482–487.
- Ahaduzzaman, M.; Nath, C.; Hossain, M.S. Evidence of Circulation of Beak and Feather Disease Virus in Captive Psittacine and Non-Psittacine Birds in Bangladesh. Arch. Virol. 2022, 167, 2567–2575.
- Todd, D.; Weston, J.; Ball, N.W.; Borghmans, B.J.; Smyth, J.A.; Gelmini, L.; Lavazza, A. Nucleotide Sequence-Based Identification of a Novel Circovirus of Canaries. Avian Pathol. 2001, 30, 321–325.
- Phenix, K.V.; Weston, J.H.; Ypelaar, I.; Lavazza, A.; Smyth, J.A.; Todd, D.; Wilcox, G.E.; Raidal, S.R. Nucleotide Sequence Analysis of a Novel Circovirus of Canaries and Its Relationship to Other Members of the Genus Circovirus of the Family Circoviridae. J. Gen. Virol. 2001, 82, 2805–2809.
- Rinder, M.; Schmitz, A.; Peschel, A.; Wörle, B.; Gerlach, H.; Korbel, R. Molecular Characterization of a Recently Identified Circovirus in Zebra Finches (Taeniopygia guttata) Associated with Immunosuppression and Opportunistic Infections. Avian Pathol. 2017, 46, 106–116.
- Stewart, M.E.; Perry, R.; Raidal, S.R. Identification of a Novel Circovirus in Australian Ravens (Corvus coronoides) with Feather Disease. Avian Pathol. 2006, 35, 86–92.
- Todd, D.; Scott, A.N.J.; Fringuelli, E.; Shivraprasad, H.L.; Gavier-Widen, D.; Smyth, J.A. Molecular Characterization of Novel Circoviruses from Finch and Gull. Avian Pathol. 2007, 36, 75–81.
- Truchado, D.A.; Diaz-Piqueras, J.M.; Gomez-Lucia, E.; Doménech, A.; Milá, B.; Pérez-Tris, J.; Schmidt-Chanasit, J.; Cadar, D.; Benítez, L. A Novel and Divergent Gyrovirus with Unusual Genomic Features Detected in Wild Passerine Birds from a Remote Rainforest in French Guiana. Viruses 2019, 11, 1148.
- Yao, Y.; Wu, H.; Sun, G.; Yang, S.; Shen, Q.; Wang, X.; Zhang, W. Identification of Diverse Novel Genomoviruses in Gut of Wild Birds. Biosaf. Health 2021, 3, 136–141.
- Hanna, Z.R.; Runckel, C.; Fuchs, J.; DeRisi, J.L.; Mindell, D.P.; Van Hemert, C.; Handel, C.M.; Dumbacher, J.P. Isolation of a Complete Circular Virus Genome Sequence from an Alaskan Black-Capped Chickadee (Poecile atricapillus) Gastrointestinal Tract Sample. Genome Announc. 2015, 3, e01081-e15.
- Schmidlin, K.; Sepp, T.; Khalifeh, A.; Smith, K.; Fontenele, R.S.; McGraw, K.J.; Varsani, A. Diverse Genomoviruses Representing Eight New and One Known Species Identified in Feces and Nests of House Finches (Haemorhous mexicanus). Arch. Virol. 2019, 164, 2345–2350.
- Sikorski, A.; Massaro, M.; Kraberger, S.; Young, L.M.; Smalley, D.; Martin, D.P.; Varsani, A. Novel Myco-like DNA Viruses Discovered in the Faecal Matter of Various Animals. Virus Res. 2013, 177, 209–216.
- De Souza, W.M.; Dennis, T.; Fumagalli, M.J.; Araujo, J.; Sabino-Santos, G.; Maia, F.G.M.; Acrani, G.O.; Carrasco, A.D.O.T.; Romeiro, M.F.; Modha, S.; et al. Novel Parvoviruses from Wild and Domestic Animals in Brazil Provide New Insights into Parvovirus Distribution and Diversity. Viruses 2018, 10, 143.
