Patients suffering from chronic gastritis and developing gastric mucosa atrophy are at increased risk of the development of gastric cancer. The diagnosis of chronic atrophic gastritis (CAG) is a complex procedure involving a detailed history taking, a thorough physical examination and the use of laboratory and instrumental diagnostic methods among which the endoscopy of the upper digestive tract is the cornerstone because it allows the assessment of the topography of gastritis and identification of erosions and areas of intestinal metaplasia with the use of narrow band imaging (NBI) endoscopy. However, the diagnosis of CAG requires morphological examination of the gastric mucosa.
- chronic atrophic gastritis
- Helicobacter pylori
- autoimmune gastritis
- intestinal metaplasia
- gastric cancer
1. Introduction
2. The Etiological Factor of Gastritis and the Atrophy Risk
The two most significant etiological factors of CAG are H. pylori infection and autoimmune inflammation, with the dominant infectious factor [17,18][2][3]. H. pylori is a gram-negative, curved or S-shaped microaerophilic bacterium with high motility due to a unipolar bundle of coated flagella [19][4]. H. pylori is thought to have been acquired by modern humans in Africa at least ~100,000 years ago, possibly being transmissed from an unknown animal [20][5]. The most ancient phylogeographic population of H. pylori is hpAfrica2, mainly found in South Africa. Other important, widespread, and recent populations include hpAfrica1, hpNEAfrica, hpEurope, hpEastAsia, hpAsia2, and hpSahul [21,22][6][7]. An important step in the evolution of H. pylori from the ancestral population of hpAfrica2 to populations that spread around the world was the acquisition of the cag pathogenicity island (cagPAI), which encodes the components of the Cag T4SS protein complex [23[8][9],24], surrounding the bacterial cell membrane and facilitating the delivery of various effector molecules into host cells after attachment. H. pylori is well adapted to colonize a unique ecological niche in the deep near-wall mucus layer of the antral mucosa. Several mechanisms, including motility, urease production, adhesion, and others, are important for H. pylori colonization [25,26,27][10][11][12]. H. pylori colonization of the gastric mucosa induces a proinflammatory response involving various immune cells in the mucosal layer resulting in chronic active gastritis [28][13]. The severity of inflammation varies greatly in individuals depending on bacterial, host and environmental factors [29,30][14][15]. The most important determinant of the pro-inflammatory activity of the H. pylori strain is its functional cagPAI [31,32][16][17]. Expression of additional host interaction factors, such as a set of adhesins that promotes strong binding to epithelial cells depends on the variable composition of host receptors [33][18]. In addition to association with H. pylori, CAG may also be primarily of autoimmune nature due to the production of autoantibodies to gastric parietal cells and/or intrinsic Castle factor. The prevalence of autoimmune gastritis (AIG) in the population ranges from 1 to 8% [54][19]. The risk group of patients with autoimmune inflammation of the gastric mucosa includes women suffering from autoimmune diseases (e.g., type 1 diabetes mellitus, autoimmune thyroiditis), as well as celiac disease [55][20]. It is noteworthy that the experts of the Maastricht Consensus addressed the problem of the diagnosis of autoimmune gastritis [42][21]. For example, one of the provisions (WG 2 Diagnostics Statement 6) states that gastric functional serology (pepsinogens I-II and gastrin levels), anti-H. pylori antibodies, anti-intrinsic factor and anti-parietal cell auto-antibodies may provide clinically valuable information on the likelihood of gastric mucosal atrophy, including its aetiology (agreement: 98%, grade 1 A). It should be noted that with