Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Jamir Pitton Rissardo.
Cardiac sympathetic denervation, as documented on 123I-metaiodobenzylguanidine (MIBG) myocardial scintigraphy, is relatively sensitive and specific for distinguishing Parkinson’s disease (PD) from other neurodegenerative causes of parkinsonism.
- neuroimaging
- cardiac imaging
- myocardium
- 123I
1. Physiological and Anatomical Changes in the Cardiovascular System of Parkinson’s Disease
Spinal cells are responsible for the sympathetic innervation of the heart. These cells originate in the upper 3–4 thoracic segments of the spinal cord. The first synapses form in the stellate (cervicothoracic) and thoracic sympathetic ganglia. Postganglionic noradrenergic sympathetic fibers accompany the blood vessels to the heart and enter into the myocardium. Parasympathetic fibers relay at ganglia located directly on the heart and short postsynaptic fibers. The main areas innervated by the parasympathetic nervous system are the atrial muscle and the sinoatrial and atrioventricular nodes. But, the ventricular myocardium is sparsely innervated by vagal efferents [42][1].
Lewy bodies are intra-cytoplasmic eosinophilic inclusions with a hyaline core and a pale halo mainly composed of aggregated α-synuclein. In the early stages of PD, Lewy bodies accumulate significantly in structures of the lower brainstem and the olfactory system [43][2]. At this stage, also known as Braak stage 1, the dorsal motor nucleus of the vagus nerve in the medulla oblongata and anterior olfactory nucleus are affected. The involvement of the vagus nerve can partially explain the denervation of some cardiac structures [44][3].
Mitsui et al. performed a neuropathological study on a 71-year-old male with a diagnosis of PD who had a cardiac MIBG performed one year before the autopsy [45][4]. The patient had a low MIBG uptake in the early and delayed phases. Some neuropathological findings on this individual can contribute to understanding cardiac involvement in the pathophysiology of PD. First, Lewy bodies were present in the intermediolateral column of the thoracic spinal cord and sympathetic ganglia. Second, immunohistochemistry with anti-phosphorylated α-synuclein antibody was positive on axons in the thoracic ventral roots, sympathetic trunk, and cardiac plexus. Third, total loss of anti-tyrosine hydroxyls immunoreactivity and a marked decrease in axons were observed in the cardiac plexus. Fourth, no valvular, coronary, or myocardial abnormality was observed in the gross and microscopic anatomy of the heart.
The findings of cardiac MIBG studies correlated with pathology reports are important for understanding the progression and involvement of the cardiac system in PD [46][5]. The uptake reduction in MIBG in the heart was found to be associated with denervation of the heart, leading to hyperdynamic cardiac contractility in response to adrenergic responses related to beta 1 [47][6]. Also, patients with reduced MIBG uptake may have reduced cardiac contractility during exercise, suggesting an impaired response to exercise capacity [48][7]. A possible explanation for this finding is the sympathetic denervation observed in individuals with PD.
Takahashi et al. studied the extent of cardiac sympathetic denervation in cases of Lewy body disease confirmed using autopsy and the relationship between residual sympathetic nerve preservation and cardiac MIBG uptake in life. The authors found a quantitative correlation between cardiac imaging and the loss of sympathetic axon loss in cardiac tissue samples [49][8].
The efficiency of vesicular sequestration in individuals with PD can provide significant information on neuronal death. In this context, cardiac MIBG cannot quantify the functional abnormality, but the accelerated loss (“washout”) of MIBG might provide a biomarker of increased sympathetically mediated exocytosis [50][9].
The morphometric study of the brain can add important information regarding indirect functionality and neuronal damage. Kikuchi et al. evaluated voxel-based morphometry and diffusion tensor imaging in relationship to cardiac MIBG in individuals with PD. They found that individuals with an abnormal H/M ratio compared with those with normal had reduced brain volume at the right inferior frontal gyrus and lower fractional anisotropy at the left anterior thalamic radiation, the left inferior fronto-occipital fasciculus, the left superior longitudinal fasciculus, and the left uncinate fasciculus [51][10].
Park et al. studied cardiac MIBG, 18F-FP-CIT, and striatal dopamine transporter imaging in individuals with PD [52][11]. Striatal 18F-FP-CIT uptake did not correlate with plasma α-synuclein levels. In individuals with early PD, plasma α-synuclein levels correlated with cardiac sympathetic denervation found in the cardiac MIBG (p = 0.01) but not with nigrostriatal degeneration (p = 0.61) in a multivariate analysis. This may suggest that plasma α-synuclein levels more readily reflect the peripheral accumulation of Lewy bodies than central. Furthermore, it may suggest that central and peripheral deposition of Lewy bodies could have, in fact, two different pathological pathways [53][12].
One of the possible pathophysiological mechanisms hypothesized in PD is related to the serotoninergic pathway [54][13]. Brain postmortem analysis of PD patients showed that the density of serotonergic neurons and the concentrations of serotonin and its metabolites were decreased in the raphe area of PD patients [55][14]. Some authors found a link between the central serotonergic and cardiac sympathetic systems. The cerebrospinal fluid 5-hydroxyindole acetic acid concentration correlated with a delayed H/M ratio (r = 0.458, p < 0.05) and the washout rate (r = −0.642, p < 0.01). Therefore, these results can support the concurrent progression of central serotonergic and cardiac sympathetic dysfunction in individuals with PD [56][15].
2. Cardiac MIBG Scintigraphy as a Supporting Criteria for PD
For many years, the main diagnostic criteria for PD were only based on clinical cardinal features. Hughes et al. performed a clinic-pathological study with one hundred individuals with PD. The U.K. PD Society Brain Bank criteria for PD diagnosis had a specificity of 93%, meaning that 7% of the individuals with PD were misdiagnosed [57][16]. To address this concern, the Movement Disorder developed diagnostic criteria for PD improving the specificity with a balanced sensitivity. These new criteria included two levels of certainty, supportive studies, absolute exclusion criteria, and red flags. One supportive criterion in diagnosing PD is either olfactory loss or cardiac sympathetic denervation on MIBG scintigraphy [6][17].
Figure 21 summarizes the cardiac MIBG results found in different studies. Most studies consider a normal H/M ratio as above two. However, several factors directly and indirectly related to the patient and the collimator can influence these results. Also, significant differences exist in the early and delayed phases of registering the H/M ratio. Interestingly, some clinical features in patients with PD are statistically associated with early and delayed cardiac MIBG uptakes. These symptoms are rapid eye movement sleep behavior disorder, urinary disturbances, rigidity, and constipation. Moreover, tremor is only associated with delayed cardiac MIBG uptake [58][18].
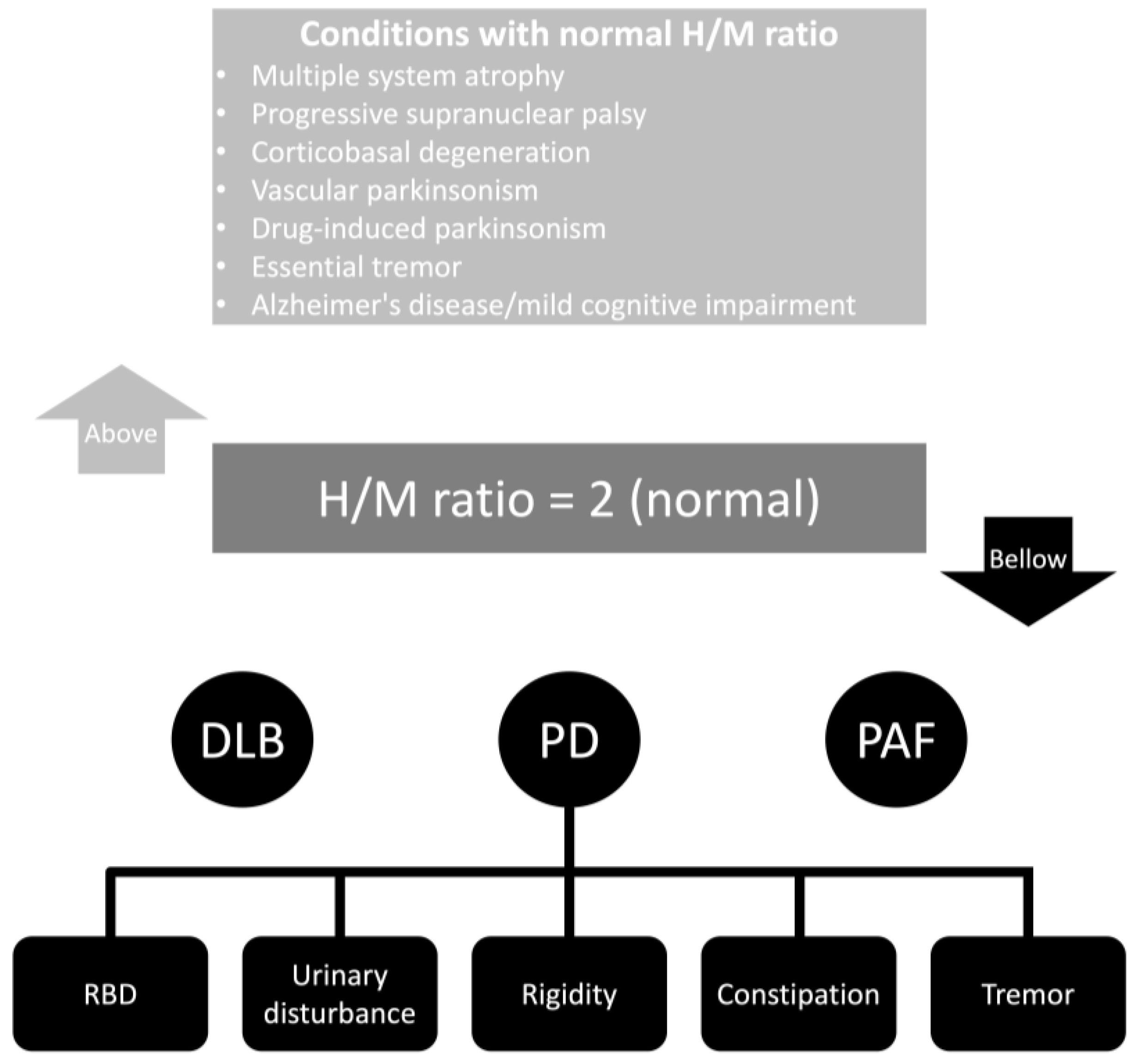
Figure 21. Cardiac MIBG results in different neurological conditions. Abbreviations: DLB, dementia with Lewy bodies; H/M, heart-to-mediastinum ratio; PAF, pure autonomic failure; PD, Parkinson’s disease; RBD, rapid eye movement sleep behavior disorder.