- Fouchier, R.A.; Bestebroer, T.M.; Herfst, S.; Van Der Kemp, L.; Rimmelzwaan, G.F.; Osterhaus, A.D. Detection of Influenza A Viruses from Different Species by PCR Amplification of Conserved Sequences in the Matrix Gene. J. Clin. Microbiol. 2000, 38, 4096–4101.
- Morishita, T.Y.; Aye, P.P.; Ley, E.C.; Harr, B.S. Survey of Pathogens and Blood Parasites in Free-Living Passerines. Avian Dis. 1999, 43, 549–552.
- Munster, V.J.; Baas, C.; Lexmond, P.; Waldenström, J.; Wallensten, A.; Fransson, T.; Rimmelzwaan, G.F.; Beyer, W.E.P.; Schutten, M.; Olsen, B.; et al. Spatial, Temporal, and Species Variation in Prevalence of Influenza A Viruses in Wild Migratory Birds. PLoS Pathog. 2007, 3, e61.
- Swayne, D.E.; Suarez, D.L. Highly Pathogenic Avian Influenza. Rev. Sci. Tech. 2000, 19, 463–482.
- Chang, H.; Dai, F.; Liu, Z.; Yuan, F.; Zhao, S.; Xiang, X.; Zou, F.; Zeng, B.; Fan, Y.; Duan, G. Seroprevalence Survey of Avian Influenza A (H5) in Wild Migratory Birds in Yunnan Province, Southwestern China. Virol. J. 2014, 11, 18.
- Fuller, T.L.; Saatchi, S.S.; Curd, E.E.; Toffelmier, E.; Thomassen, H.A.; Buermann, W.; DeSante, D.F.; Nott, M.P.; Saracco, J.F.; Ralph, C.; et al. Mapping the Risk of Avian Influenza in Wild Birds in the US. BMC Infect. Dis. 2010, 10, 187.
- Kou, Z.; Lei, F.M.; Yu, J.; Fan, Z.J.; Yin, Z.H.; Jia, C.X.; Xiong, K.J.; Sun, Y.H.; Zhang, X.W.; Wu, X.M.; et al. New Genotype of Avian Influenza H5N1 Viruses Isolated from Tree Sparrows in China. J. Virol. 2005, 79, 15460–15466.
- Peterson, A.T.; Bush, S.E.; Spackman, E.; Swayne, D.E.; Ip, H.S. Influenza A Virus Infections in Land Birds, People’s Republic of China. Emerg. Infect. Dis. 2008, 14, 1644–1646.
- Shriner, S.A.; Root, J.J. A Review of Avian Influenza A Virus Associations in Synanthropic Birds. Viruses 2020, 12, 1209.
- European Food Safety Authority; European Centre for Disease Prevention and Control; European Union Reference Laboratory for Avian Influenza; Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Niqueux, É.; Staubach, C.; et al. Avian Influenza Overview June–September 2022. EFS2 2022, 20, e7597.
- Schnebel, B.; Dierschke, V.; Rautenschlein, S.; Ryll, M.; Neumann, U. Investigations on Infection Status with H5 and H7 Avian Influenza Virus in Short-Distance and Long-Distance Migrant Birds in 2001. Avian Dis. 2007, 51, 432–433.
- Turan, N.; Ozsemir, C.; Yilmaz, A.; Cizmecigil, U.Y.; Aydin, O.; Bamac, O.E.; Gurel, A.; Kutukcu, A.; Ozsemir, K.; Tali, H.E.; et al. Identification of Newcastle Disease Virus Subgenotype VII.2 in Wild Birds in Turkey. BMC Vet. Res. 2020, 16, 277.
- Silva, B.R.; Gamon, T.H.; Campos, A.C.A.; Thomazelli, L.M.; Serafini, P.P.; Chiarani, E.; Silva, T.W.; Locatelli-Dittrich, R. Molecular Diagnosis of Avian Viruses in Grassland Passerines and Captive Yellow Cardinals Gubernatrix cristata in Brazil. Pesq. Vet. Bras. 2021, 41, e06840.