primary AIG, the risk of neuroendocrine tumors increases compared with other etiological factors of gastritis, but the risk of gastric adenocarcinoma is lower than with multifocal atrophy of the gastric mucosa due to H. pylori infection (involving the mucous membrane of the antrum and body). Epidemiological studies estimate the incidence of gastric adenocarcinoma among patients with autoimmune gastritis as 14.2 cases per 1000 person-years [56][22]. A study carried out in Sweden reported that the risk of developing gastric cancer in patients with autoimmune gastritis was 7.4 versus 1.4 cases per 1000 patient-years in the general population [57][23], and a study performed in Finland reported a similar value risk with a standardized incidence rate of 5.0 [58][24].3. Possibilities of Endoscopic Examination and Sampling of Biopsy Specimens for the Diagnosis of Gastric Mucosa Atrophy
Endoscopic examination plays a key role in the diagnosis of CAG, since the competency of the endoscopist and the adequacy of gastrobiopsy sampling determine the subsequent morphological assessment of the lesion of the gastric mucosa and verification of the diagnosis [59][25]. The key data for the diagnosis of chronic atrophic gastritis, which can be obtained from the results of endoscopic examination, are the topography of gastritis and the actual identification of atrophy and metaplasia zones [60][26]. The inflammation of the gastric mucosa is usually considered from the standpoint of a predominant lesion of an organ part: body gastritis, antrum gastritis, pangastritis [61,62][27][28]. Clinical interpretation of the topography of inflammation can be as follows. The body gastric mucosa predominantly involved in the inflammatory process may indicate the presence of AIG [63][29]. In adults, and in cases of introduction of H. pylori infection during adolescence, inflammation begins in the antrum, in the so-called “ecological niche” of the H. pylori bacterium [20][5]. Further spread of the H. pylori infection in the proximal direction results in the additional involvement of the body of the stomach in the inflammatory process. Pangastritis is formed in this way. Regardless of the etiology, the dominance of gastric lesions is an unfavorable sign in terms of the risk of developing gastric cancer [44][30].
According to the data published in the literature, the sensitivity and specificity of conventional white light endoscopy for diagnosing gastric mucosal atrophy are 53–59%, and those of high-definition white light endoscopy with magnification are 70–74% [64][31]. The study by Zhang Q. et al. presents findings of a meta-analysis of the data collected from 1724 patients which indicate that the combined sensitivity and specificity of white light endoscopy for diagnosing early gastric cancer were 48% and 67%, respectively [65][32]. Moreover, a meta-analysis of 22 studies showed that almost 10% of gastric cancers could potentially be missed during white light endoscopy, mainly it relates to adenocarcinoma of the body of the stomach [66][33]. To overcome the diagnostic limitations of standard white light endoscopy in detecting premalignant changes in the gastric mucosa, various imaging enhanced endoscopy (IEE) techniques have been developed, including dye chromoscopy, high-resolution imaging, virtual chromoscopy, and artificial intelligence [65,67,68][32][34][35]. Chromoendoscopy (CE) is an IEE technique that sprays dyes onto the surface of the gastric mucosa to improve visualization of the lesions under study. The use of CE in the screening of malignancies and premalignant changes in the gastric mucosa can increase the detection rate and provide more accurate visualization of the boundaries of the lesion, which helps to differentiate benign or inflammatory changes from suspected precancerous or malignant ones and determine the zones for biopsy [60,69][26][36]. Virtual or electronic chromoendoscopy are