Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jason Zhu and Version 1 by Pawel Pietkiewicz.
Pityriasis versicolor (PV), also known as tinea versicolor, is a mild, non-contagious chronic, superficial fungal skin infection caused by lipid-dependent yeast-like fungus Malassezia. It manifests as poorly to well-demarcated discoloured or light pink scaly patches, usually affecting the trunk and arms. The disease occurs worldwide but is most prevalent in humid and warm tropical regions. PV tends to be more active in summer seasons.
- Malassezia
- Pitryrosporum
- yeast
- tinea versicolor
- fungal infections
- dermatoscopy
- confocal reflectance microscopy
- ultraviolet-induced fluorescence dermatoscopy
- Wood's lamp
- dermoscopy
1. Introduction
Pityriasis versicolor (PV), also known as tinea versicolor, is a mild, non-contagious chronic, superficial fungal skin infection caused by lipid-dependent yeast-like fungus Malassezia [1]. It manifests as poorly to well-demarcated discoloured or light pink scaly patches, usually affecting the trunk and arms. The disease occurs worldwide but is most prevalent in humid and warm tropical regions. PV tends to be more active in summer seasons [2][3][4].
Skin discoloration, associated with enzymatic activity of the yeast and concomitant bacterial colonisation, is transient. However, in many cases, recurrence of the disease may occur despite effective treatment, which adds to the impact on the quality of life of PV patients [5]. Therefore, long-term maintenance treatment is often necessary [1][6][7][8][9].
The diagnosis of PV is often simple. It solely relies on the clinical appearance and hardly ever requires biopsy. However, in clinically ambiguous cases, additional non-invasive work-up (e.g., dermatoscopy, ultraviolet-induced fluorescence dermatoscopy, Wood’s light examination or direct microscopy) may facilitate the diagnostic process [4].
2. Culture
Microbiological culture in PV is not recommended [10]. The process is difficult for the required use of synthetic mycological media enriched with olive oil, and 1–4 weeks of incubation at 32 °C [11][12].
3. Direct Microscopic Examination
Direct microscopic examination is particularly used when the patient presents a typical clinical appearance of PV, but in Wood’s lamp examination, there is no characteristic fluorescence (e.g., after using shampoos with ketoconazole). Skin scrapings are dissolved with a mixture of potassium hydroxide (KOH) and dimethyl sulfoxide (DMSO) and evaluated under light microscopy and phase contrast microscopy. A characteristic appearance of roundish spores and elongated fungal hyphae resembles spaghetti and meatballs (Figure 1) [1][13]. As the highest concentration of fungi is noted at the lesion’s periphery [1][13], researchers recommend taking the sample from this area to achieve optimal reliability. As adding KOH provides no stain, methylene green, periodic acid–Schiff (PAS; with methylene green or fuchsin), or simply common blue ink can be used for better visualisation [1][14]. A simple scotch-test, involving the adhesion of cellophane foil to the scales, offers an alternative approach for collecting material suitable for staining and subsequent microscopic analysis [15]. Nevertheless, it should be kept in mind that direct microscopic examination with KOH does not provide 100% sensitivity [1][14], and that this method has not been standardised [3]. Thus, supplementary diagnostic tools may be used to obtain a wider context.
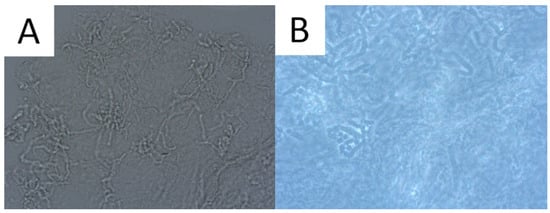
Figure 1. Direct microscopic examination of the skin scrapings reveals yeast cells and hyphal fragments (spaghetti and meatballs appearance) (A). Lactophenol cotton blue stain (LPCB) used to enhance the visibility of hyphae and spores (B).