- Orynbayev, M.B.; Fereidouni, S.; Sansyzbai, A.R.; Seidakhmetova, B.A.; Strochkov, V.M.; Nametov, A.M.; Sadikaliyeva, S.O.; Nurgazieva, A.; Tabynov, K.K.; Rametov, N.M.; et al. Genetic Diversity of Avian Avulavirus 1 (Newcastle Disease Virus Genotypes VIg and VIIb) Circulating in Wild Birds in Kazakhstan. Arch. Virol. 2018, 163, 1949–1954.
- Kariithi, H.M.; Christy, N.; Decanini, E.L.; Lemiere, S.; Volkening, J.D.; Afonso, C.L.; Suarez, D.L. Detection and Genome Sequence Analysis of Avian Metapneumovirus Subtype a Viruses Circulating in Commercial Chicken Flocks in Mexico. Vet. Sci. 2022, 9, 579.
- Greenacre, C.B. Viral Diseases of Companion Birds. Vet. Clin. N. Am. Exot. Anim. Pract. 2005, 8, 85–105.
- Fleury, H.J.; Alexander, D.J. Isolation of Twenty-Three Yucaipa-like Viruses from 616 Wild Birds in Senegal, West Africa. Avian Dis. 1979, 23, 742–744.
- Mbugua, H.C.; Karstad, L.; Thorsen, J. Isolation of Avian Paramyxoviruses (Yucaipa-like) from Wild Birds in Kenya, 1980–1982. J. Wildl. Dis. 1985, 21, 52–54.
- Nymadava, P.; Konstantinow-Siebelist, I.; Schulze, P.; Starke, S. Isolation of Paramyxoviruses from Free-Flying Birds of the Order Passeriformes in the German Democratic Republic. Acta Virol. 1977, 21, 443.
- Maldonado, A.; Arenas, A.; Tarradas, M.C.; Luque, I.; Astorga, R.; Perea, J.A.; Miranda, A. Serological Survey for Avian Paramyxoviruses from Wildfowl in Aquatic Habitats in Andalusia. J. Wildl. Dis. 1995, 31, 66–69.
- Goodman, B.B.; Hanson, R.P. Isolation of Avian Paramyxovirus-2 from Domestic and Wild Birds in Costa Rica. Avian Dis. 1988, 32, 713–717.
- Račnik, J.; Slavec, B.; Trilar, T.; Zadravec, M.; Dovč, A.; Krapež, U.; Barlič-Maganja, D.; Zorman Rojs, O. Evidence of Avian Influenza Virus and Paramyxovirus Subtype 2 in Wild-Living Passerine Birds in Slovenia. Eur. J. Wildl. Res. 2008, 54, 529–532.
- Schemera, B.; Toro, H.; Kaleta, E.F.; Herbst, W. A Paramyxovirus of Serotype 3 Isolated from African and Australian Finches. Avian Dis. 1987, 31, 921–925.
- Shihmanter, E.; Weisman, Y.; Lublin, A.; Mahani, S.; Panshin, A.; Lipkind, M. Isolation of Avian Serotype 3 Paramyxoviruses from Imported Caged Birds in Israel. Avian Dis. 1998, 42, 829–831.
- Muzyka, D.; Pantin-Jackwood, M.; Stegniy, B.; Rula, O.; Bolotin, V.; Stegniy, A.; Gerilovych, A.; Shutchenko, P.; Stegniy, M.; Koshelev, V.; et al. Wild Bird Surveillance for Avian Paramyxoviruses in the Azov-Black Sea Region of Ukraine (2006 to 2011) Reveals Epidemiological Connections with Europe and Africa. Appl. Environ. Microbiol. 2014, 80, 5427–5438.
- Bennett, R.S.; Nezworski, J.; Velayudhan, B.T.; Nagaraja, K.V.; Zeman, D.H.; Dyer, N.; Graham, T.; Lauer, D.C.; Njenga, M.K.; Halvorson, D.A. Evidence of Avian Pneumovirus Spread beyond Minnesota among Wild and Domestic Birds in Central North America. Avian Dis. 2004, 48, 902–908.
- Shin, H.-J.; Njenga, M.K.; McComb, B.; Halvorson, D.A.; Nagaraja, K.V. Avian Pneumovirus (APV) RNA from Wild and Sentinel Birds in the United States Has Genetic Homology with RNA from APV Isolates from Domestic Turkeys. J. Clin. Microbiol. 2000, 38, 4282–4284.