imaging methods allowing a detailed examination of the gastric mucosa. Their use increases the efficiency of diagnosing precancerous changes and makes it easier for the endoscopist to select areas “suspicious” for intestinal metaplasia or dysplasia for taking gastrobiopsy specimens without any dye techniques. The methods are easy to use and less time-consuming than when using dyes [60,71,72][26][37][38]. One of the virtual chromoendoscopy methods is narrow band imaging (NBI) developed by Olympus (Olympus Medical Systems Co., Ltd., Tokyo, Japan). The principle of NBI endoscopy is based on an optical phenomenon in which the depth of penetration of light into tissues depends on the wavelength. NBI uses narrow spectra of blue light (415 nm) and green light (540 nm) due to light filters installed in the illuminator, which allows you to get a clearer, more detailed image of the gastric mucosa [60][26]. The sensitivity and specificity of NBI endoscopy for the diagnosis of the gastric mucosa atrophy reach 95 and 98.5%, and for the diagnosis of early gastric cancer, they are 83% and 96%, respectively [64][31]. The standard for the study of biopsy specimens is the OLGA-system protocol which involves taking two fragments from the body of the stomach, two fragments from the antrum and one fragment from the incisura angularis of the stomach, and allows assessing the stage of the process and the risk of developing gastric cancer [75][39]. In a detailed presentation, modern recommendations are indicated as follows: two biopsies from the antrum of the stomach at a distance of 2 cm from the pylorus along the lesser and greater curvatures, one biopsy from the incisura angularis and two biopsies from the body of the stomach at a distance of 8 cm from the rosette of the cardia along the lesser and greater curvatures [76][40]. The use of special adhesive strips can be one of the effective approaches to solve the problem of orientation and fragmentation of biopsies. Orientation using specialized adhesive strips made of cellulose acetate can be carried out by fixing tissue fragments to the strip using manual pressing for 5 s. In this case, the first biopsy specimen is placed at the pointed end of the strip, which provides intuitive recognition of the serial number of the biopsy tissue sample (Figure 1).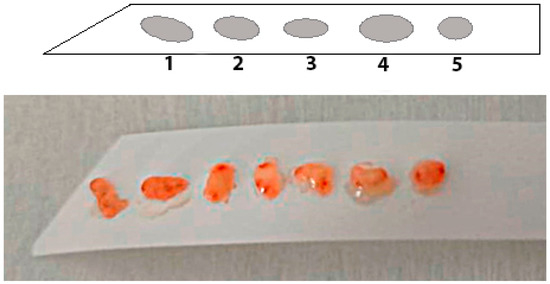
4. Histological Examination—“Pitfalls” of a Standard Study
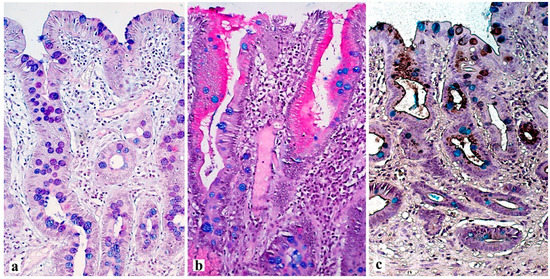
5. Conclusions
References
- Correa, P. Human gastric carcinogenesis: A multistep and multifactorial process—First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992, 52, 6735–6740.
- Kumar, S.; Metz, D.C.; Ellenberg, S.; Kaplan, D.E.; Goldberg, D.S. Risk Factors and Incidence of Gastric Cancer After Detection of Helicobacter pylori Infection: A Large Cohort Study. Gastroenterology 2020, 158, 527–536.e7.
- Shah, S.C.; Piazuelo, M.B.; Kuipers, E.J.; Li, D. AGA Clinical Practice Update on the Diagnosis and Management of Atrophic Gastritis: Expert Review. Gastroenterology 2021, 161, 1325–1332.e7.
- Suerbaum, S.; Michetti, P. Helicobacter pylori infection. N. Engl. J. Med. 2022, 347, 1175–1186.
- Malfertheiner, P.; Camargo, M.C.; El-Omar, E.; Liou, J.M.; Peek, R.; Schulz, C.; Smith, S.I.; Suerbaum, S. Helicobacter pylori infection. Nat. Rev. Dis. Primers 2023, 9, 19.
- Ailloud, F.; Estibariz, I.; Suerbaum, S. Evolved to vary: Genome and epigenome variation in the human pathogen Helicobacter pylori. FEMS Microbiol. Rev. 2021, 45, fuaa042.
- Moodley, Y.; Linz, B.; Yamaoka, Y.; Windsor, H.M.; Breurec, S.; Wu, J.Y.; Maady, A.; Bernhöft, S.; Thiberge, J.M.; Phuanukoonnon, S.; et al. The peopling of the Pacific from a bacterial perspective. Science 2009, 323, 527–530.
- Censini, S.; Lange, C.; Xiang, Z.; Crabtree, J.E.; Ghiara, P.; Borodovsky, M.; Rappuoli, R.; Covacci, A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. USA 1996, 93, 14648–14653.