The most common form of imaging acquisition of cardiac MIBG is two-dimensional planar images. In this context, three-dimensional imaging using single-photon emission tomography (SPECT) may provide a more complete understanding of regional cardiac sympathetic innervation with an assessment of the polar model (Figure 3). The conventional 17-segment/five-point model used for SPECT myocardial perfusion imaging is usually used [59][19]. Studying cardiac segments with SPECT, in addition to the H/M ratio, is important for patients with particular anatomical deformities. Also, they may provide additional information to develop scores with lower variability between patients and be less dependent on the quality of the imaging acquired. The literature about the SPECT of cardiac segments involved in differentiating parkinsonian syndromes is scarce. However, there are many studies in patients with structural heart conditions.
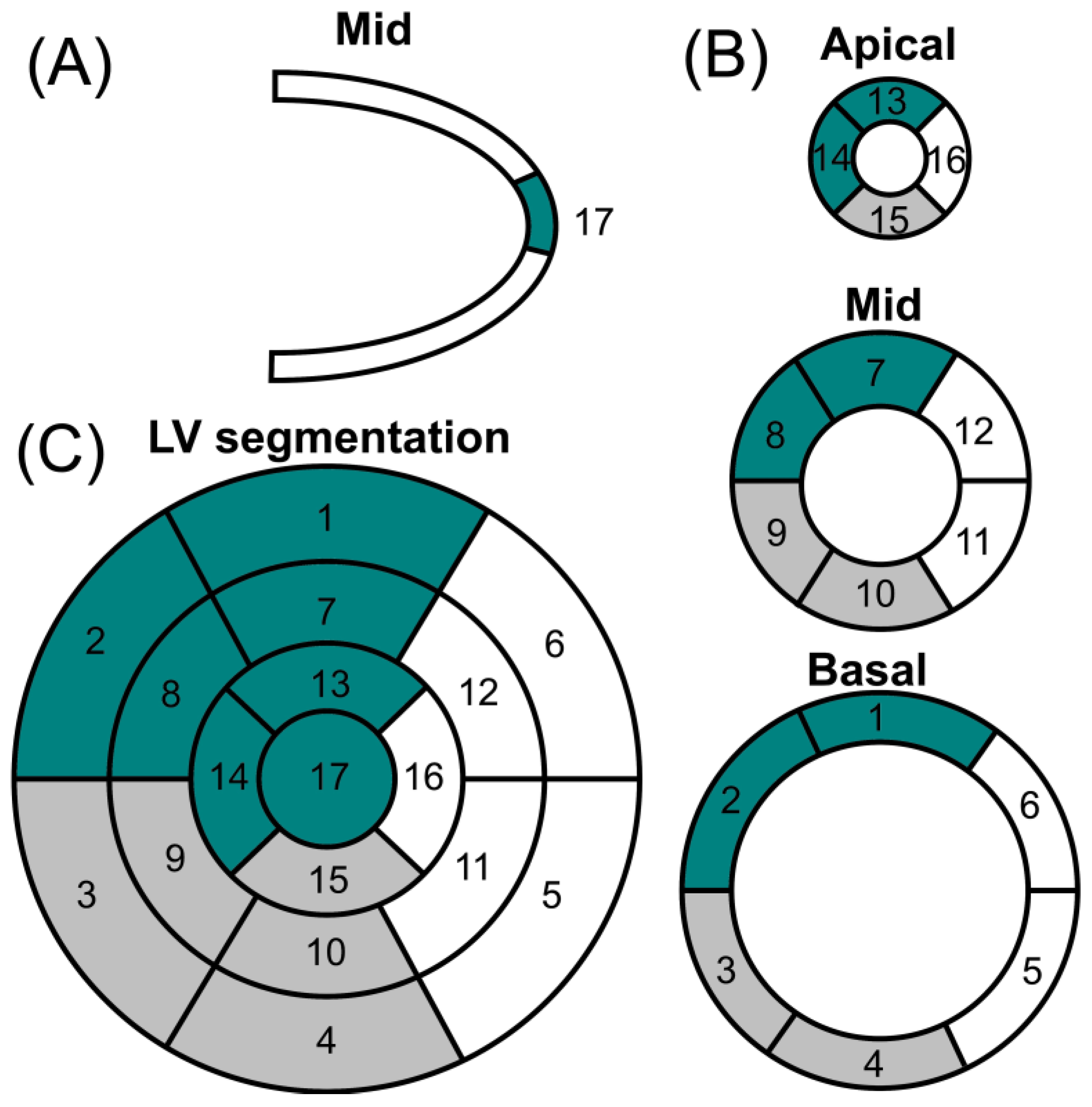
Figure 32. Polar map of cardiac perfusion. (A) Vertical long axis; (B) short axis; and (C) left ventricular (LV) segmentation. Coronary artery territories: left anterior descending artery (dark green); right coronary artery (gray); left circumflex artery (white). Segments: (1) basal anterior; (2) basal anteroseptal; (3) basal inferoseptal; (4) basal inferior; (5) basal inferolateral; (6) basal anterolateral; (7) mid anterior; (8) mid anteroseptal; (9) mid inferoseptal; (10) mid inferior; (11) mid inferolateral; (12) mid anterolateral; (13) apical anterior; (14) apical septal; (15) apical inferior; (16) apical lateral; (17) apex.
Kwon et al. showed that the summed defect score and defect scores in the anteroseptal and inferior regions are statistically significant in differentiating PD from essential tremors [60][20]. Furthermore, Courbon et al. described a prominent regional reduction in MIBG uptake in the apex region in patients with PD compared with multiple systemic atrophy [61][21]. The results of both studies suggest that cardiac denervation might affect mainly the anteroseptal region, including the apex, in PD patients, which is the main distribution of sympathetic fibers in the heart.
The inferior wall of the ventricles was also associated with a decreased uptake of MIBG in individuals with PD [60][20]. However, this area should be cautiously analyzed. The inferior wall is related to many artifacts because images are rendered to a planar surface on a different axis. Also, the liver is one of the areas with high uptake of MIBG, causing difficulties in the reconstruction due to artifacts. In an elderly population, studies showed a strong association between decreased uptake of MIBG in the inferior wall region and the aging effect [62][22].
The cardiac MIBG SPECT uptake distribution can be homogeneous, non-homogeneous, or absent. In the non-homogenous distribution, interesting patterns of uptake can be observed. The uptake of the apex is commonly observed in PD and multiple system atrophy, but apparently, uptake can only be complete in PD. Also, the dysfunction of multiple system atrophy usually affects the left circumflex artery territory more than the other coronary territories [63][23]. Interestingly, a similar non-homogeneous pattern has been found with 6-[18F]- fluorodopamine [64][24] and [11C]-hydroxyephedrine [65][25] PET scanning in patients with PD.
3. Cardiac MIBG Scintigraphy in Parkinson’s Disease
In the 1990s, it was observed that patients with PD disclosed a significantly lower myocardial MIBG uptake than healthy controls. This was especially observed in the H/M ratio, where patients with PD had a significantly lower H/M ratio than healthy controls [66][26]. Reduced myocardial MIBG uptake reflects sympathetic myocardial degeneration in PD and the results from a Lewy body type degeneration of the cardiac plexus. Noteworthy, there are significant discrepancies in the literature results regarding the correlation between MIBG and age, sex, years of the disease, and Hoehn and Yahr stage.
Patients with a mild Hoehn Yahr stage or short-duration disease, compared with healthy individuals, will have reduced myocardial MIBG uptake. Interestingly, one study investigated individuals with different Hoehn and Yahr stages, and the authors observed that in patients with more advanced PD (Hoehn and Yahr stages II–V), compared with early PD (Hoehn and Yahr stages) and healthy individuals, myocardial MIBG uptake will have high sensitivity and specificity [67][27].
Table 21 shows the sensitivity and specificity of cardiac MIBG scintigraphy in PD to differentiate it from other forms of parkinsonism. Fifty-four studies involved 3114 individuals with PD with a mean Hoehn and Yahr (H&Y) stage of 2.5. The mean early and delayed registration H/M ratios were 1.70 and 1.51, respectively. The mean cutoff for the early and delayed phases were 1.89 and 1.86. The sensitivity for the early and delayed phases was 0.81 and 0.83, respectively. The specificity for the early and delayed phases were 0.86 and 0.80, respectively.
Table 21.
Sensitivity, specificity, and H/M ratio of cardiac MIBG scintigraphy in Parkinson’s disease.