- Berg, M.; Johansson, M.; Montell, H.; Berg, A.L. Wild Birds as a Possible Natural Reservoir of Borna Disease Virus. Epidemiol. Infect. 2001, 127, 173–178.
- Rubbenstroth, D.; Rinder, M.; Stein, M.; Höper, D.; Kaspers, B.; Brosinski, K.; Horie, M.; Schmidt, V.; Legler, M.; Korbel, R.; et al. Avian Bornaviruses Are Widely Distributed in Canary Birds (Serinus canaria f. domestica). Vet. Microbiol. 2013, 165, 287–295.
- Rubbenstroth, D.; Schmidt, V.; Rinder, M.; Legler, M.; Corman, V.M.; Staeheli, P. Discovery of a New Avian Bornavirus Genotype in Estrildid Finches (Estrildidae) in Germany. Vet. Microbiol. 2014, 168, 318–323.
- Kato, M.; Okanoya, K. Molecular Characterization of the Song Control Nucleus HVC in Bengalese Finch Brain. Brain Res. 2010, 1360, 56–76.
- Hasegawa, T.; Takehara, Y.; Takahashi, K. Natural and Experimental Infections of Japanese Tree Sparrows with Japanese Encephalitis Virus. Arch. Virol. 1975, 49, 373–376.
- Day, J.F.; Stark, L.M. Avian Serology in a St. Louis Encephalitis Epicenter Before, During, and After a Widespread Epidemic in South Florida, USA. J. Med. Entomol. 1999, 36, 614–624.
- Díaz, A.; Flores, F.S.; Quaglia, A.I.; Contigiani, M.S. Evaluation of Argentinean Bird Species as Amplifying Hosts for St. Louis Encephalitis Virus (Flavivirus, Flaviviridae). Am. J. Trop. Med. Hyg. 2018, 99, 216–221.
- Reisen, W.K.; Chiles, R.E.; Martinez, V.M.; Fang, Y.; Green, E.N. Experimental Infection of California Birds with Western Equine Encephalomyelitis and St. Louis Encephalitis Viruses. J. Med. Entomol. 2003, 40, 968–982.
- Gruwell, J.A.; Fogarty, C.L.; Bennett, S.G.; Challet, G.L.; Vanderpool, K.S.; Jozan, M.; Webb, J.P., Jr. Role of Peridomestic Birds on the Transmission of St. Louis Encephalitis Virus in Southern California. J. Wildl. Dis. 2000, 36, 13–34.
- Curren, E.J.; Lindsey, N.P.; Fischer, M.; Hills, S.L. St. Louis Encephalitis Virus Disease in the United States, 2003–2017. Am. J. Trop. Med. Hyg. 2018, 99, 1074–1079.
- Chevalier, V.; Marsot, M.; Molia, S.; Rasamoelina, H.; Rakotondravao, R.; Pedrono, M.; Lowenski, S.; Durand, B.; Lecollinet, S.; Beck, C. Serological Evidence of West. Nile and Usutu Viruses Circulation in Domestic and Wild Birds in Wetlands of Mali. and Madagascar in 2008. Int. J. Environ. Res. Public Health 2020, 17, 1998.
- Nikolay, B.; Diallo, M.; Boye, C.S.B.; Sall, A.A. Usutu Virus in Africa. Vector Borne Zoonotic Dis. 2011, 11, 1417–1423.
- Chvala, S.; Bakonyi, T.; Bukovsky, C.; Meister, T.; Brugger, K.; Rubel, F.; Nowotny, N.; Weissenböck, H. Monitoring of Usutu Virus Activity and Spread by Using Dead Bird Surveillance in Austria, 2003–2005. Vet. Microbiol. 2007, 122, 237–245.
- Bernard, K.A.; Maffei, J.G.; Jones, S.A.; Kauffman, E.B.; Ebel, G.; Dupuis, A.P.; Ngo, K.A.; Nicholas, D.C.; Young, D.M.; Shi, P.Y.; et al. West Nile Virus Infection in Birds and Mosquitoes, New York State, 2000. Emerg. Infect. Dis. 2001, 7, 679–685.