- Olbermann, P.; Josenhans, C.; Moodley, Y.; Uhr, M.; Stamer, C.; Vauterin, M.; Suerbaum, S.; Achtman, M.; Linz, B. A global overview of the genetic and functional diversity in the Helicobacter pylori cag pathogenicity island. PLoS Genet. 2010, 6, e1001069.
- Gu, H. Role of Flagella in the Pathogenesis of Helicobacter pylori. Curr. Microbiol. 2017, 74, 863–869.
- Sharndama, H.C.; Mba, I.E. Helicobacter pylori: An up-to-date overview on the virulence and pathogenesis mechanisms. Braz. J. Microbiol. 2022, 53, 33–50.
- Ansari, S.; Yamaoka, Y. Helicobacter pylori Virulence Factors Exploiting Gastric Colonization and its Pathogenicity. Toxins 2019, 11, 677.
- Cook, K.W.; Letley, D.P.; Ingram, R.J.; Staples, E.; Skjoldmose, H.; Atherton, J.C.; Robinson, K. CCL20/CCR6-mediated migration of regulatory T cells to the Helicobacter pylori-infected human gastric mucosa. Gut 2014, 63, 1550–1559.
- Robinson, K.; Kenefeck, R.; Pidgeon, E.L.; Shakib, S.; Patel, S.; Polson, R.J.; Zaitoun, A.M.; Atherton, J.C. Helicobacter pylori-induced peptic ulcer disease is associated with inadequate regulatory T cell responses. Gut 2008, 57, 1375–1385.
- Kao, C.Y.; Sheu, B.S.; Wu, J.J. Helicobacter pylori infection: An overview of bacterial virulence factors and pathogenesis. Biomed. J. 2016, 39, 14–23.
- Backert, S.; Haas, R.; Gerhard, M.; Naumann, M. The Helicobacter pylori Type IV Secretion System Encoded by the cag Pathogenicity Island: Architecture, Function, and Signaling. Curr. Top. Microbiol. Immunol. 2017, 413, 187–220.
- Chen, B.; Zhang, J.; Ma, Q. The relationship between the simultaneity present of cagA and hopQI genes in Helicobacter pylori and the risk of gastric cancer. Cell Mol. Biol. 2021, 67, 121–126.
- Chmiela, M.; Kupcinskas, J. Review: Pathogenesis of Helicobacter pylori infection. Helicobacter 2019, 24 (Suppl. S1), e12638.
- Toh, B.H. Diagnosis and classification of autoimmune gastritis. Autoimmun. Rev. 2014, 13, 459–462.
- Rodriguez-Castro, K.I.; Franceschi, M.; Miraglia, C.; Russo, M.; Nouvenne, A.; Leandro, G.; Meschi, T.; De’Angelis, G.L.; Di Mario, F. Autoimmune diseases in autoimmune atrophic gastritis. Acta Biomed. 2018, 89, 100–103.
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022, 71, 1724–1762.
- Mahmud, N.; Stashek, K.; Katona, B.W.; Tondon, R.; Shroff, S.G.; Roses, R.; Furth, E.E.; Metz, D.C. The incidence of neoplasia in patients with autoimmune metaplastic atrophic gastritis: A renewed call for surveillance. Ann. Gastroenterol. 2019, 32, 67–72.
- Hsing, A.W.; Hansson, L.E.; McLaughlin, J.K.; Nyren, O.; Blot, W.J.; Ekbom, A.; Fraumeni, J.F., Jr. Pernicious anemia and subsequent cancer. A population-based cohort study. Cancer 1993, 71, 745–750.
- Kokkola, A.; Sjöblom, S.M.; Haapiainen, R.; Sipponen, P.; Puolakkainen, P.; Järvinen, H. The risk of gastric carcinoma and carcinoid tumours in patients with pernicious anaemia. A prospective follow-up study. Scand. J. Gastroenterol. 1998, 33, 88–92.