| Reference | n | n PD | H&Y Stage a | H/M Ratio | Sensitivity c | Specificity c | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PD b | Cutoff | Early Phase | Delayed Phase | Early Phase | Delayed Phase | ||||||
| Early Phase | Delayed Phase | Early Phase | Delayed Phase | ||||||||
| Yoshita et al. [68][28] | 54 | 25 | 2.1 | 1.36 ± 0.15 | 1.19 ± 0.15 | NA | 1.00 | 0.79 | |||
| Braune et al. [69][29] | 10 | 10 | NA | NA | 1.06 ± 0.06 | NA | NA | NA | |||
| Iwasa et al. [70][30] | 12 | 12 | 2.5 | 1.55 ± 0.17 | 1.37 ± 0.15 | NA | NA | NA | |||
| Braune et al. [71][31] | 20 | 15 | NA | NA | 1.08 ± 0.13 | NA | NA | NA | |||
| Orimo et al. [72][32] | 68 | 45 | 3.0 | 1.71 ± 0.36 | 1.53 ± 0.36 | NA | 0.80 | 0.87 | |||
| Druschky et al. [73][33] | 30 | 10 | 1.6 | NA | 1.25 ± 0.61 | NA | NA | NA | |||
| Taki et al. [74][34] | 70 | 41 | 1.9 | 1.61 ± 0.29 | 1.47 ± 0.34 | 1.89 | 2.02 | 0.83 | 0.90 | 0.83 | 0.76 |
| Reinhardt et al. [75][35] | 28 | 21 | 3.4 | 1.05 | NA | 1.00 | 1.00 | ||||
| Takatsu et al. [76][36] | 32 | 32 | 2.84 | 1.58 ± 0.37 | 1.33 ± 0.28 | NA | 0.93 | 1.00 | |||
| Courbon et al. [61][21] | 28 | 18 | 2.34 | NA | 1.50 ± 0.53 | NA | 1.30 | 0.80 | 1.00 | ||
| Hamada et al. [77][37] | 113 | 88 | 2.61 | 1.51 ± 0.32 | 1.39 ± 0.33 | NA | NA | NA | |||
| Orimo et al. [78][38] | 98 | 90 | 2.73 | 1.72 ± 0.33 | 1.54 ± 0.35 | NA | NA | NA | |||
| Saiki et al. [79][39] | 45 | 34 | 2.55 | 1.45 ± 0.20 | 1.33 ± 0.27 | 1.38 | 1.25 | 0.83 | 0.66 | 0.86 | 0.73 |
| Nagayama et al. [80][40] | 391 | 122 | 3.0 | NA | 1.38 ± 0.29 | NA | 1.84 | NA | 0.87 | NA | 0.37 |
| Kashihara et al. [81][41] | 204 | 130 | 3.1 | 1.63 ± 0.29 | 1.37 ± 0.27 | NA | 0.84 | 1.00 | |||
| Kim et al. [82][42] | 65 | 30 | NA | NA | 1.27 ± 0.13 | NA | 1.00 | 0.84 | |||
| Lee et al. [83][43] | 93 | 51 | 1.61 | NA | 1.28 ± 0.35 | NA | 0.98 | 1.00 | |||
| Miyamoto et al. [84][44] | 34 | 12 | 2.3 | NA | 1.43 ± 0.20 | NA | NA | NA | |||
| Shin et al. [85][45] | 119 | 40 | 2.25 | 1.34 ± 0.15 | 1.29 ± 0.15 | 1.38 | 1.36 | 0.65 | 0.80 | 0.95 | 1.00 |
| Köllensperger et al. [86][46] | 18 | 9 | 3.2 | 1.51 ± 0.24 | 1.32 ± 0.25 | 1.93 | 1.68 | 0.44 | 0.55 | 0.88 | 0.88 |
| Spiegel et al. [87][47] | 102 | 102 | 1.7 | NA | 1.45 ± 0.29 | NA | NA | 0.93 | NA | ||
| Miyamoto et al. [88][48] | 95 | 26 | NA | 2.08 ± 0.55 | 1.80 ± 0.68 | 1.82 | NA | 0.65 | NA | 0.77 | NA |
| Chung et al. [89][49] | 51 | 27 | 2.5 | 1.53 ± 0.27 | 1.35 ± 0.24 | 1.74 | 1.79 | 0.85 | 1.00 | 0.54 | 0.68 |
| Novellino et al. [90][50] | 70 | 20 | NA | NA | 1.10 ± 0.09 | NA | 1.0 | 1.0 | |||
| Sawada et al. [91][51] | 400 | 267 | 3.2 | 1.66 ± 0.33 | 1.44 ± 0.39 | 1.92 | 1.68 | 0.81 | 0.84 | 0.85 | 0.89 |
| Fröhlich et al. [92][52] | 50 | 39 | NA | NA | 1.48 ± 0.46 | NA | 1.60 | NA | 0.87 | NA | 0.46 |
| Ishibashi et al. [93][53] | 39 | 24 | 2.41 | 1.66 ± 0.45 | 1.46 ± 0.41 | 1.95 | 1.60 | 0.79 | 0.93 | 0.70 | 0.93 |
| Izawa et al. [94][54] | 80 | 44 | NA | 1.67 ± 0.37 | NA | 1.66 | NA | 0.60 | NA | NA | |
| Kikuchi et al. [95][55] | 84 | 42 | NA | NA | 1.55 ± 0.30 | NA | 1.75 | NA | 0.85 | NA | 0.76 |
| Kurata et al. [96][56] | 295 | 166 | 2.96 | 1.74 ± 0.41 | 1.53 ± 0.48 | NA | NA | NA | |||
| Muxí et al. [97][57] | 28 | 14 | 1.57 | 1.28 ± 0.11 | 1.12 ± 0.11 | 1.48 | 1.43 | 0.86 | 0.93 | 0.92 | 1.00 |
| Südmeyer et al. [98][58] | 48 | 31 | NA | NA | 1.34 ± 0.27 | NA | 1.34 | NA | 0.88 | NA | 0.65 |
| Behnke et al. [99][59] | 42 | 42 | 1.47 | NA | 1.47 ± 0.31 | NA | NA | NA | |||
| Chiaravalloti et al. [100][60] | 37 | 37 | 1.67 | 1.72 ± 0.33 | 1.6 ± 0.32 | NA | NA | NA | |||
| Umemura et al. [101][61] | 138 | 118 | NA | NA | 1.75 ± 0.63 | NA | 1.85 | NA | 0.67 | NA | 0.80 |
| Leite et al. [102][62] | 21 | 21 | 2 | 1.53 ± 0.27 | 1.46 ± 0.29 | 1.8 | 1.7 | NA | NA | ||
| Katagiri et al. [66][26] | 100 | 50 | 2.3 | 2.05 ± 0.68 | 1.84 ± 0.88 | NA | NA | NA | |||
| Mochizuki et al. [103][63] | 357 | 191 | 2.3 | 1.91 ± 0.51 | 1.62 ± 0.60 | NA | NA | NA | |||
| Rocchi et al. [104][64] | 27 | 27 | 2.4 | NA | 1.53 ± 0.39 | NA | NA | NA | 0.70 | ||
| Tsujikawa et al. [49][8] | 70 | 70 | 2.1 | 1.83 ± 0.40 | 1.69 ± 0.48 | 1.90 | 1.97 | 1.00 | 0.64 | 1.00 | 0.71 |
| Fujita et al. [105][65] | 139 | 101 | 2.7 | NA | 1.9 ± 0.1 | NA | 2.00 | NA | 0.70 | NA | 0.86 |
| Uyama et al. [106][66] | 34 | 15 | NA | NA | 2.19 ± 0.55 | NA | 2.74 | NA | 0.86 | NA | 0.79 |
| Yang et al. [107][67] | 64 | 25 | 2 | 1.65 ± 0.36 | 1.50 ± 0.43 | NA | NA | NA | |||
| Gabilondo et al. [108][68] | 194 | 85 | NA | 1.80 ± 0.51 | 1.60 ± 0.46 | 2.16 | NA | 0.87 | NA | 0.89 | NA |
| Brandl et al. [109][69] | 167 | 104 | 2.0 | NA | 1.26 ± 0.24 | NA | NA | 0.94 | NA | 0.65 | |
| Kawazoe et al. [58][18] | 600 | 272 | 2.39 | NA | NA | 2.00 | 2.00 | 0.74 | 0.82 | 0.75 | 0.84 |
| Skowronek et al. [110][70] | 36 | 11 | NA | NA | 1.5 ± 0.5 | NA | NA | NA | 0.73 | ||
| Jeong et al. [35][71] | 60 | 60 | 2.2 | 1.39 ± 0.15 | 1.31 ± 0.15 | NA | NA | NA | |||
| Sakuramoto et al. [111][72] | 96 | 70 | 2.6 | NA | 1.99 ± 0.89 | NA | 2.00 | NA | 0.67 | NA | 1.00 |
| Brumberg et al. [112][73] | 42 | 21 | NA | NA | 1.94 ± 0.63 | NA | 2.76 | NA | 0.90 | NA | 0.66 |
| Jang et al. [113][74] | 31 | 31 | NA | 2.10 ± 0.87 | 1.85 ± 1.22 | NA | NA | NA | |||
| Iwabuchi et al. [114][75] | 216 | 90 | NA | 1.99 ± 0.44 | 1.82 ± 0.54 | NA | 2.26 | NA | NA | ||
| Eckhardt et al. [63][23] | 31 | 19 | 2.42 | NA | 1.18 ± 0.19 | NA | NA | 0.89 | NA | 0.67 | |
| Miyagi et al. [115][76] | 28 | 17 | 2.0 | 1.92 ± 0.56 | 1.69 ± 0.71 | 2.2 | 2.2 | NA | NA | ||
Abbreviations: H/M, heart-to-mediastinum; H&Y, Hoehn and Yahr stages; N, number of participants; NA, not available/not applicable; PD, Parkinson’s disease. a The mean scores of Hoehn and Yahr stages; b the mean scores and standard deviation of H/M ratio; c the sensitivity and specificity were described according to early and delayed phase registration. When only a number is provided, the results represent the authors’ description of general data.
4. Autonomic Function in PD and Cardiac MIBG
The investigation of autonomic function in the clinical setting is based on measuring blood pressure, heart rate, variability in the heart rate, and the effect of different body positions on blood pressure. The vagus nerve influences parasympathetic activity in the heart, which has a fast response with higher frequency modulation. On the other hand, sympathetic activity in the heart is associated with the paravertebral ganglia and has a slow response with lower frequency modulation. There are several tests to investigate autonomic function, including orthostatic reaction, deep breathing, and the Valsalva maneuver [116][77]. Cardiac MIBG is a method that can particularly assess sympathetic myocardial function.
The assessment of autonomic function with different methods and cardiac MIBG in individuals with PD has contradictory results. Nakahara et al. assessed the correlation between heart rate variability analysis and cardiac MIBG uptake in patients with PD and found no association between these two variables [117][78]. In this context, some authors observed a significant correlation between cardiac MIBG uptake and sympathetic and parasympathetic function [33][79]. However, other authors found no statistically significant correlation [118][80]. Berganzo et al. studied the relationship between Scales for Outcomes in PD–Autonomic chapter (SCOPA-AUT) scores and cardiac MIBG uptake. The severity of dysautonomia measured using SCOPA-AUT was not correlated with clinical severity, time since onset, or the early and delayed registrations of the H/M ratio. In patients with PD, the only variable associated with a delayed H/M ratio was the age at the onset of the disease [119][81].
Manabe et al. found an interesting correlation between systematic blood pressure and cardiac MIBG uptake [120][82]. They monitored the circadian blood pressure patterns of 37 patients with PD. The authors found that a nocturnal percentage decline in arterial blood pressure was associated with the H/M ratio on early and delayed images (p < 0.01). In this context, Kim et al. discovered that orthostatic hypotension was closely associated with cardiac sympathetic denervation observed on cardiac MIBG in patients with early and mild PD [121][83]. This is interesting because ambulatory blood pressure can be easily accessed in clinical practice. Also, the measurement of systematic blood pressure can be used as a primary step for assessing the significance of further workup. In cases without neurogenic orthostatic hypotension, baroreflex sensitivity and low-frequency diastolic blood pressure are the best predictors of cardiac sympathetic denervation at rest [122][84].
Individuals with PD presenting with syncope/presyncope have more common dysfunction of the cardiovascular autonomic system. In this way, some authors proposed that cardiac MIBG should be used to help identify patients with an elevated risk of syncope episodes and that the results should be used to choose the best management [123][85]. Other studies found an association between the body mass index and the development of cardiac MIBG uptake abnormality in individuals with PD [124][86]. However, these data should be cautiously analyzed due to the possible influence of metabolic diseases in the autonomic system, leading to abnormal cardiac MIBG results.