4. Histopathology
In the vast majority of PV cases, skin biopsy remains unnecessary and does not play a role in a daily practice other than for ruling out any of the differential diagnosis [1][11][16]. Thus, the authors advise considering faster and less expensive and less invasive alternatives described below. Histological findings include minimal epidermal changes, some grade of hyperkeratosis, and acanthosis [1][11]. Of note, contrary to what may be appreciated in other superficial mycoses caused by Trichophyton or Microsporon species, PV features spongiotic changes, whereas the superficial perivascular infiltrate in the dermis is absent or minimal [1][11][17]. Fungal elements can be visualised within the stratum corneum and can be present even in routine haematoxylin–eosin staining as, contrary to other superficial epidermal fungal infections, the spores and hyphal elements are basophilic [17]. Periodic acid–Schiff or Grocott–Gomori methenamine silver stains may further enhance the diagnostic accuracy [1][11] (Figure 2). The classic spaghetti and meatballs appearance may also be appreciated with this method (Figure 3). Hyperpigmented lesions tend to contain more hyphae and spores than the hypopigmented ones [4]. Also, hyperpigmented PV exhibits single, abnormally large melanosomes [18]. On the other hand, hypopigmented lesions feature mild hyperkeratosis and exhibit fewer and smaller melanosomes in the spinous layer when compared to healthy skin [4].
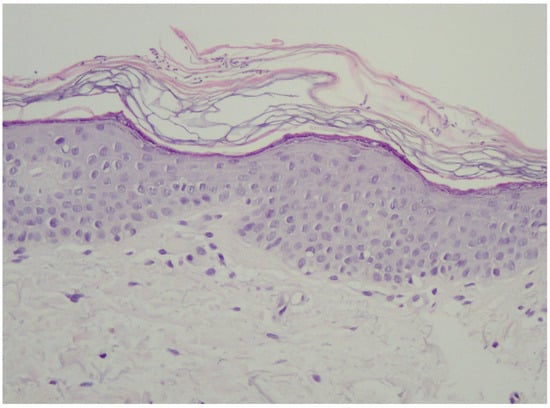
Figure 2. Typical epidermis and dermis with no signs of inflammatory infiltrates or other pathological characteristics. Notably, there is a thickened but loosely arranged stratum corneum. Within this layer, clusters of short longitudinal filaments (resembling hyphae or mycelium) are visible. These filaments occasionally form short branching chains (reminiscent of spaghetti) and round spores resembling yeast cells (meatballs), which can be observed interspersed between the keratin layers. Magnification ×20.
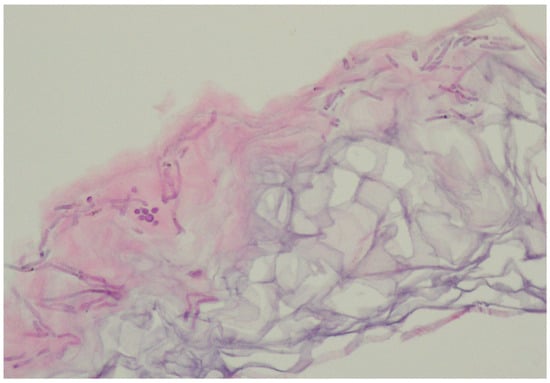
Figure 3.
Characteristic basophilic hyphae and spores responsible for spaghetti and meatballs appearance in histopathology.
5. Wood’s Light
Wood’s light is an ultraviolet light (UV) emitter (wavelength range: 320–450 nm; peak wavelength: 365 nm). The examination requires a dark environment. UV radiation has the ability to induce excited fluorescence in various substances, called chromophores, including the ones that are present within/on the surface of the skin. If the excited fluorescence belongs to the visible light spectrum, it can be appreciated using visual examination [19][20].
The device can be of aid in PV, as UV light may be used to determine the extent of M. furfur infection. Typically, a yellow-white or copper-orange excited fluorescence can be observed in an active classical infection and bluish-white fluorescence in the active follicular form (Figure 4) [4][20]. Of note, it has been shown that only one third of the PV lesions display fluorescence with Wood’s lamp [11]. One of the factors seemingly responsible for this number could be washing out the chromophores with a shower/bath prior to the consultation, just as in the case of porphyrin-dependent excited fluorescence in Corynebacterial dermatoses [21].
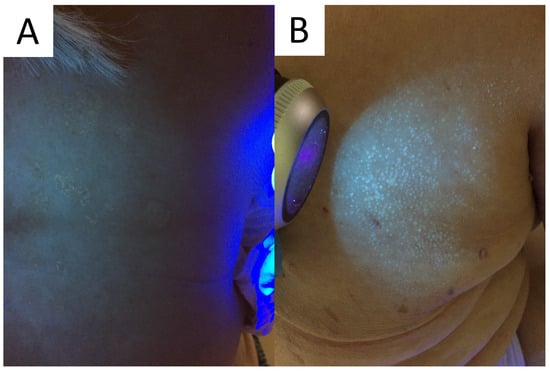
Figure 4. Excited ochre and blue fluorescence seen with Wood’s lamp examination in classic (A) and follicular (B) forms of pityriasis versicolor, respectively.