- Săftoiu, A.; Hassan, C.; Areia, M.; Bhutani, M.S.; Bisschops, R.; Bories, E.; Cazacu, I.M.; Dekker, E.; Deprez, P.H.; Pereira, S.P.; et al. Role of gastrointestinal endoscopy in the screening of digestive tract cancers in Europe: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2020, 52, 293–304.
- Martins, B.C.; Moura, R.N.; Kum, A.S.T.; Matsubayashi, C.O.; Marques, S.B.; Safatle-Ribeiro, A.V. Endoscopic Imaging for the Diagnosis of Neoplastic and Pre-Neoplastic Conditions of the Stomach. Cancers 2023, 15, 2445.
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 1996, 20, 1161–1181.
- Correa, P.; Yardley, J.H. Grading and classification of chronic gastritis: One American response to the Sydney system. Gastroenterology 1992, 102, 355–359.
- Yagi, K.; Nakamura, A.; Sekine, A.; Graham, D. Features of the atrophic corpus mucosa in three cases of autoimmune gastritis revealed by magnifying endoscopy. Case Rep. Med. 2012, 2012, 368160.
- Shichijo, S.; Hirata, Y.; Niikura, R.; Hayakawa, Y.; Yamada, A.; Ushiku, T.; Fukayama, M.; Koike, K. Histologic intestinal metaplasia and endoscopic atrophy are predictors of gastric cancer development after Helicobacter pylori eradication. Gastrointest. Endosc. 2016, 84, 618–624.
- Pimentel-Nunes, P.; Libânio, D.; Marcos-Pinto, R.; Areia, M.; Leja, M.; Esposito, G.; Garrido, M.; Kikuste, I.; Megraud, F.; Matysiak-Budnik, T.; et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 2019, 51, 365–388.
- Zhang, Q.; Wang, F.; Chen, Z.Y.; Wang, Z.; Zhi, F.C.; Liu, S.D.; Bai, Y. Comparison of the diagnostic efficacy of white light endoscopy and magnifying endoscopy with narrow band imaging for early gastric cancer: A meta-analysis. Gastric Cancer 2016, 19, 543–552.
- Pimenta-Melo, A.R.; Monteiro-Soares, M.; Libânio, D.; Dinis-Ribeiro, M. Missing rate for gastric cancer during upper gastrointestinal endoscopy: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1041–1049.
- Zhao, Z.; Yin, Z.; Wang, S.; Wang, J.; Bai, B.; Qiu, Z.; Zhao, Q. Meta-Analysis: The Diagnostic Efficacy of Chromoendoscopy for Early Gastric Cancer and Premalignant Gastric Lesions. J. Gastroenterol. Hepatol. 2016, 31, 1539–1545.
- Fiuza, F.; Maluf-Filho, F.; Ide, E.; Furuya, C.K., Jr.; Fylyk, S.N.; Ruas, J.N.; Stabach, L.; Araujo, G.A.; Matuguma, S.E.; Uemura, R.S.; et al. Association between Mucosal Surface Pattern under near Focus Technology and Helicobacter Pylori Infection. World J. Gastrointest. Endosc. 2021, 13, 518–528.
- Vu, N.T.H.; Quach, D.T.; Dang, N.L.B.; Le, Q.D.; Nguyen, D.T.N.; Le, H.M.; Le, N.Q.; Hiyama, T. Performance of chromoendoscopy and narrow-band imaging in the diagnosis of gastric intestinal metaplasia. Scand. J. Gastroenterol. 2022, 57, 1005–1010.
- Pal, P.; Singh, A.P.; Kanuri, N.D.; Banerjee, R. Electronic chromo-endoscopy: Technical details and a clinical perspective. Transl. Gastroenterol. Hepatol. 2022, 7, 6.
- Pimentel-Nunes, P.; Libânio, D.; Lage, J.; Abrantes, D.; Coimbra, M.; Esposito, G.; Hormozdi, D.; Pepper, M.; Drasovean, S.; White, J.R.; et al. A multicenter prospective study of the real-time use of narrow-band imaging in the diagnosis of premalignant gastric conditions and lesions. Endoscopy 2016, 48, 723–730.