The Valsalva maneuver is divided into four phases based on systolic blood pressure and heart rate. The last phase is associated with an overshoot of blood pressure due to activation of the sympathetic system [125][87]. Also, the overshoot of blood pressure leads to baroreflex stimulation, leading to bradycardia and the return of blood pressure to the baseline. The fourth phase is directly related to sympathetic function, so in patients with PD, the most affected phase will be the last. One study found that cardiac MIBG uptake was moderate (r = 0.648, p = 0.0003) and associated with a reduction in the overshoot of the fourth phase of Valsalva maneuvers in individuals with PD [126][88]. This association could reflect postganglionic sympathetic noradrenergic impairment but also central baroreflex-sympathoneural failure. Other authors found an association between cardiac MIBG uptake and the coefficient of variation for intervals in resting and deep breathing, suggesting parasympathetic dysfunction [127][89]. Therefore, sleep behavior disorder may be not directly associated with cardiac MIBG; instead, it is autonomic dysfunction that plays a role in the study of both sleep behavior disorder and cardiac MIBG.
Autonomic dysfunction may be a confounding factor in many studies investigating specific clinical manifestations of PD with cardiac MIBG. For example, sleep behavior disorder in PD was already considered an independent risk factor for abnormal cardiac MIBG uptake. However, this could be a confounding factor between autonomic dysfunction and PD progression without peripheral signs. Therefore, sleep behavior disorder maybe not be directly associated with cardiac MIBG. Instead, it is autonomic dysfunction that plays a role in the study of both sleep behavior disorder and cardiac MIBG.
5. Parkinson’s Disease Subtypes and Cardiac MIBG
Three main types of PD are characterized by tremor-dominant, akinetic-rigid form, and mixed condition. There are significant clinical differences among these historical subtypes. Chung et al. revealed that individuals with tremor-dominant PD usually have normal cardiac MIBG uptake, but the akinetic-rigid and mixed subtypes have lower levels of cardiac MIBG uptake. No statistical difference was observed between akinetic-rigid and mixed subtypes regarding cardiac scintigraphy [128][90]. Noteworthy, Chiaravalloti et al. presented opposite findings, where the cardiac sympathetic system was more severely impaired in the tremor-dominant subtype than in the akinetic-rigid subtype [129][91]. But the Chiaravalloti et al. cohort of individuals with tremor-dominant was older, which can be associated with more confounding variables such as aging and possible comorbidities influencing the autonomic nervous system.
Some indirect assumptions can be made from the findings of Chung et al. In this way, the tremor-dominant subtype may be a less severe disease with minimal progression to the periphery. Interestingly, patients presenting with the predominant akinetic-rigid form have a more severe motor impairment, more severe disabilities, and more commonly present non-motor symptoms [130][92].
6. Genetic Causes of Parkinson’s Disease
The neuropathological and clinical manifestation findings vary among the different forms of genetic parkinsonism. Parkinson’s disease-1 (PARK1) carriers usually have an aggressive Lewy body pathology [131][93]. On the other hand, in patients with leucine-rich repeat kinase 2 (LRRK2) gene mutation (PARK8), variable degrees of Lewy body pathology are observed [132][94]. Interestingly, some patients, mainly those with PARK2, are considered almost free of Lewy body lesions. Therefore, the study of cardiac MIBG in these pathologies can significantly contribute to understanding the clinical manifestations and spectrum of these conditions.
The results found in the literature regarding genetic causes of PD are controversial. Two studies found that individuals with hereditary PD, compared with idiopathic PD, had normal or less impaired cardiac MIBG uptake. However, the studies did not perform a subgroup analysis of the different genetic forms of PD with cardiac MIBG uptake [133,134][95][96]. Gabilondo et al. studied 194 patients with suspected synucleinopathy or atypical parkinsonism, in which 34 individuals had a genetic diagnosis of PD. The authors found a significantly reduced uptake for individuals with PARK1 but not for PARK2 or PARK 8 [108][68]. Tijero et al. found similar results: cardiac MIBG abnormalities were less common in PARK2 mutation carriers than in patients with idiopathic PD [135][97].
Ruiz Martínez et al. investigated olfactory dysfunction and the changes in cardiac MIBG uptake in patients with PD carrying R1441G and G2019S mutations in LRRK2 [136][98]. The authors found that olfactory and cardiac impairments are less prevalent when PD is associated with mutations in LRRK2. In early registration, the difference was statistically significant, where LRRK2 noncarriers had a H/M of 1.49 ± 0.28 and LRRK2 carriers had 1.75 ± 0.38 [136][98]. Interestingly, Valldeoriola et al. [137][99] found similar results as the study of Ruiz Martínez et al. [136][98]. Also, Valldeoriola et al. revealed a moderate relationship between olfactory testing and the H/M ratio on early and late cardiac MIBG uptake (early: r = 0.62 p < 0.02; late: r = 0.68 p = 0.01) [137][99].
Patients with PD with glucocerebrosidase gene mutations are known to show more rapid clinical progression than sporadic PD without glucocerebrosidase gene mutation [138][100]. Kim et al. studied patients with PD with and without this mutation. They found that cardiac sympathetic denervation and non-motor symptoms (orthostatic hypotension, rapid eye movement sleep behavior disorder) were more common in individuals with glucocerebrosidase gene mutations. Also, the delayed H/M ratio between the two groups was significant (p = 0.043), in which individuals with the mutation had 1.58 ± 0.29 and the participants with sporadic PD had 1.82 ± 0.25 [139][101].
7. Normal Cardiac MIBG Uptake in Parkinson’s Disease
An abnormal result in cardiac MIBG uptake in individuals with parkinsonism can be a supporting criterion for diagnosing PD. However, a normal result of cardiac MIBG in individuals with suspicion of parkinsonian syndrome does not exclude the diagnosis of PD.
Tsujikawa et al. particularly evaluated individuals with normal results in a cardiac MIBG study. They found that patients with PD with normal or mildly low MIBG uptakes at the early stages of illness were predominantly females, with young disease onset, slow disease progression in motor dysfunctions, and preserved cognitive function [140][102]. It is worth mentioning that the authors did not perform genetic analysis in their study, which can lead to misinterpretations. One possible explanation is that the variety of anatomical distributions of Lewy bodies could influence the variety of MIBG uptakes among patients with PD [141][103].
An interesting fact related to normal MIBG scans is that this group of individuals apparently have more significant damage from synuclein pathology in motor function than in non-motor manifestations compared with those with decreased MIBG, even when this variable was adjusted. Also, individuals with PD and normal cardiac MIBG have a relatively low disease burden compared with those with abnormal MIBG [142][104]. Interestingly, the relationship between disease burden and abnormal cardiac MIBG uptake was shown to be independent of orthostatic blood pressure fluctuations, suggesting that extracranial cardiac markers might reflect disease burden and disease progression in the central nervous system [143][105].
Oh et al. investigated dopamine transporter activity of the corpus striatum and thalamus according to cardiac MIBG uptake in patients with PD. They found that individuals with PD with normal cardiac MIBG uptake had preserved dopamine reserve in the globus pallidus region [144][106]. In this way, the progression of the disease to the brainstem can explain cardiac sympathetic denervation. And dopaminergic loss in the globus pallidus may be associated with the complex circuitry in the midbrain.
8. Sleep Behavior Disorder, Cognition, and Dysphagia
Isolated rapid-eye-movement sleep behavior disorder (iRBD) is a prodromal stage of Lewy body disease and multiple system atrophy. Park et al. investigated plasma neurofilament light chain and cardiac MIBG uptake as predictors for phenoconversion. They found that elevated plasma neurofilament light chain levels may suggest imminent phenoconversion to multiple system atrophy, whereas low cardiac MIBG uptake suggests phenoconversion to Lewy body disease [145][107]. A possible explanation for the association between the plasma neurofilaments light chain levels and multiple system atrophy is the fact that neurodegeneration in this condition usually involves pontine nuclei leading to axonal degeneration in projecting fibers, which have large myelinated axons that are abundant in neurofilaments light chain [146][108].
Cognitive impairment in individuals with Lewy body pathology can be related to a cardiovascular autonomic dysfunction or the direct lesion of Lewy bodies in the neocortex [147][109]. Interestingly, in individuals with PD, there is a strong relationship between cognitive impairment and the degree of autonomic dysfunction in MIBG, which can suggest impaired microvascular circulation or invasion of α-synuclein in the central nervous system. Noteworthy, abnormal cognitive function is not associated with autonomic dysfunction in individuals with dementia with Lewy bodies, which may suggest initial involvement of Lewy body pathology in the neocortex [148][110].
Youn et al. found an association between cardiac sympathetic denervation and the presence and severity of dysphagia in patients with PD. The mean early and late H/M ratios were significantly lower in the PD with dysphagia group than the without dysphagia group (1.39 ± 0.21 vs. 1.86 ± 0.21, p < 0.01; 1.26 ± 0.18 vs. 1.82 ± 0.29, p < 0.01). The mechanism of dysphagia in PD is poorly understood, and these findings may suggest a cholinergic and noradrenergic dysfunction associated with striatonigral lesions [149][111].
9. Cardiac MIBG Scintigraphy in Pre-Motor Parkinson’s Disease
A relationship exists between motor symptoms and Lewy bodies and a-synuclein aggregation in the substantia nigra pars compacta. However, non-motor symptoms are believed to be independent of substantia nigra pathology, suggesting possible different mechanisms for the degenerative processes. Therefore, methods to predict the development of PD should be studied. In the following table, there is a list of symptoms that were already assessed with cardiac MIBG for the understanding and development of PD (Table 32).
Table 32.
Cardiac MIBG scintigraphy in pre-motor Parkinson’s disease.
| Symptom | Description | Differential Diagnosis | Reference |
|---|---|---|---|
| Constipation | Constipation and abnormal cardiac MIBG were associated with the initial manifestation of PD. One in every four individuals with this presentation will have a diagnosis of PD. | DLB | [22,150][112][113] |
| Postural hypotension | Pure autonomic failure may be a risk factor for the development of PD. There is a significant association between reduced cardiac MIBG in patients with pure autonomic failure and the development of PD. | MSA | [81,151][41][114] |
| Cognitive impairment | Most patients who present with cognitive impairment and abnormal cardiac MIBG will develop dementia with Lewy bodies; only a small percentage of individuals will develop PD. Reduced cardiac MIBG may be associated with a subsequent risk of dementia and may reflect the wider extension of alpha-synuclein pathology. | DLB | [152,153][115][116] |
| Depression/ anxiety | Depression is among the most common neuropsychiatric disorders in PD. Of 13 patients presenting with depression and anxiety and abnormal cardiac MIBG, 11 developed PD years later. | DLB | [154,155][117][118] |
| Visual hallucinations | Visual hallucinations are a possible risk factor for the development of PD. Four individuals presented with visual hallucinations and abnormal cardiac MIBG, but no discussion is given in the follow-up of these individuals. Also, visual hallucinations is an independent risk factor for abnormal cardiac MIBG uptake. | Corticobasal degeneration | [154,156][117][119] |
| Sleep disorders | Low MIBG uptake and rapid eye movement sleep behavior disorder was associated with PD severity. Also, REM sleep behavior disorder is an independent risk factor associated with abnormal MIBG uptake in individuals with PD. | DLBMSA | [157,158,159][120][121][122] |
| Olfactory | A relationship between abnormal MIBG uptake and hyposmia in the early prodromal stage of PD (before nigrostriatal degeneration) was observed. Degeneration in broad aspects of the cardiovascular sympathetic system occurs concurrently with olfactory system degeneration during the premotor phase of PD. | DLB | [160,161][123][124] |
| Gait | Abnormal MIBG uptake was already associated with a higher incidence of falls. This may be an indirect finding of autonomic dysfunction. Also, cardiac MIBG uptake correlated significantly with the annual progress of rigidity and axial symptoms. | MSA | [162,163][125][126] |
| Fatigue | The results in the literature are contradictory. Some authors found, and others did not, a correlation between cardiac MIBG uptake and PD-related fatigue. | MSA | [164,165][127][128] |
Abbreviations: DLB, dementia with Lewy bodies; MSA, multiple system atrophy; PD, Parkinson’s disease.