It should also be kept in mind that cosmetics, sunscreens, topical medications, and other intentionally and unintentionally applied exogenous pigments might potentially interfere with examination, being a potential source of false-positive fluorescence. Nevertheless, this low-cost, fast, and easy-to-apply method has gained great popularity not only in aiding in the diagnostic process and determining the extent of disease, but also in disease monitoring and confirming therapeutic success.
6. Reflectance Confocal Microscopy
There are scarce data on the reflectance confocal microscopy (RCM) features of PV. Clusters of roundish bright structures and tortuous hyperreflective structures (corresponding to the spaghetti and meatballs appearance seen in conventional direct microscopy) at the level of the horny layer can be seen [22]. Other unspecific findings include the presence of hyperkeratosis, elongated vessels, and spongiotic changes (Figure 5).
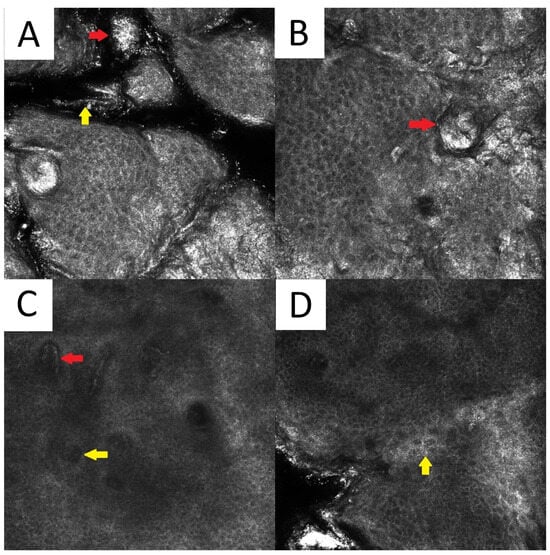
Figure 5. Reflectance confocal microscopy of pityriasis versicolor. Malassezia spores/meatballs (red arrow) and hyphae/spaghetti (yellow arrow) present in the stratum corneum (A). Presence of fungi can induce secondary morphological changes: hyperkeratotic plug (red arrow) inside the hair follicle (B); elongated vessels (red arrow) and vessels inside papillae (yellow arrow) present at the same level as the lower epidermal layers (C); epidermal spongiosis (yellow arrow) (D).
7. Dermatoscopy
PV is usually diagnosed based on clinical features related to the characteristic appearance and distribution of skin lesions. However, cases with atypical distribution or morphology may pose a diagnostic challenge and require differentiating with various pigmentation disorders. Non-contact polarised dermatoscopy is a gold standard in inflammoscopy (Figure 6) [23]. Both hypopigmented and hyperpigmented PV lesions display particular clues [2]. PV patches typically display relatively well-defined depigmented (hypopigmented variant), tan, or red-brownish (hyperpigmented variant) structureless areas covered with fine scales. In darker skin types, these areas display physiological hyper- or hypopigmented reticular lines [13]. It has been noted that in both hypopigmented and hyperpigmented lesions, the pigmentation is usually nonuniform [2]. The scales typically occur in the skin furrows and around the hair follicle openings, due to the higher humidity of these areas [23]. Scaling is more frequently observed in hypopigmented lesions, while furrowed scaling is more common in dermatoscopy of hyperpigmented lesions [2], possibly due to the better contrast between the bright scale and darker background. Of notice, the scale can be completely removed with alcohol solution [24]. Other clues observed in PV may include the peripheral area of hyperpigmentation surrounding the central hypopigmented area or the peripheral area of hypopigmentation surrounding the hyperpigmented centre (described by the authors as a “contrast halo sign”), peripheral hyper/hypopigmented satellite globules, folliculocentricity of the lesions, and hypopigmentation of the affected hair follicle opening (due to fungal invasion) [2][13][25].