- Bacha, D.; Walha, M.; Ben Slama, S.; Ben Romdhane, H.; Bouraoui, S.; Bellil, K.; Lahmar, A. Chronic gastritis classifications. Tunis Med. 2018, 96, 405–410.
- Rugge, M.; Savarino, E.; Sbaraglia, M.; Bricca, L.; Malfertheiner, P. Gastritis: The clinico-pathological spectrum. Dig. Liver Dis. 2021, 53, 1237–1246.
- Rugge, M.; Genta, R.M. OLGA Group Staging gastritis: An international proposal. Gastroenterology 2005, 129, 1807–1808.
- Ramírez-Mendoza, P.; González-Angulo, J.; Angeles-Garay, U.; Segovia-Cueva, G.A. Evaluaciónhistopatológica de gastritis atrófica. Comparación de lossistemas Sidney y OLGA . Rev. Med. Inst. Mex Seguro Soc. 2008, 46, 135–139.
- Yue, H.; Shan, L.; Bin, L. The significance of OLGA and OLGIM staging systems in the risk assessment of gastric cancer: A systematic review and meta-analysis. Gastric Cancer 2018, 21, 579–587.
- Aruin, L.I.; Kononov, A.V.; Mozgovoy, S.I. New classification of chronic gastritis. Actual problems of pathological anatomy: Proceedings of the III Congress of the Russian Academy of Sciences. Total pathologists. Samara 2009, 1, 5–8.
- Satoh, K.; Osawa, H.; Yoshizawa, M.; Nakano, H.; Hirasawa, T.; Kihira, K.; Sugano, K. Assessment of atrophic gastritis using the OLGA system. Helicobacter 2008, 13, 225–229.
- Isajevs, S.; Liepniece-Karele, I.; Janciauskas, D.; Moisejevs, G.; Putnins, V.; Funka, K.; Kikuste, I.; Vanags, A.; Tolmanis, I.; Leja, M. Gastritis staging: Interobserver agreement by applying OLGA and OLGIM systems. Virchows Arch. 2014, 464, 403–407.
- Kim, H.J.; Kim, N.; Yun, C.Y.; Lee, H.S. The clinical meaning of the “indefinite for atrophy” lesions within gastric mucosa biopsy specimens in a region with a high prevalence of gastric cancer. Helicobacter 2019, 24, e12605.
- Rugge, M.; Sacchi, D.; Genta, R.M.; Zanco, F.; Guzzinati, S.; Pizzi, M.; Fassan, M.; Di Sabatino, A.; El-Serag, H. Histological assessment of gastric pseudopyloric metaplasia: Intra- and inter-observer consistency. Dig. Liver Dis. 2021, 53, 61–65.
- Cho, S.J.; Choi, I.J.; Kook, M.C.; Nam, B.H.; Kim, C.G.; Lee, J.Y.; Ryu, K.W.; Kim, Y.W. Staging of intestinal- and diffuse-type gastric cancers with the OLGA and OLGIM staging systems. Aliment. Pharmacol. Ther. 2013, 38, 1292–1302.
- Cheng, H.C.; Tsai, Y.C.; Yang, H.B.; Yeh, Y.C.; Chang, W.L.; Kuo, H.Y.; Lu, C.C.; Sheu, B.S. The corpus-predominant gastritis index can be an early and reversible marker to identify the gastric cancer risk of Helicobacter pylori-infected nonulcer dyspepsia. Helicobacter 2017, 22, e12385.
- Genta, R.M.; Rugge, M. Assessing risks for gastric cancer: New tools for pathologists. World J. Gastroenterol. 2006, 12, 5622–5627.
- Wang, Y.K.; Li, C.; Zhou, Y.M.; Zeng, L.; Li, Y.Y.; Huang, S.L.; Zhu, C.Y.; Wang, Y.; Wang, S.N.; Chen, X.D. Histopathological Features of Helicobacter pylori Infection in Gastric Mucosa. J. Inflamm. Res. 2022, 15, 6231–6243.