References
- Mitchell, G.A. The Innervation of the Heart. Br. Heart J. 1953, 15, 159–171.
- Burke, R.E.; Dauer, W.T.; Vonsattel, J.P. A Critical Evaluation of the Braak Staging Scheme for Parkinson’s Disease. Ann. Neurol. 2008, 64, 485–491.
- Mantri, S.; Morley, J.F.; Siderowf, A.D. The Importance of Preclinical Diagnostics in Parkinson Disease. Park. Relat. Disord. 2019, 64, 20–28.
- Mitsui, J.; Saito, Y.; Momose, T.; Shimizu, J.; Arai, N.; Shibahara, J.; Ugawa, Y.; Kanazawa, I.; Tsuji, S.; Murayama, S. Pathology of the Sympathetic Nervous System Corresponding to the Decreased Cardiac Uptake in 123I-Metaiodobenzylguanidine (MIBG) Scintigraphy in a Patient with Parkinson Disease. J. Neurol. Sci. 2006, 243, 101–104.
- Watanabe, H.; Sobue, G. Filling in the Missing Puzzle Piece between Cardiac MIBG Scintigraphy Findings and Parkinson’s Disease Pathology. J. Neurol. Neurosurg. Psychiatry 2015, 86, 937.
- Nakamura, T.; Hirayama, M.; Ito, H.; Takamori, M.; Hamada, K.; Takeuchi, S.; Watanabe, H.; Koike, Y.; Sobue, G. Dobutamine Stress Test Unmasks Cardiac Sympathetic Denervation in Parkinson’s Disease. J. Neurol. Sci. 2007, 263, 133–138.
- Nakamura, T.; Hirayama, M.; Yamashita, F.; Uchida, K.; Hama, T.; Watanabe, H.; Sobue, G. Lowered Cardiac Sympathetic Nerve Performance in Response to Exercise in Parkinson’s Disease. Mov. Disord. 2010, 25, 1183–1189.
- Takahashi, M.; Ikemura, M.; Oka, T.; Uchihara, T.; Wakabayashi, K.; Kakita, A.; Takahashi, H.; Yoshida, M.; Toru, S.; Kobayashi, T.; et al. Quantitative Correlation between Cardiac MIBG Uptake and Remaining Axons in the Cardiac Sympathetic Nerve in Lewy Body Disease. J. Neurol. Neurosurg. Psychiatry 2015, 86, 939–944.
- Lamotte, G.; Goldstein, D.S. What New Can We Learn from Cardiac Sympathetic Neuroimaging in Synucleinopathies? Clin. Auton. Res. 2022, 32, 95–98.
- Kikuchi, K.; Hiwatashi, A.; Togao, O.; Yamashita, K.; Somehara, R.; Kamei, R.; Baba, S.; Yamaguchi, H.; Kira, J.I.; Honda, H. Structural Changes in Parkinson’s Disease: Voxel-Based Morphometry and Diffusion Tensor Imaging Analyses Based on 123I-MIBG Uptake. Eur. Radiol. 2017, 27, 5073–5079.
- Park, D.G.; Kang, J.; An, Y.-S.; Chang, J.; Yoon, J.H. Association of Plasma α-Synuclein with Cardiac 123I-MIBG Scintigraphy in Early Parkinson’s Disease. Neurosci. Lett. 2022, 770, 136399.
- Yamada, M.; Komatsu, J.; Nakamura, K.; Sakai, K.; Samuraki-Yokohama, M.; Nakajima, K.; Yoshita, M. Diagnostic Criteria for Dementia with Lewy Bodies: Updates and Future Directions. J. Mov. Disord. 2020, 13, 1–10.
- Politis, M.; Niccolini, F. Serotonin in Parkinson’s Disease. Behav. Brain Res. 2015, 277, 136–145.
- Politis, M.; Loane, C. Serotonergic Dysfunction in Parkinson’s Disease and Its Relevance to Disability. Sci. World J. 2011, 11, 1726–1734.
- Murakami, H.; Yamamoto, K.; Yasumoto, T.; Kimura, A.; Sakae, Y.; Nomoto, S.; Kubota, S.; Watanabe, D.; Watanabe, K.; Saito, Y.; et al. Cerebrospinal Fluid 5-HIAA Concentrations Correlate with Cardiac Uptake of 123I-MIBG during Myocardial Scintigraphy in Drug Naïve Parkinson’s Disease. J. Neural. Transm. 2018, 125, 1511–1514.
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of Clinical Diagnosis of Idiopathic Parkinson’s Disease: A Clinico-Pathological Study of 100 Cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184.
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS Clinical Diagnostic Criteria for Parkinson’s Disease. Mov. Disord. 2015, 30, 1591–1601.
- Kawazoe, M.; Arima, H.; Maeda, T.; Tsuji, M.; Mishima, T.; Fujioka, S.; Tsugawa, J.; Tsuboi, Y. Sensitivity and Specificity of Cardiac 123I-MIBG Scintigraphy for Diagnosis of Early-Phase Parkinson’s Disease. J. Neurol. Sci. 2019, 407, 116409.
- Wang, T.; Wu, K.Y.; Miner, R.C.; Renaud, J.M.; Beanlands, R.S.B.; deKemp, R.A. Reproducible Quantification of Cardiac Sympathetic Innervation Using Graphical Modeling of Carbon-11-Meta-Hydroxyephedrine Kinetics with Dynamic PET-CT Imaging. EJNMMI Res. 2018, 8, 63.
- Kwon, S.H.; Yoon, J.-K.; Yoon, J.H.; Lee, S.J.; Jo, K.S.; Lee, D.H.; An, Y.-S. The Utility of Segmental Analysis in Cardiac I-123 MIBG SPECT in Parkinson’s Disease. Nucl. Med. Mol. Imaging 2015, 49, 298–302.
- Courbon, F.; Brefel-Courbon, C.; Thalamas, C.; Alibelli, M.-J.; Berry, I.; Montastruc, J.-L.; Rascol, O.; Senard, J.-M. Cardiac MIBG Scintigraphy Is a Sensitive Tool for Detecting Cardiac Sympathetic Denervation in Parkinson’s Disease. Mov. Disord. 2003, 18, 890–897.
- Somsen, G.A.; Verberne, H.J.; Fleury, E.; Righetti, A. Normal Values and Within-Subject Variability of Cardiac I-123 MIBG Scintigraphy in Healthy Individuals: Implications for Clinical Studies. J. Nucl. Cardiol. 2004, 11, 126–133.
- Eckhardt, C.; Krismer, F.; Donnemiller, E.; Eschlböck, S.; Fanciulli, A.; Raccagni, C.; Bösch, S.; Mair, K.; Scherfler, C.; Djamshidian, A.; et al. Cardiac Sympathetic Innervation in Parkinson’s Disease versus Multiple System Atrophy. Clin. Auton. Res. 2022, 32, 103–114.
- Goldstein, D.S.; Holmes, C.S.; Dendi, R.; Bruce, S.R.; Li, S.T. Orthostatic Hypotension from Sympathetic Denervation in Parkinson’s Disease. Neurology 2002, 58, 1247–1255.
- Wong, K.K.; Raffel, D.M.; Koeppe, R.A.; Frey, K.A.; Bohnen, N.I.; Gilman, S. Pattern of Cardiac Sympathetic Denervation in Idiopathic Parkinson Disease Studied with 11C Hydroxyephedrine PET. Radiology 2012, 265, 240–247.
- Katagiri, A.; Asahina, M.; Araki, N.; Poudel, A.; Fujinuma, Y.; Yamanaka, Y.; Kuwabara, S. Myocardial 123I-MIBG Uptake and Cardiovascular Autonomic Function in Parkinson’s Disease. Park. Dis. 2015, 2015, 805351.
- Goetz, C.G.; Poewe, W.; Rascol, O.; Sampaio, C.; Stebbins, G.T.; Counsell, C.; Giladi, N.; Holloway, R.G.; Moore, C.G.; Wenning, G.K.; et al. Movement Disorder Society Task Force Report on the Hoehn and Yahr Staging Scale: Status and Recommendations. Mov. Disord. 2004, 19, 1020–1028.
- Yoshita, M. Differentiation of Idiopathic Parkinson’s Disease from Striatonigral Degeneration and Progressive Supranuclear Palsy Using Iodine-123 Meta-Iodobenzylguanidine Myocardial Scintigraphy. J. Neurol. Sci. 1998, 155, 60–67.
- Braune, S.; Reinhardt, M.; Bathmann, J.; Krause, T.; Lehmann, M.; Lücking, C.H. Impaired Cardiac Uptake of Meta-Iodobenzylguanidine in Parkinson’s Disease with Autonomic Failure. Acta Neurol. Scand. 1998, 97, 307–314.
- Iwasa, K.; Nakajima, K.; Yoshikawa, H.; Tada, A.; Taki, J.; Takamori, M. Decreased Myocardial 123I-MIBG Uptake in Parkinson’s Disease. Acta Neurol. Scand. 1998, 97, 303–306.
- Braune, S.; Reinhardt, M.; Schnitzer, R.; Riedel, A.; Lücking, C.H. Cardiac Uptake of MIBG Separates Parkinson’s Disease from Multiple System Atrophy. Neurology 1999, 53, 1020–1025.
- Orimo, S.; Ozawa, E.; Nakade, S.; Sugimoto, T.; Mizusawa, H. 123I-Metaiodobenzylguanidine Myocardial Scintigraphy in Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 1999, 67, 189–194.