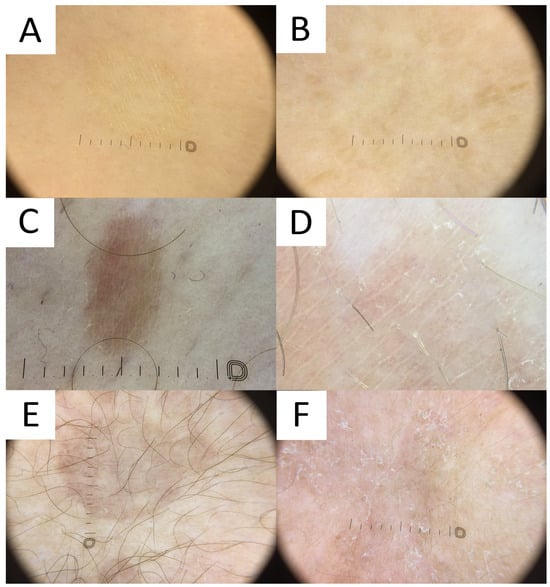
Figure 6. Dermatoscopy in pityriasis versicolor. (A) Classic hypopigmented lesion of pityriasis versicolor (PV) displaying typical furrow scaling. (B) Follicular hyperpigmented PV displaying small, roundish, folliculocentric tan areas with discrete scaling. (C) Hyperpigmented folliculocentric PV lesion exhibiting reddish-tan area with perifollicular, furrow, and peripheral inward scaling. (D) Hyperpigmented PV with subtle furrow scales and perifollicular scale. (E) Hyperpigmented classic PV lesion with no scale. Peripheral hypopigmented area surrounding the lighter centre (“contrast halo sign”) can be appreciated. (F) Hypopigmented PV exhibits diffuse scaling and peripheral hyperpigmented areas (“contrast halo sign”).
8. Ultraviolet-Induced Fluorescence Dermatoscopy
Ultraviolet-induced fluorescence dermatoscopy (UVFD) is a novel dermatoscopic method utilising UV light. Similarly to Wood’s lamp, UVFD produces colourful images due to the excited fluorescence emitted by the chromophores [19][26]. The main sources of luminescence in PV are blue background elastin and collagen [19][27], bright blue keratin [27], yellow fungal pityrialactone [28], and orange, pink-to-coral-red porphyrins (esp. coproporphyrin III and protoporphyrin IX) [19][27][29].
Even though no literature data exist on UVFD clues to PV, hypopigmented lesions can be characterised by light greenish structureless areas, whereas hyperpigmented lesions feature dark greenish structureless areas (Figure 7). In seborrheic areas, the affected sites become deprived of the background red follicular fluorescence of Cutibacteria spp., likely due to the antiseptic properties of azelaic acid produced by Malassezia spp.. UVFD enhances the visibility of the light greenish scale in the skin furrows (that may display a double edge) and bright greenish perifollicular scaling even in non-obvious lesions (revealing their folliculotropic nature). Some of the lesions might be surrounded by the darker area visible only with this method (a UVFD equivalent of “contrast halo sign”). The aforementioned observations require confirmation with a larger study group, optimally in an international study representing different ethnic groups. As the scaling and fluorescence are likely to disappear with the elimination of the Malassezia fungus and the background cutibacterial follicular fluorescence is likely to reappear with the cessation of azelaic acid production, UVFD can be utilised not only in the diagnostic process, but also in disease monitoring and confirmation of successful treatment. Nevertheless, up until now, there have been no observations on specific time frames when these phenomena occur. Luminescent neon-like images shown on the monitor/smartphone screen are visually attractive to the patients. Moreover, the instant confirmation of the fungal infection becomes more perceptible, contributing to stronger compliance.