- Bakonyi, T.; Ferenczi, E.; Erdélyi, K.; Kutasi, O.; Csörgő, T.; Seidel, B.; Weissenböck, H.; Brugger, K.; Bán, E.; Nowotny, N. Explosive Spread of a Neuroinvasive Lineage 2 West Nile Virus in Central Europe, 2008/2009. Vet. Microbiol. 2013, 165, 61–70.
- Jourdain, E.; Olsen, B.; Lundkvist, A.; Hubálek, Z.; Sikutová, S.; Waldenström, J.; Karlsson, M.; Wahlström, M.; Jozan, M.; Falk, K.I. Surveillance for West Nile Virus in Wild Birds from Northern Europe. Vector Borne Zoonotic Dis. 2011, 11, 77–79.
- López, G.; Jiménez-Clavero, M.A.; Tejedor, C.G.; Soriguer, R.; Figuerola, J. Prevalence of West Nile Virus Neutralizing Antibodies in Spain Is Related to the Behavior of Migratory Birds. Vector Borne Zoonotic Dis. 2008, 8, 615–621.
- Drennan, J.E. Northern Goshawk Food Habits and Goshawk Prey Species Habitats|Searchable Ornithological Research Archive. Stud. Avian Biol. 2006, 31, 198–218.
- Centers for Disease Control and Prevention. Species of Dead Birds in Which West Nile Virus Has Been Detected, United States, 1999–2016. Available online: https://www.cdc.gov/westnile/resources/pdfs/birdspecies1999-2016.pdf (accessed on 27 June 2023).
- Estep, L.K.; McClure, C.J.W.; Burkett-Cadena, N.D.; Hassan, H.K.; Hicks, T.L.; Unnasch, T.R.; Hill, G.E. A Multi-Year Study of Mosquito Feeding Patterns on Avian Hosts in a Southeastern Focus of Eastern Equine Encephalitis Virus. Am. J. Trop. Med. Hyg. 2011, 84, 718–726.
- Molaei, G.; Armstrong, P.M.; Graham, A.C.; Kramer, L.D.; Andreadis, T.G. Insights into the Recent Emergence and Expansion of Eastern Equine Encephalitis Virus in a New Focus in the Northern New England USA. Parasit. Vectors 2015, 8, 516.
- Molaei, G.; Thomas, M.C.; Muller, T.; Medlock, J.; Shepard, J.J.; Armstrong, P.M.; Andreadis, T.G. Dynamics of Vector-Host Interactions in Avian Communities in Four Eastern Equine Encephalitis Virus Foci in the Northeastern U.S. PLoS Negl. Trop. Dis. 2016, 10, e0004347.
- Andreadis, T.G.; Anderson, J.F.; Tirrell-Peck, S.J. Multiple Isolations of Eastern Equine Encephalitis and Highlands J Viruses from Mosquitoes (Diptera: Culicidae) during a 1996 Epizootic in Southeastern Connecticut. J. Med. Entomol. 1998, 35, 296–302.
- Hesson, J.C.; Lundström, J.O.; Tok, A.; Östman, Ö.; Lundkvist, Å. Temporal Variation in Sindbis Virus Antibody Prevalence in Bird Hosts in an Endemic Area in Sweden. PLoS ONE 2016, 11, e0162005.
- Lundström, J.O.; Lindström, K.M.; Olsen, B.; Dufva, R.; Krakower, D.S. Prevalence of Sindbis Virus Neutralizing Antibodies among Swedish Passerines Indicates That Thrushes Are the Main Amplifying Hosts. J. Med. Entomol. 2001, 38, 289–297.
- Whitehead, R.H.; Doderty, R.L.; Domrow, R.; Standfast, H.A.; Wetters, E.J. Studies of the Epidemiology of Arthropod-Borne Virus Infections at Mitchell River Mission, Cape York Peninsula, North Queensland. 3. Virus Studies of Wild Birns, 1964–1967. Trans. R Soc. Trop. Med. Hyg. 1968, 62, 439–445.
- Jones, A.; Lowry, K.; Aaskov, J.; Holmes, E.C.; Kitchen, A. Molecular Evolutionary Dynamics of Ross River Virus and Implications for Vaccine Efficacy. J. Gen. Virol. 2010, 91, 182–188.