- Druschky, A.; Hilz, M.J.; Platsch, G.; Radespiel-Tröger, M.; Druschky, K.; Kuwert, T.; Neundörfer, B. Differentiation of Parkinson’s Disease and Multiple System Atrophy in Early Disease Stages by Means of I-123-MIBG-SPECT. J. Neurol. Sci. 2000, 175, 3–12.
- Taki, J.; Nakajima, K.; Hwang, E.H.; Matsunari, I.; Komai, K.; Yoshita, M.; Sakajiri, K.; Tonami, N. Peripheral Sympathetic Dysfunction in Patients with Parkinson’s Disease without Autonomic Failure Is Heart Selective and Disease Specific. Eur. J. Nucl. Med. 2000, 27, 566–573.
- Reinhardt, M.J.; Jüngling, F.D.; Krause, T.M.; Braune, S. Scintigraphic Differentiation between Two Forms of Primary Dysautonomia Early after Onset of Autonomic Dysfunction: Value of Cardiac and Pulmonary Iodine-123 MIBG Uptake. Eur. J. Nucl. Med. 2000, 27, 595–600.
- Takatsu, H.; Nishida, H.; Matsuo, H.; Watanabe, S.; Nagashima, K.; Wada, H.; Noda, T.; Nishigaki, K.; Fujiwara, H. Cardiac Sympathetic Denervation from the Early Stage of Parkinson’s Disease: Clinical and Experimental Studies with Radiolabeled MIBG. J. Nucl. Med. 2000, 41, 71–77.
- Hamada, K.; Hirayama, M.; Watanabe, H.; Kobayashi, R.; Ito, H.; Ieda, T.; Koike, Y.; Sobue, G. Onset Age and Severity of Motor Impairment Are Associated with Reduction of Myocardial 123I-MIBG Uptake in Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 2003, 74, 423–426.
- Orimo, S.; Ozawa, E.; Nakade, S.; Hattori, H.; Tsuchiya, K.; Taki, K.; Takahashi, A. Meta-Iodobenzylguanidine Myocardial Scintigraphy Differentiates Corticobasal Degeneration from Parkinson’s Disease. Intern. Med. 2003, 42, 127–128.
- Saiki, S.; Hirose, G.; Sakai, K.; Kataoka, S.; Hori, A.; Saiki, M.; Kaito, M.; Higashi, K.; Taki, S.; Kakeshita, K.; et al. Cardiac 123I-MIBG Scintigraphy Can Assess the Disease Severity and Phenotype of PD. J. Neurol. Sci. 2004, 220, 105–111.
- Nagayama, H.; Hamamoto, M.; Ueda, M.; Nagashima, J.; Katayama, Y. Reliability of MIBG Myocardial Scintigraphy in the Diagnosis of Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 2005, 76, 249–251.
- Kashihara, K.; Ohno, M.; Kawada, S.; Okumura, Y. Reduced Cardiac Uptake and Enhanced Washout of 123I-MIBG in Pure Autonomic Failure Occurs Conjointly with Parkinson’s Disease and Dementia with Lewy Bodies. J. Nucl. Med. 2006, 47, 1099–1101.
- Kim, J.S.; Lee, P.H.; Lee, K.S.; Park, J.W.; Kim, Y.I.; Chung, Y.A.; Kim, S.H.; Kim, S.H.; Kim, J.; Choi, Y.Y.; et al. Cardiac Metaiodobenzylguanidine Scintigraphy for Vascular Parkinsonism. Mov. Disord. 2006, 21, 1990–1994.
- Lee, P.H.; Kim, J.W.; Bang, O.Y.; Joo, I.S.; Yoon, S.-N.; Huh, K. Cardiac 123I-MIBG Scintigraphy in Patients with Essential Tremor. Mov. Disord. 2006, 21, 1235–1238.
- Miyamoto, T.; Miyamoto, M.; Inoue, Y.; Usui, Y.; Suzuki, K.; Hirata, K. Reduced Cardiac 123I-MIBG Scintigraphy in Idiopathic REM Sleep Behavior Disorder. Neurology 2006, 67, 2236–2238.
- Shin, D.H.; Lee, P.H.; Bang, O.Y.; Joo, I.S.; Huh, K. Clinical Implications of Cardiac-MIBG SPECT in the Differentiation of Parkinsonian Syndromes. J. Clin. Neurol. 2006, 2, 51–57.
- Köllensperger, M.; Seppi, K.; Liener, C.; Boesch, S.; Heute, D.; Mair, K.J.; Mueller, J.; Sawires, M.; Scherfler, C.; Schocke, M.F.; et al. Diffusion Weighted Imaging Best Discriminates PD from MSA-P: A Comparison with Tilt Table Testing and Heart MIBG Scintigraphy. Mov. Disord. 2007, 22, 1771–1776.
- Spiegel, J.; Hellwig, D.; Farmakis, G.; Jost, W.H.; Samnick, S.; Fassbender, K.; Kirsch, C.-M.; Dillmann, U. Myocardial Sympathetic Degeneration Correlates with Clinical Phenotype of Parkinson’s Disease. Mov. Disord. 2007, 22, 1004–1008.
- Miyamoto, T.; Miyamoto, M.; Suzuki, K.; Nishibayashi, M.; Iwanami, M.; Hirata, K. 123I-MIBG Cardiac Scintigraphy Provides Clues to the Underlying Neurodegenerative Disorder in Idiopathic REM Sleep Behavior Disorder. Sleep 2008, 31, 717–723.
- Chung, E.J.; Lee, W.Y.; Yoon, W.T.; Kim, B.J.; Lee, G.H. MIBG Scintigraphy for Differentiating Parkinson’s Disease with Autonomic Dysfunction from Parkinsonism-Predominant Multiple System Atrophy. Mov. Disord. 2009, 24, 1650–1655.
- Novellino, F.; Arabia, G.; Bagnato, A.; Cascini, G.L.; Salsone, M.; Nicoletti, G.; Messina, D.; Morelli, M.; Paglionico, S.; Giofrè, L.; et al. Combined Use of DAT-SPECT and Cardiac MIBG Scintigraphy in Mixed Tremors. Mov. Disord. 2009, 24, 2242–2248.
- Sawada, H.; Oeda, T.; Yamamoto, K.; Kitagawa, N.; Mizuta, E.; Hosokawa, R.; Ohba, M.; Nishio, R.; Yamakawa, K.; Takeuchi, H.; et al. Diagnostic Accuracy of Cardiac Metaiodobenzylguanidine Scintigraphy in Parkinson Disease. Eur. J. Neurol. 2009, 16, 174–182.
- Fröhlich, I.; Pilloy, W.; Vaillant, M.; Diederich, N.J. Myocardial MIBG Scintigraphy: A Useful Clinical Tool?: A Retrospective Study in 50 Parkinsonian Patients. Neurol. Sci. 2010, 31, 403–406.
- Ishibashi, K.; Saito, Y.; Murayama, S.; Kanemaru, K.; Oda, K.; Ishiwata, K.; Mizusawa, H.; Ishii, K. Validation of Cardiac 123I-MIBG Scintigraphy in Patients with Parkinson’s Disease Who Were Diagnosed with Dopamine PET. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 3–11.
- Izawa, M.O.; Miwa, H.; Kajimoto, Y.; Kondo, T. Combination of Transcranial Sonography, Olfactory Testing, and MIBG Myocardial Scintigraphy as a Diagnostic Indicator for Parkinson’s Disease. Eur. J. Neurol. 2012, 19, 411–416.
- Kikuchi, A.; Baba, T.; Hasegawa, T.; Sugeno, N.; Konno, M.; Takeda, A. Differentiating Parkinson’s Disease from Multiple System Atrophy by Meta-Iodobenzylguanidine Myocardial Scintigraphy and Olfactory Test. Park. Relat. Disord. 2011, 17, 698–700.
- Kurata, T.; Kametaka, S.; Ohta, Y.; Morimoto, N.; Deguchi, S.; Deguchi, K.; Ikeda, Y.; Takao, Y.; Ohta, T.; Manabe, Y.; et al. PSP as Distinguished from CBD, MSA-P and PD by Clinical and Imaging Differences at an Early Stage. Intern. Med. 2011, 50, 2775–2781.
- Muxí, A.; Paredes, P.; Navales, I.; Valldeoriola, F.; Gaig, C.; Lomeña, F.; de la Cerda, A.; Solà, O.; Domenech, B.; Tolosa, E.; et al. Diagnostic Cutoff Points for 123I-MIBG Myocardial Scintigraphy in a Caucasian Population with Parkinson’s Disease. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1139–1146.
- Südmeyer, M.; Antke, C.; Zizek, T.; Beu, M.; Nikolaus, S.; Wojtecki, L.; Schnitzler, A.; Müller, H.-W. Diagnostic Accuracy of Combined FP-CIT, IBZM, and MIBG Scintigraphy in the Differential Diagnosis of Degenerative Parkinsonism: A Multidimensional Statistical Approach. J. Nucl. Med. 2011, 52, 733–740.
- Behnke, S.; Hellwig, D.; Bürmann, J.; Runkel, A.; Farmakis, G.; Kirsch, C.M.; Fassbender, K.; Becker, G.; Dillmann, U.; Spiegel, J. Evaluation of Transcranial Sonographic Findings and MIBG Cardiac Scintigraphy in the Diagnosis of Idiopathic Parkinson’s Disease. Park. Relat. Disord. 2013, 19, 995–999.
- Chiaravalloti, A.; Stefani, A.; Di Biagio, D.; Pierantozzi, M.; Tavolozza, M.; Di Pietro, B.; Stanzione, P.; Schillaci, O. Cardiac Sympathetic Denervation Is Not Related to Nigrostriatal Degeneration in Parkinson’s Disease. Ann. Nucl. Med. 2013, 27, 444–451.
- Umemura, A.; Oeda, T.; Hayashi, R.; Tomita, S.; Kohsaka, M.; Yamamoto, K.; Sawada, H. Diagnostic Accuracy of Apparent Diffusion Coefficient and 123I-Metaiodobenzylguanidine for Differentiation of Multiple System Atrophy and Parkinson’s Disease. PLoS ONE 2013, 8, e61066.
- Leite, M.A.A.; Nascimento, O.J.M.; Pereira, J.S.; Amaral, C.; Mesquita, C.T.; Azevedo, J.C.; de Brito, A.S.X.; Pedras, F.V. Cardiac 123I-MIBG Uptake in de Novo Brazilian Patients with Parkinson’s Disease without Clinically Defined Dysautonomia. Arq. Neuropsiquiatr. 2014, 72, 430–434.