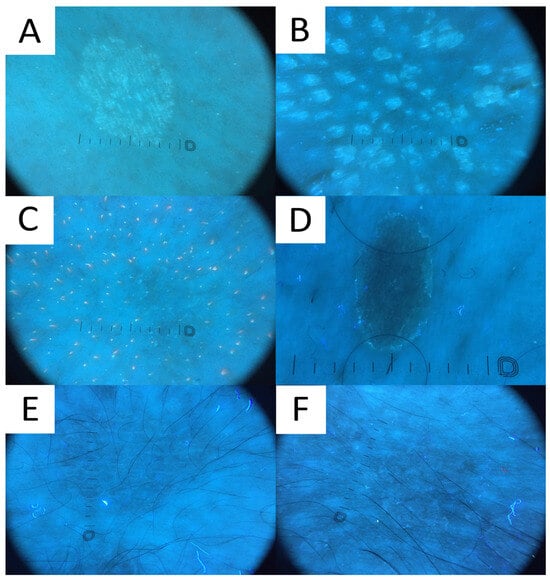
Figure 7. Ultraviolet-induced fluorescence dermatoscopy (UVFD) of pityriasis versicolor (PV). (A) Light greenish excited fluorescence is emitted by fungal chromophore (pityrialactone), which results in emergence of single- or double-edged furrow scale and perifollicular scaling, which seem to be better seen in hypopigmented PV lesions. The image matches Figure 7A. (B) Light greenish perifollicular scale can be appreciated in active follicular PV strongly enhancing the diagnostic clues of scale. The figure matches Figure 7B. (C) UVFD in a case of achromic PV at seborrheic site displays “blackout areas”—areas deprived of background pink-orange porphyrin fluorescence, possibly due to antibacterial properties of azelaic acid produced by Malassezia spp. (D) Dark greenish fluorescence of folliculocentric hyperpigmented PV lesion. Subtle peripheral free edge of scale is seen better with UVFD. The figure matches Figure 7C (E) A case of hyperpigmented PV lesion showing no fluorescence in UVFD. The figure matches Figure 7D. (F) “UVFD contrast halo sign” showing dark curvilinear border enclosing hypopigmented PV.
References
- Karray, M.; McKinney, W.P. Tinea Versicolor; StatPearls Publishing: Treasure Island, FL, USA, 2023.
- Mathur, M.; Acharya, P.; Karki, A.; Kc, N.; Shah, J. Dermoscopic pattern of pityriasis versicolor. Clin. Cosmet. Investig. Dermatol. 2019, 12, 303–309.
- Krueger, L.; Saizan, A.; Stein, J.A.; Elbuluk, N. Dermoscopy of acquired pigmentary disorders: A comprehensive review. Int. J. Dermatol. 2022, 61, 7–19.
- Leung, A.K.; Barankin, B.; Lam, J.M.; Leong, K.F.; Hon, K.L. Tinea versicolor: An updated review. Drugs Context 2022, 11.
- Kaymak, Y.; Taner, E. Anxiety and depression in patients with pityriasis rosea compared to patients with tinea versicolor. Dermatol. Nurs. 2008, 20, 367–370+377.
- Gupta, A.K.; Foley, K.A. Antifungal Treatment for Pityriasis Versicolor. J. Fungi 2015, 1, 13–29.
- Gupta, A.K.; Lyons, D.C. Pityriasis versicolor: An update on pharmacological treatment options. Expert Opin. Pharmacother. 2014, 15, 1707–1713.
- Theelen, B.; Cafarchia, C.; Gaitanis, G.; Bassukas, I.D.; Boekhout, T.; Dawson, T.L., Jr. Malassezia ecology, pathophysiology, and treatment. Med. Mycol. 2018, 56, S10–S25.
- Vijaya Chandra, S.H.; Srinivas, R.; Dawson, T.L., Jr.; Common, J.E. Cutaneous Malassezia: Commensal, Pathogen, or Protector? Front. Cell. Infect. Microbiol. 2020, 10, 614446.
- Saunte, D.M.L.; Gaitanis, G.; Hay, R.J. Malassezia-Associated Skin Diseases, the Use of Diagnostics and Treatment. Front. Cell. Infect. Microbiol. 2020, 10, 112.
- Crespo-Erchiga, V.; Florencio, V.D. Malassezia yeasts and pityriasis versicolor. Curr. Opin. Infect. Dis. 2006, 19, 139–147.
- Hamdino, M.; Saudy, A.A.; El-Shahed, L.H.; Taha, M. Identification of Malassezia species isolated from some Malassezia associated skin diseases. J. Mycol. Med. 2022, 32, 101301.
- Kaur, I.; Jakhar, D.; Singal, A. Dermoscopy in the Evaluation of Pityriasis Versicolor: A Cross Sectional Study. Indian Dermatol. Online J. 2019, 10, 682–685.
- Shah, A.; Koticha, A.; Ubale, M.; Wanjare, S.; Mehta, P.; Khopkar, U. Identification and speciation of malassezia in patients clinically suspected of having pityriasis versicolor. Indian J. Dermatol. 2013, 58, 239.
