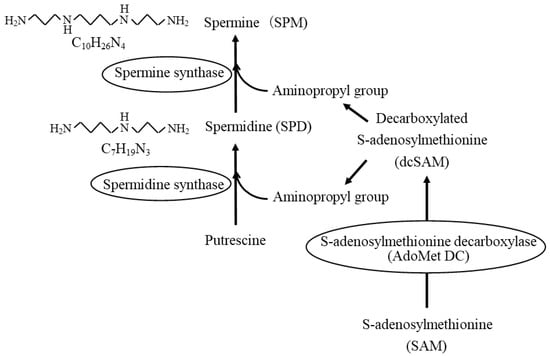
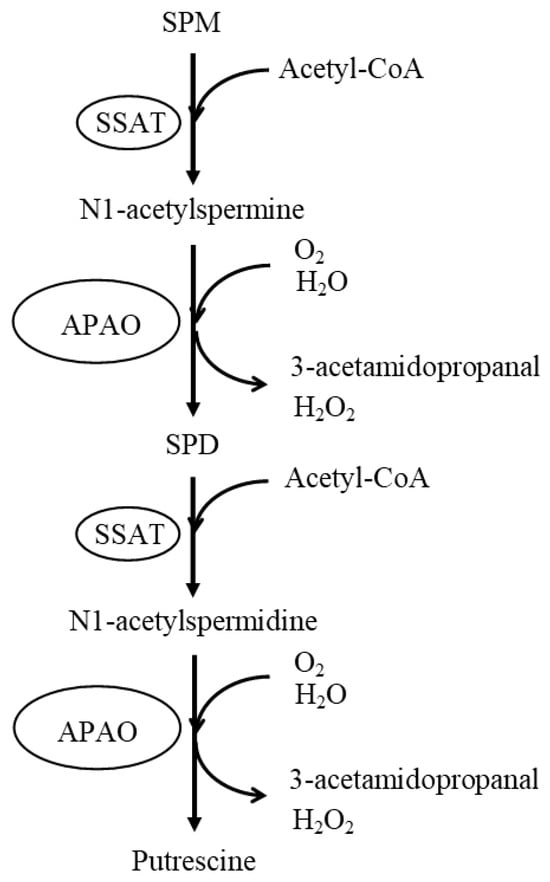
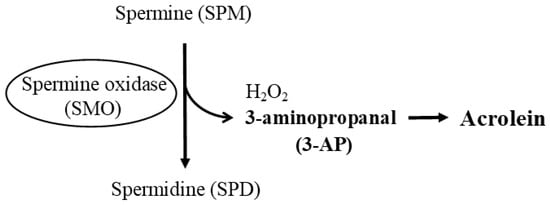
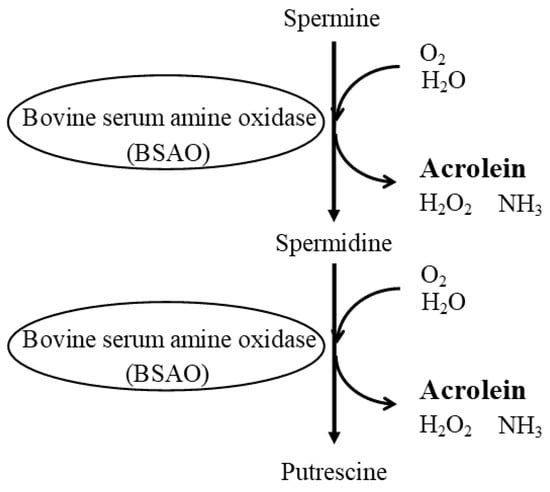
4. Age- and Disease-Related Changes in the Ratio of Spermine to Spermidine
In adults, changes in tissue and blood polyamine concentrations with age are not pronounced and do not decrease with age. However, SPM concentrations show a slight tendency to gradually decrease with age, so that the SPM/SPD ratio tends to decrease
[16][18][19][23,26,27]. And this decline tends to be more pronounced in patients with age- and lifestyle-related diseases
[16][18][23,26].
Similar changes in polyamine levels, i.e., a decrease in the SPM/SPD ratio and/or an increase in SPD levels, have been reported in other age-related diseases such as cerebral infarction, neurodegenerative diseases, and sarcopenia
[18][19][20][21][26,27,29,30]. In patients with neurodegenerative diseases such as Alzheimer’s disease, SPD levels were elevated in the frontal and parietal lobes of the brain
[22][31]. Plasma concentrations of PUT and SPD increased in stroke patients, while SPM concentrations remained unchanged, resulting in a significant decrease in the SPM/SPD ratio
[23][32].