- Mochizuki, H.; Ebihara, Y.; Ugawa, Y.; Ishii, N.; Taniguchi, A.; Nagamachi, S.; Shiomi, K.; Nakazato, M. PR Prolongation and Cardiac 123I-MIBG Uptake Reduction in Parkinson’s Disease. Eur. Neurol. 2015, 74, 107–111.
- Rocchi, C.; Pierantozzi, M.; Galati, S.; Chiaravalloti, A.; Pisani, V.; Prosperetti, C.; Lauretti, B.; Stampanoni Bassi, M.; Olivola, E.; Schillaci, O.; et al. Autonomic Function Tests and MIBG in Parkinson’s Disease: Correlation to Disease Duration and Motor Symptoms. CNS Neurosci. Ther. 2015, 21, 727–732.
- Fujita, H.; Suzuki, K.; Numao, A.; Watanabe, Y.; Uchiyama, T.; Miyamoto, T.; Miyamoto, M.; Hirata, K. Usefulness of Cardiac MIBG Scintigraphy, Olfactory Testing and Substantia Nigra Hyperechogenicity as Additional Diagnostic Markers for Distinguishing between Parkinson’s Disease and Atypical Parkinsonian Syndromes. PLoS ONE 2016, 11, e0165869.
- Uyama, N.; Otsuka, H.; Shinya, T.; Otomi, Y.; Harada, M.; Sako, W.; Izumi, Y.; Kaji, R.; Watanabe, Y.; Takashi, S.; et al. The Utility of the Combination of a SPECT Study with -FP-CIT of Dopamine Transporters and -MIBG Myocardial Scintigraphy in Differentiating Parkinson Disease from Other Degenerative Parkinsonian Syndromes. Nucl. Med. Commun. 2017, 38, 487–492.
- Yang, T.; Wang, L.; Li, Y.; Cheng, M.; Jiao, J.; Wang, Q.; Guo, H. 131I-MIBG Myocardial Scintigraphy for Differentiation of Parkinson’s Disease from Multiple System Atrophy or Essential Tremor in Chinese Population. J. Neurol. Sci. 2017, 373, 48–51.
- Gabilondo, I.; Llorens, V.; Rodriguez, T.; Fernández, M.; Concha, T.P.; Acera, M.; Tijero, B.; Murueta-Goyena, A.; Del Pino, R.; Cortés, J.; et al. Myocardial MIBG Scintigraphy in Genetic Parkinson’s Disease as a Model for Lewy Body Disorders. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 376–384.
- Brandl, S.J.; Braune, S. Sensitivity and Specificity of Cardiac Metaiodobenzylguanidine Scintigraphy in the Early Diagnosis of Parkinson’s Disease. Clin. Auton. Res. 2019, 29, 567–574.
- Skowronek, C.; Zange, L.; Lipp, A. Cardiac 123I-MIBG Scintigraphy in Neurodegenerative Parkinson Syndromes: Performance and Pitfalls in Clinical Practice. Front. Neurol. 2019, 10, 152.
- Jeong, Y.J.; Jeong, J.E.; Cheon, S.M.; Yoon, B.A.; Kim, J.W.; Kang, D.Y. Relationship between the Washout Rate of I-123 MIBG Scans and Autonomic Function in Parkinson’s Disease. PLoS ONE 2020, 15, e0229860.
- Sakuramoto, H.; Fujita, H.; Suzuki, K.; Matsubara, T.; Watanabe, Y.; Hamaguchi, M.; Hirata, K. Combination of Midbrain-to-Pontine Ratio and Cardiac MIBG Scintigraphy to Differentiate Parkinson’s Disease from Multiple System Atrophy and Progressive Supranuclear Palsy. Clin. Park. Relat. Disord. 2020, 2, 20–24.
- Brumberg, J.; Kuzkina, A.; Lapa, C.; Mammadova, S.; Buck, A.; Volkmann, J.; Sommer, C.; Isaias, I.U.; Doppler, K. Dermal and Cardiac Autonomic Fiber Involvement in Parkinson’s Disease and Multiple System Atrophy. Neurobiol. Dis. 2021, 153, 105332.
- Jang, W.; Lee, J.Y.; Kim, J.Y.; Lee, S.J.; Kim, T.Y.; Choi, Y.Y.; Kim, H.-T.; Kim, C.K. Intrasubject Relationship between Striatal 18F-FP-CIT Uptake and Cardiac 123I-MIBG Uptake Differs by Motor Subtype in Early Parkinson Disease. Medicine 2021, 100, e26995.
- Iwabuchi, Y.; Kameyama, M.; Matsusaka, Y.; Narimatsu, H.; Hashimoto, M.; Seki, M.; Ito, D.; Tabuchi, H.; Yamada, Y.; Jinzaki, M. A Diagnostic Strategy for Parkinsonian Syndromes Using Quantitative Indices of DAT SPECT and MIBG Scintigraphy: An Investigation Using the Classification and Regression Tree Analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1833–1841.
- Miyagi, T.; Yamazato, M.; Nakamura, T.; Tokashiki, T.; Namihira, Y.; Kokuba, K.; Ishihara, S.; Sakima, H.; Ohya, Y. Power Spectral Analysis of Heart Rate Variability Is Useful as a Screening Tool for Detecting Sympathetic and Parasympathetic Nervous Dysfunctions in Parkinson’s Disease. BMC Neurol. 2022, 22, 339.
- Low, P.A.; Tomalia, V.A.; Park, K.-J. Autonomic Function Tests: Some Clinical Applications. J. Clin. Neurol. 2013, 9, 1–8.
- Nakahara, K.; Nakane, S.; Ando, Y. Correlation of heart rate variability analysis and MIBG myocardial scintigraphy in patients with Parkinson’s disease. Amyloid 2019, 26, 146–147.
- Spiegel, J. Diagnostic and Pathophysiological Impact of Myocardial MIBG Scintigraphy in Parkinson’s Disease. Park. Dis. 2010, 2010, 295346.
- Haensch, C.-A.; Lerch, H.; Jörg, J.; Isenmann, S. Cardiac Denervation Occurs Independent of Orthostatic Hypotension and Impaired Heart Rate Variability in Parkinson’s Disease. Park. Relat. Disord. 2009, 15, 134–137.
- Berganzo, K.; Tijero, B.; Somme, J.H.; Llorens, V.; Sánchez-Manso, J.C.; Low, D.; Iodice, V.; Vichayanrat, E.; Mathias, C.J.; Lezcano, E.; et al. SCOPA-AUT Scale in Different Parkinsonisms and Its Correlation with (123) I-MIBG Cardiac Scintigraphy. Park. Relat. Disord. 2012, 18, 45–48.
- Manabe, Y.; Fujii, D.; Kono, S.; Sakai, Y.; Tanaka, T.; Narai, H.; Omori, N.; Imai, Y.; Abe, K. Systemic Blood Pressure Profile Correlates with Cardiac 123I-MIBG Uptake in Patients with Parkinson’s Disease. J. Neurol. Sci. 2011, 307, 153–156.
- Kim, J.-S.; Park, H.-E.; Oh, Y.-S.; Lee, S.-H.; Park, J.-W.; Son, B.-C.; Lee, K.-S. Orthostatic Hypotension and Cardiac Sympathetic Denervation in Parkinson Disease Patients with REM Sleep Behavioral Disorder. J. Neurol. Sci. 2016, 362, 59–63.
- Pérez, T.; Tijero, B.; Gabilondo, I.; Luna, A.; Llorens, V.; Berganzo, K.; Acera, M.; Gonzalez, A.; Sanchez-Ferro, A.; Lezcano, E.; et al. Cardiocirculatory Manifestations in Parkinson’s Disease Patients without Orthostatic Hypotension. J. Hum. Hypertens. 2015, 29, 604–609.
- Leńska-Mieciek, M.; Derecka-Charzyńska, I.; Fiszer, U.; Królicki, L.; Kułakowski, P. Syncope and Autonomic Cardiovascular Dysfunction in Parkinson Disease. Neurol. Neurochir. Pol. 2011, 45, 335–341.
- Mochizuki, H.; Taniguchi, A.; Nakazato, Y.; Ishii, N.; Ebihara, Y.; Sugiyama, T.; Shiomi, K.; Nakazato, M. Increased Body Mass Index Associated with Autonomic Dysfunction in Parkinson’s Disease. Park. Relat. Disord. 2016, 24, 129–131.
- Goldstein, D.S.; Cheshire, W.P.J. Beat-to-Beat Blood Pressure and Heart Rate Responses to the Valsalva Maneuver. Clin. Auton. Res. 2017, 27, 361–367.
- Oka, H.; Toyoda, C.; Yogo, M.; Mochio, S. Reduced Cardiac 123I-MIBG Uptake Reflects Cardiac Sympathetic Dysfunction in de Novo Parkinson’s Disease. J. Neural. Transm. 2011, 118, 1323–1327.
- Suzuki, M.; Nakamura, T.; Hirayama, M.; Ueda, M.; Katsuno, M.; Sobue, G. Cardiac Parasympathetic Dysfunction in the Early Phase of Parkinson’s Disease. J. Neurol. 2017, 264, 333–340.
- Chung, E.J.; Kim, E.G.; Kim, M.S.; Bae, S.K.; Seog, D.H.; Oh, S.J.; Oh, M.; Kim, S.J. Differences in Myocardial Sympathetic Degeneration and the Clinical Features of the Subtypes of Parkinson’s Disease. J. Clin. Neurosci. 2011, 18, 922–925.
- Chiaravalloti, A.; Stefani, A.; Tavolozza, M.; Pierantozzi, M.; Di Biagio, D.; Olivola, E.; Di Pietro, B.; Stampanoni, M.; Danieli, R.; Simonetti, G.; et al. Different Patterns of Cardiac Sympathetic Denervation in Tremor-Type Compared to Akinetic-Rigid-Type Parkinson’s Disease: Molecular Imaging with 123I-MIBG. Mol. Med. Rep. 2012, 6, 1337–1342.
- Marras, C.; Rochon, P.; Lang, A.E. Predicting Motor Decline and Disability in Parkinson Disease: A Systematic Review. Arch. Neurol. 2002, 59, 1724–1728.
- Aasly, J.O. Long-Term Outcomes of Genetic Parkinson’s Disease. J. Mov. Disord. 2020, 13, 81–96.
- Kalia, L.V.; Lang, A.E.; Hazrati, L.-N.; Fujioka, S.; Wszolek, Z.K.; Dickson, D.W.; Ross, O.A.; Van Deerlin, V.M.; Trojanowski, J.Q.; Hurtig, H.I.; et al. Clinical Correlations with Lewy Body Pathology in LRRK2-Related Parkinson Disease. JAMA Neurol. 2015, 72, 100–105.