- Porto, J.A. The use of cellophane tape in the diagnosis of Tinea versicolor. J. Investig. Dermatol. 1953, 21, 229–231.
- Maleszka, R.; Adamski, Z.; Szepietowski, J.; Baran, E. Treatment of superficial fungal infections—Recommendations of experts of Mycological Section of Polish Dermatological Society. Dermatol. Rev. 2015, 4, 305–315.
- Guarner, J.; Brandt, M.E. Histopathologic diagnosis of fungal infections in the 21st century. Clin. Microbiol. Rev. 2011, 24, 247–280.
- Allen, H.B.; Charles, C.R.; Johnson, B.L. Hyperpigmented tinea versicolor. Arch. Dermatol. 1976, 112, 1110–1112.
- Pietkiewicz, P.; Navarrete-Dechent, C.; Goldust, M.; Korecka, K.; Todorovska, V.; Errichetti, E. Differentiating Fordyce Spots from Their Common Simulators Using Ultraviolet-Induced Fluorescence Dermatoscopy—Retrospective Study. Diagnostics 2023, 13, 985.
- Mojeski, J.A.; Almashali, M.; Jowdy, P.; Fitzgerald, M.E.; Brady, K.L.; Zeitouni, N.C.; Colegio, O.R.; Paragh, G. Ultraviolet imaging in dermatology. Photodiagn. Photodyn. Ther. 2020, 30, 101743.
- Pietkiewicz, P.; Navarrete-Dechent, C.; Salwowska, N.; Cantisani, C.; Goldust, M.; Errichetti, E. Ultraviolet-Induced Fluorescence Dermoscopy Reveals Fluorescent Clues in Pitted Keratolysis. Dermatol. Pract. Concept. 2023, 13, e2023242.
- Pimenta, R.; Soares-de-Almeida, L.; Arzberger, E.; Ferreira, J.; Leal-Filipe, P.; Bastos, P.M.; Oliveira, A.L. Reflectance confocal microscopy for the diagnosis of skin infections and infestations. Dermatol. Online J. 2020, 26.
- Errichetti, E.; Zalaudek, I.; Kittler, H.; Apalla, Z.; Argenziano, G.; Bakos, R.; Blum, A.; Braun, R.P.; Ioannides, D.; Lacarrubba, F.; et al. Standardization of dermoscopic terminology and basic dermoscopic parameters to evaluate in general dermatology (non-neoplastic dermatoses): An expert consensus on behalf of the International Dermoscopy Society. Br. J. Dermatol. 2020, 182, 454–467.
- Kapadia, F.; Kharkar, V.; Vishwanath, T. Dermoscopy to the Rescue in an Annular Enigma: A Rare Case of Annular Pityriasis Versicolor Presenting in an Unusual Location. Dermatol. Pract. Concept. 2022, 12, e2022057.
- Al-Refu, K. Dermoscopy is a new diagnostic tool in diagnosis of common hypopigmented macular disease: A descriptive study. Dermatol. Rep. 2019, 11, 7916.
- Al-Nasiri, M.; Navarrete-Dechent, C.; Korecka, K.; Salwowska, N.; Goldust, M.; Pietkiewicz, P. Ultraviolet-Induced Fluorescence Dermatoscopy of Trichobacteriosis Axillaris Reveals Peripilar Yellow-Green Luminescent Concretions. Dermatol. Pract. Concept. 2023, 13, e2023169.
- Asawanonda, P.; Taylor, C.R. Wood’s light in dermatology. Int. J. Dermatol. 1999, 38, 801–807.
- Mayser, P.; Stapelkamp, H.; Krämer, H.J.; Podobinska, M.; Wallbott, W.; Irlinger, B.; Steglich, W. Pityrialactone—A new fluorochrome from the tryptophan metabolism of Malassezia furfur. Antonie Van Leeuwenhoek 2003, 84, 185–191.
- Patwardhan, S.V.; Richter, C.; Vogt, A.; Blume-Peytavi, U.; Canfield, D.; Kottner, J. Measuring acne using Coproporphyrin III, Protoporphyrin IX, and lesion-specific inflammation: An exploratory study. Arch. Dermatol. Res. 2017, 309, 159–167.
More