- Degallier, N.; Rosa, A.; Vasconcelos, P.F.C.; Hervé, J.-P.; Sá Filho, G.C.; Rosa, J.F.; Rosa, E.S.; Rodrigues, S.G. Modifications of Arbovirus Transmission in Relation to Construction of Dams in Brazilian Amazonia. Ciênc. Cult. 1992, 44, 124–135.
- Hoch, A.L.; Peterson, N.E.; LeDuc, J.W.; Pinheiro, F.P. An Outbreak of Mayaro Virus Disease in Belterra, Brazil. III. Entomological and Ecological Studies. Am. J. Trop. Med. Hyg. 1981, 30, 689–698.
- Sanmartín, C.; Mackenzie, R.B.; Trapido, H.; Barreto, P.; Mullenax, C.H.; Gutiérrez, E.; Lesmes, C. Encefalitis Equina Venezolana En Colombia. Boletín Oficina Sanit. Panam. 1973, 74, 108–137.
- Calisher, C.H.; Gutiérrez, E.; Maness, K.S.; Lord, R.D. Isolation of Mayaro Virus from a Migrating Bird Captured in Louisiana in 1967. Bull. Pan. Am. Health Organ. 1974, 8, 243–248.
- Scott, T.W.; Bowen, G.S.; Monath, T.P. A Field Study on the Effects of Fort Morgan Virus, an Arbovirus Transmitted by Swallow Bugs, on the Reproductive Success of Cliff Swallows and Symbiotic House Sparrows in Morgan County, Colorado, 1976. Am. J. Trop. Med. Hyg. 1984, 33, 981–991.
- Fassbinder-Orth, C.A.; Killpack, T.L.; Goto, D.S.; Rainwater, E.L.; Shearn-Bochsler, V.I. High Costs of Infection: Alphavirus Infection Reduces Digestive Function and Bone and Feather Growth in Nestling House Sparrows (Passer domesticus). PLoS ONE 2018, 13, e0195467.
- O’Brien, V.A.; Meteyer, C.U.; Ip, H.S.; Long, R.R.; Brown, C.R. Pathology and Virus Detection in Tissues of Nestling House Sparrows Naturally Infected with Buggy Creek Virus (Togaviridae). J. Wildl. Dis. 2010, 46, 23–32.
- Miłek, J.; Blicharz-Domańska, K. Coronaviruses in Avian Species—Review with Focus on Epidemiology and Diagnosis in Wild Birds. J. Vet. Res. 2018, 62, 249–255.
- Woo, P.C.Y.; Lau, S.K.P.; Lam, C.S.F.; Lai, K.K.Y.; Huang, Y.; Lee, P.; Luk, G.S.M.; Dyrting, K.C.; Chan, K.-H.; Yuen, K.-Y. Comparative Analysis of Complete Genome Sequences of Three Avian Coronaviruses Reveals a Novel Group 3c Coronavirus. J. Virol. 2009, 83, 908–917.
- Woo, P.C.Y.; Lau, S.K.P.; Lam, C.S.F.; Lau, C.C.Y.; Tsang, A.K.L.; Lau, J.H.N.; Bai, R.; Teng, J.L.L.; Tsang, C.C.C.; Wang, M.; et al. Discovery of Seven Novel Mammalian and Avian Coronaviruses in the Genus Deltacoronavirus Supports Bat Coronaviruses as the Gene Source of Alphacoronavirus and Betacoronavirus and Avian Coronaviruses as the Gene Source of Gammacoronavirus and Deltacoronavirus. J. Virol. 2012, 86, 3995–4008.
- Woo, P.C.Y.; Lau, S.K.P.; Huang, Y.; Lam, C.S.F.; Poon, R.W.S.; Tsoi, H.-W.; Lee, P.; Tse, H.; Chan, A.S.L.; Luk, G.; et al. Comparative Analysis of Six Genome Sequences of Three Novel Picornaviruses, Turdiviruses 1, 2 and 3, in Dead Wild Birds, and Proposal of Two Novel Genera, Orthoturdivirus and Paraturdivirus, in the Family Picornaviridae. J. Gen. Virol. 2010, 91, 2433–2448.