- Quattrone, A.; Bagnato, A.; Annesi, G.; Novellino, F.; Morgante, L.; Savettieri, G.; Zappia, M.; Tarantino, P.; Candiano, I.C.C.; Annesi, F.; et al. Myocardial 123metaiodobenzylguanidine Uptake in Genetic Parkinson’s Disease. Mov. Disord. 2008, 23, 21–27.
- Orimo, S.; Amino, T.; Yokochi, M.; Kojo, T.; Uchihara, T.; Takahashi, A.; Wakabayashi, K.; Takahashi, H.; Hattori, N.; Mizuno, Y. Preserved Cardiac Sympathetic Nerve Accounts for Normal Cardiac Uptake of MIBG in PARK2. Mov. Disord. 2005, 20, 1350–1353.
- Tijero, B.; Gabilondo, I.; Lezcano, E.; Teran-Villagrá, N.; Llorens, V.; Ruiz-Martinez, J.; Marti-Masso, J.F.; Carmona, M.; Luquin, M.R.; Berganzo, K.; et al. Autonomic Involvement in Parkinsonian Carriers of PARK2 Gene Mutations. Park. Relat. Disord. 2015, 21, 717–722.
- Ruiz-Martínez, J.; Gorostidi, A.; Goyenechea, E.; Alzualde, A.; Poza, J.J.; Rodríguez, F.; Bergareche, A.; Moreno, F.; López de Munain, A.; Martí Massó, J.F. Olfactory Deficits and Cardiac 123I-MIBG in Parkinson’s Disease Related to the LRRK2 R1441G and G2019S Mutations. Mov. Disord. 2011, 26, 2026–2031.
- Valldeoriola, F.; Gaig, C.; Muxí, A.; Navales, I.; Paredes, P.; Lomeña, F.; De la Cerda, A.; Buongiorno, M.; Ezquerra, M.; Santacruz, P.; et al. 123I-MIBG Cardiac Uptake and Smell Identification in Parkinsonian Patients with LRRK2 Mutations. J. Neurol. 2011, 258, 1126–1132.
- Smith, L.; Schapira, A.H.V. GBA Variants and Parkinson Disease: Mechanisms and Treatments. Cells 2022, 11, 1261.
- Kim, M.S.; Park, D.G.; An, Y.S.; Yoon, J.H. Dual-Phase 18F-FP-CIT Positron Emission Tomography and Cardiac 123I-MIBG Scintigraphy of Parkinson’s Disease Patients with GBA Mutations: Evidence of the Body-First Type? Eur. J. Neurol. 2023, 30, 344–352.
- Tsujikawa, K.; Hasegawa, Y.; Yokoi, S.; Yasui, K.; Nanbu, I.; Yanagi, T.; Takahashi, A. Chronological Changes of 123I-MIBG Myocardial Scintigraphy and Clinical Features of Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 2015, 86, 945–951.
- Rissardo, J.P.; Caprara, A.L.F. Risk Factors for Parkinson’s Disease Dementia. Ann. Mov. Disord. 2023.
- Kim, J.S.; Park, H.E.; Park, I.S.; Oh, Y.S.; Ryu, D.W.; Song, I.U.; Jung, Y.A.; Yoo, I.R.; Choi, H.-S.; Lee, P.H.; et al. Normal “heart” in Parkinson’s Disease: Is This a Distinct Clinical Phenotype? Eur. J. Neurol. 2017, 24, 349–356.
- Yoo, S.W.; Kim, J.S.; Oh, Y.S.; Ryu, D.W.; Ha, S.; Yoo, J.Y.; Lee, K.S. Cardiac Sympathetic Burden Reflects Parkinson Disease Burden, Regardless of High or Low Orthostatic Blood Pressure Changes. NPJ Park. Dis. 2021, 7, 71.
- Oh, Y.S.; Kim, J.S.; Yoo, S.W.; Hwang, E.J.; Lyoo, C.H.; Lee, K.S. Striatal Dopamine Activity and Myocardial 123I-Metaiodobenzylguanidine Uptake in Early Parkinson’s Disease. Park. Relat. Disord. 2019, 63, 156–161.
- Park, D.G.; Kim, J.Y.; Kim, M.S.; Kim, M.H.; An, Y.-S.; Chang, J.; Yoon, J.H. Neurofilament Light Chain and Cardiac MIBG Uptake as Predictors for Phenoconversion in Isolated REM Sleep Behavior Disorder. J. Neurol. 2023, 270, 4393–4402.
- Jellinger, K.A. Multiple System Atrophy—A Clinicopathological Update. Free Neuropathol. 2020, 1, 1–17.
- Gomperts, S.N. Lewy Body Dementias: Dementia with Lewy Bodies and Parkinson Disease Dementia. Continuum 2016, 22, 435–463.
- Oka, H.; Umehara, T.; Nakahara, A.; Matsuno, H. Comparisons of Cardiovascular Dysautonomia and Cognitive Impairment between de Novo Parkinson’s Disease and de Novo Dementia with Lewy Bodies. BMC Neurol. 2020, 20, 350.
- Youn, J.; Umemoto, G.; Oh, E.; Park, J.; Jang, W.; Oh, Y.-S.; Kim, H.-T.; Cho, J.W.; Fujioka, S.; Tsuboi, Y. Cardiac Sympathetic Denervation Could Be Associated with Dysphagia in Parkinson’s Disease. Front. Neurol. 2022, 13, 1010006.
- Sakakibara, R.; Tateno, F.; Kishi, M.; Tsuyusaki, Y.; Terada, H.; Inaoka, T. MIBG Myocardial Scintigraphy in Pre-Motor Parkinson’s Disease: A Review. Park. Relat. Disord. 2014, 20, 267–273.
- Matsui, H.; Nishinaka, K.; Oda, M.; Komatsu, K.; Kubori, T.; Udaka, F. Does Cardiac Metaiodobenzylguanidine (MIBG) Uptake in Parkinson’s Disease Correlate with Major Autonomic Symptoms? Park. Relat. Disord. 2006, 12, 284–288.
- Yoshida, M.; Fukumoto, Y.; Kuroda, Y.; Ohkoshi, N. Sympathetic Denervation of Myocardium Demonstrated by 123I-MIBG Scintigraphy in Pure Progressive Autonomic Failure. Eur. Neurol. 1997, 38, 291–296.
- Sakakibara, R.; Ogata, T.; Haruta, M.; Kishi, M.; Tsuyusaki, Y.; Tateno, A.; Tateno, F.; Mouri, T. Amnestic Mild Cognitive Impairment with Low Myocardial Metaiodobenzylguanidine Uptake. Am. J. Neurodegener. Dis. 2012, 1, 146–151.
- Choi, M.H.; Yoon, J.H.; Yong, S.W. Cardiac Sympathetic Denervation and Dementia in de Novo Parkinson’s Disease: A 7-Year Follow-up Study. J. Neurol. Sci. 2017, 381, 291–295.
- Kobayashi, K.; Sumiya, H.; Nakano, H.; Akiyama, N.; Urata, K.; Koshino, Y. Detection of Lewy Body Disease in Patients with Late-Onset Depression, Anxiety and Psychotic Disorder with Myocardial Meta-Iodobenzylguanidine Scintigraphy. Int. J. Geriatr. Psychiatry 2010, 25, 55–65.
- Roberts, G.; Durcan, R.; Donaghy, P.C.; Lawley, S.; Ciafone, J.; Hamilton, C.A.; Colloby, S.J.; Firbank, M.J.; Allan, L.; Barnett, N.; et al. Accuracy of Cardiac Innervation Scintigraphy for Mild Cognitive Impairment with Lewy Bodies. Neurology 2021, 96, e2801–e2811.
- Kitayama, M.; Wada-Isoe, K.; Irizawa, Y.; Nakashima, K. Association of Visual Hallucinations with Reduction of MIBG Cardiac Uptake in Parkinson’s Disease. J. Neurol. Sci. 2008, 264, 22–26.
- Salsone, M.; Labate, A.; Quattrone, A. Cardiac Denervation Precedes Nigrostriatal Damage in Idiopathic Rapid Eye Movement Sleep Behavior Disorder. Mov. Disord. 2012, 27, 1068–1069.
- Nomura, T.; Inoue, Y.; Högl, B.; Uemura, Y.; Kitayama, M.; Abe, T.; Miyoshi, H.; Nakashima, K. Relationship between 123I-MIBG Scintigrams and REM Sleep Behavior Disorder in Parkinson’s Disease. Park. Relat. Disord. 2010, 16, 683–685.
- Miyamoto, T.; Miyamoto, M.; Iwanami, M.; Hirata, K. Follow-up Study of Cardiac 123I-MIBG Scintigraphy in Idiopathic REM Sleep Behavior Disorder. Eur. J. Neurol. 2011, 18, 1275–1278.
- Janzen, A.; Vadasz, D.; Booij, J.; Luster, M.; Librizzi, D.; Henrich, M.T.; Timmermann, L.; Habibi, M.; Sittig, E.; Mayer, G.; et al. Progressive Olfactory Impairment and Cardiac Sympathetic Denervation in REM Sleep Behavior Disorder. J. Park. Dis. 2022, 12, 1921–1935.
- Mizutani, Y.; Nakamura, T.; Okada, A.; Suzuki, J.; Watanabe, H.; Hirayama, M.; Sobue, G. Hyposmia and Cardiovascular Dysautonomia Correlatively Appear in Early-Stage Parkinson’s Disease. Park. Relat. Disord. 2014, 20, 520–524.
- Murakami, N.; Sako, W.; Haji, S.; Furukawa, T.; Otomi, Y.; Otsuka, H.; Izumi, Y.; Harada, M.; Kaji, R. Potential Utility of 123I-MIBG Scintigraphy as a Predictor of Falls in Parkinson’s Disease. Front. Neurol. 2019, 10, 376.
- Dorschner, J.; Farmakis, G.; Behnke, S.; Hellwig, D.; Schneider, S.; Fassbender, K.; Kirsch, C.-M.; Dillmann, U.; Spiegel, J. Myocardial MIBG Scintigraphy May Predict the Course of Motor Symptoms in Parkinson’s Disease. Park. Relat. Disord. 2011, 17, 372–375.
- Olivola, E.; Brusa, L.; Rocchi, C.; Schillaci, O.; Liguori, C.; Cerroni, R.; Pierantozzi, M.; Chiaravalloti, A.; Stefani, A.; Stocchi, F. Does Fatigue in Parkinson’s Disease Correlate with Autonomic Nervous System Dysfunction? Neurol. Sci. 2018, 39, 2169–2174.
- Nakamura, T.; Hirayama, M.; Hara, T.; Hama, T.; Watanabe, H.; Sobue, G. Does Cardiovascular Autonomic Dysfunction Contribute to Fatigue in Parkinson’s Disease? Mov. Disord. 2011, 26, 1869–1874.
More