Chronic inflammation has been implicated in the background of these age-related chronic diseases
[24][33]. This means that in patients with these diseases, inflammation-induced increases in SMO activity and 3-AP or acrolein levels should be noted. In fact, urinary acrolein levels were significantly higher in patients with diabetes mellitus than in those without
[25][34]. An increase in plasma acrolein concentration and SMO activity has been observed in patients with chronic renal failure, such as diabetic nephropathy, chronic glomerulonephritis, and nephrosclerosis
[26][35].
Chronic inflammation has been implicated in the onset and progression of several age- and lifestyle-related diseases, as well as protein-energy depletion leading to cardiovascular disease and sarcopenia
[27][28][29][39,40,41]. In addition, the presence of chronic inflammation was a strong predictor of poor outcomes in dialysis patients
[30][42]. In light of these scientific facts, the cytotoxic activity of acrolein, which results from the degradation of SPM via activated SMO in the presence of inflammation, may contribute to the development and progression of these diseases and the prognosis of patients
[31][32][43,44].
5. Polyamines as Nutritional Contributors to the Prevention of Age- and Lifestyle-Related Disease Development
Considering that the main source of polyamines is thought to be the gastrointestinal tract, i.e., polyamines in food and polyamines synthesized by intestinal bacteria are important, and that cells can take up extracellular polyamines, it is likely that polyamine levels in the body are affected by food intake and the state of intestinal bacteria. In fact, many studies have shown that reducing polyamine intake, as well as inhibiting the activity of gut bacteria with antibiotics, reduces blood polyamine levels
[33][34][45,46].
When SPD was mixed with drinking water and administered to mice, an increase in blood SPD levels was reported
[7][2]. SPM concentrations also appeared to have increased, as seen in the figure in the paper, but specific data are not shown.
There are not many human intervention studies using high polyamine diets. The results of studies using high SPD supplements have been reported
[35][49]. There was a 12-month study of supplementation in elderly patients between the ages of 60 and 90. This study showed a 10–20% increase in polyamine intake compared to normal dietary polyamine intake, yet the blood SPD levels did not change at all
[35][49]. Thus, if there are clinical changes after being on a high SPD diet, it is not at all clear whether they are due to SPD or to other components that occurred at the same time
[36][50].
Because polyamines are absorbed from the gastrointestinal tract without being broken down, and because many foods generally contain more SPD than SPM, it was thought that a diet high in polyamines would increase blood SPD. However, although limited research can be confirmed, there is very little evidence that continuous consumption of a high polyamine diet increases the blood levels of SPD. Instead, prolonged consumption of a diet high in polyamines (richer in SPD than in SPM) or even a short period of high SPD intake appears to increase SPM concentration, although there are individual differences. It is interesting to note that the age- and lifestyle-related diseases, which are associated with shorter life expectancy, decrease the SPM/SPD ratio, whereas diets rich in polyamines, which contribute to life extension, such as soy products, increase the SPM/SPD ratio.
6. Biological Activity of Polyamines in Human Health and Disease
Polyamines are known to possess many biological activities that may counteract age-related conditions and senescence
[1][6]. For example, they have anti-inflammatory and antioxidant properties
[37][38][52,53] and protect cells and genes from damaging stimuli such as ionizing radiation, ultraviolet rays, toxic chemicals, and other stresses
[1][6]. And some researchers, including us, have reported that increasing polyamine intake extends the lifespan of animals
[10][39][40][1,5,54].
The anti-inflammatory effects of polyamines include the suppression of proinflammatory cytokine production by immune cells upon the stimulation and suppression of LFA-1 expression in the cell membrane
[37][38][52,53]. Increased LFA-1 protein causes immune cells to respond to even minor stimuli, triggering the production of proinflammatory cytokines and provoking inflammation. SPM has strong physiological activity and therefore shows anti-inflammatory activity over a range of physiological concentration changes. SPD also shows similar biological activity to SPM, but requires a concentration change well beyond the physiological concentration change to confirm the effect
[37][38][52,53]. Furthermore, the suppression of LFA-1 expression on immune cells by SPM is specific
[37][52].
The amount of LFA-1 has been found to be related to the methylation status of the ITGAL, where the gene for LFA-1 is encoded. Increased levels of LFA-1 protein on immune cells with aging are associated with the progressive demethylation of ITGAL
[41][42][55,56].
Gene methylation is a change that only occurs in cytosine, one of the four bases that make up a gene’s information, and is a mechanism that alters the reading of genetic information by adding or removing methyl groups from cytosine. In front of the genetic information, there is a site called the CpG land, which contains repeated sequences of cytosine and guanine. The methylation of cytosines within the CpG island results in decreased transcription and consequently decreased production of the protein encoded by the gene. Conversely, when cytosines within the CpG island are demethylated, transcription is more likely to occur, resulting in increased synthesis of the protein encoded by the gene. DNA methylation is regulated by DNA methyltransferases (DNMTs). DNMTs control the methylation state of cytosines by using methyl groups provided by SAM.