- Pankovics, P.; Boros, Á.; Phan, T.G.; Delwart, E.; Reuter, G. A Novel Passerivirus (Family Picornaviridae) in an Outbreak of Enteritis with High Mortality in Estrildid Finches (Uraeginthus sp.). Arch. Virol. 2018, 163, 1063–1071.
- Zylberberg, M.; Van Hemert, C.; Handel, C.M.; DeRisi, J.L. Avian Keratin Disorder of Alaska Black-Capped Chickadees Is Associated with Poecivirus Infection. Virol. J. 2018, 15, 100.
- Zylberberg, M.; Van Hemert, C.; Handel, C.M.; Liu, R.M.; DeRisi, J.L. Poecivirus Is Present in Individuals with Beak Deformities in Seven Species of North American Birds. J. Wildl. Dis. 2021, 57, 273–281.
- Fernández-Correa, I.; Truchado, D.A.; Gomez-Lucia, E.; Doménech, A.; Pérez-Tris, J.; Schmidt-Chanasit, J.; Cadar, D.; Benítez, L. A Novel Group of Avian Astroviruses from Neotropical Passerine Birds Broaden the Diversity and Host Range of Astroviridae. Sci. Rep. 2019, 9, 9513.
- Mendenhall, I.H.; Smith, G.J.D.; Vijaykrishna, D. Ecological Drivers of Virus Evolution: Astrovirus as a Case Study. J. Virol. 2015, 89, 6978–6981.
- Mendenhall, I.H.; Yaung, K.N.; Joyner, P.H.; Keatts, L.; Borthwick, S.; Neves, E.S.; San, S.; Gilbert, M.; Smith, G.J. Detection of a Novel Astrovirus from a Black-Naped Monarch (Hypothymis azurea) in Cambodia. Virol. J. 2015, 12, 182.
- French, R.K.; Stone, Z.L.; Parker, K.A.; Holmes, E.C. Novel Viral and Microbial Species in a Translocated Toutouwai (Petroica longipes) Population from Aotearoa/New Zealand. One Health Outlook 2022, 4, 16.
- Chang, W.-S.; Rose, K.; Holmes, E.C. Meta-Transcriptomic Analysis of the Virome and Microbiome of the Invasive Indian Myna (Acridotheres tristis) in Australia. One Health 2021, 13, 100360.
- Ursu, K.; Papp, H.; Kisfali, P.; Rigó, D.; Melegh, B.; Martella, V.; Bányai, K. Monitoring of Group A Rotaviruses in Wild-Living Birds in Hungary. Avian Dis. 2011, 55, 123–127.
- Duarte Júnior, J.W.B.; Chagas, E.H.N.; Serra, A.C.S.; Souto, L.C.D.S.; da Penha Júnior, E.T.; Bandeira, R.D.S.; e Guimarães, R.J.D.P.S.; Oliveira, H.G.D.S.; Sousa, T.K.S.; Lopes, C.T.D.A.; et al. Ocurrence of Rotavirus and Picobirnavirus in Wild and Exotic Avian from Amazon Forest. PLoS Negl. Trop. Dis. 2021, 15, e0008792.
- Varejka, F.; Tomsik, F. The Role of House Sparrow (Passer domesticus L.) in the Spread of Leukosis Viruses in Poultry. I. Determination of Neutralizing Antibodies. Acta Vet. Brno 1974, 43, 367–370.
- Jiang, L.; Zeng, X.; Hua, Y.; Gao, Q.; Fan, Z.; Chai, H.; Wang, Q.; Qi, X.; Wang, Y.; Gao, H.; et al. Genetic Diversity and Phylogenetic Analysis of Glycoprotein Gp85 of Avian Leukosis Virus Subgroup J Wild-Bird Isolates from Northeast China. Arch. Virol. 2014, 159, 1821–1826.
- Han, C.; Hao, R.; Liu, L.; Zeng, X. Molecular Characterization of 3’UTRs of J Subgroup Avian Leukosis Virus in Passerine Birds in China. Arch. Virol. 2015, 160, 845–849.
