Indocyanine green (ICG) is an important kind of near infrared (NIR) photosensitive molecules for photothermal therapy (PTT/PDT)/photodynamic therapy (PDT) therapy as well as imaging. When exposed to NIR light, ICG can produce reactive oxygen species (ROS), which can kill cancer cells and pathogenic bacteria. Moreover, the absorbed light can also be converted into heat by ICG molecules to eliminate cancer cells. In addition, it performs exceptionally well in optical imaging-guided tumor therapy and antimicrobial therapy due to its deeper tissue penetration and low photobleaching properties in the near-infrared region compared to other dyes. In order to solve the problems of water and optical stability and multi-function problem of ICG molecules, composite nanomaterials based on ICG have been designed and widely used, especially in the fields of tumors and sterilization.
- ICG
- composite nanoparticles
- light therapy
- tumor treatment
- antibacterial treatment
1. Introduction
2. Molecular Structure
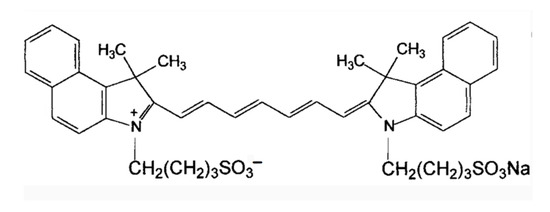
ICG is an amphiphilic molecule, meaning it has both hydrophilic and lipophilic properties [4,9][4][9]. It is composed of two polycyclic parts (benzindotricycin) that are predominantly lipophilic and connected by carbon chains as depicted in Figure 21. Each polycyclic part is bound to a sulfate group, which provides some water solubility to ICG. This property allows ICG to bind to both hydrophilic and lipophilic substances, including phospholipids, thereby enhancing its fluorescence intensity. The fluorescence yield of ICG is influenced by these binding interactions. At concentrations below 5 µM, ICG exists as a monomer. However, at concentrations exceeding 100 µM, aggregation occurs, the absorption peak of the monomeric form of ICG is around 785 nm, whereas the maximum absorbance of the aggregates shifts to approximately 690 nm at concentrations greater than 100 µM [9]. ICG J-aggregates (IJA), a derivative of ICG, exhibits a dark green color visible to the naked eye, while ICG appears light- green. IJA also undergoes a significant red shift of approximately 100 nm compared to ICG. In various media, both IJA and ICG display a characteristic peak at 892 nm. IJA demonstrates greater water stability and does not undergo significant spectral changes induced by salt [10], unlike ICG. Under physiological conditions, ICG molecules undergo aggregation and polymerization with plasma proteins or lipoproteins after intravenous injection, this results in the main peak of the absorption spectrum red-shifting to around 805 nm or 810 nm, leading to a relatively stable spectrum [9].3. Design of ICG and Composite Nanomaterials
ICG molecules offer significant advantages in terms of biosafety and metabolic modalities. ICG has been clinically approved and is considered safe for use. However, its practical application is limited due to drawbacks such as photobleaching and short blood circulation half-life (t1/2 = 2~4 min). Moreover, ICG NIR fluorescent dyes are water-soluble compounds that exhibit chemical instability in aqueous media. They tend to aggregate at high concentrations, bind to proteins under physiological conditions, and undergo photodegradation, thermal degradation, or photobleaching. Therefore, achieving stability in aqueous media is a prerequisite for their biomedical application to maximize their benefits. To address these challenges, efforts have been made to incorporate or polymerize ICG NIR fluorescent dyes into nanoparticles. Compared to free ICG molecules, ICG encapsulated in nanostructures offer several advantages:-
Enhanced stability in physiological environments;
-
Substantially higher photothermal conversion efficiency;
-
Prolonged circulation duration in the bloodstream due to the development of nanostructures. Additionally, the enhanced permeability and retention (EPR) effect of solid tumors can potentially facilitate tumor targeting;
-
The nanoparticle platform allows for the combination of various diagnostic and therapeutic tools. For instance, simultaneous loading of chemotherapeutic drugs and NIR dyes can be achieved, enabling the concurrent use of photothermal therapy and photothermal-regulated drug therapy to enhance tumor treatment.
3.1. ICG/MOFs Nanoparticles
Metal-organic frameworks (MOFs) are hybrid porous polymers constructed from metal ions/clusters and multi-layered organic ligands. They possess unique properties such as a large surface area, tunable pore size and shape, tunable composition, and functionalized pore surfaces, which make them highly advantageous over conventional porous materials. These properties have led to their utility in applications such as drug adsorption, delivery, and release [11,12][11][12]. One example of MOF application is ZIF-8 addressing challenges related to photosensitizers. ZIF-8 relieves hypoxia, slows the self-aggregation of photosensitizers, and permits photodynamic ROS production to permeate deeper into the tissue [13]. Fang C et al. designed ZIF-8-coated ZnS nanoparticles for the co-delivery ICG and tirapazamine (TPZ) for H2S amplified cooperative therapy [14]. ZIF-8 is capable of obtaining high ICG loading, decomposing, and releasing ICG in acidic environments due to its ultra-high porosity, adjustable structure, and biodegradable qualities under acidic circumstances. Hollow nanostructures have also attracted interest in drug delivery systems due to their high drug-carrying capacity and unique physicochemical properties. Sun et al. formed a shell of Zr-based porphyrin MOF through the digestion of prefabricated ZIF-8 nanoparticle templates and coordination-driven self-assembly of tetra (4-carboxyphenyl) porphyrin (TCPP) ligands and Zr4+ ions. They encapsulated DOX and ICG into the resulting hollow spherical nanoparticles with mesoporous MOF shells (DIHP) and further encapsulated them with mouse breast cancer (4T1) cell membranes to achieve imaging-guided synergistic cancer therapy [16][15].3.2. ICG/Polymers
The encapsulation of ICG molecules within various biodegradable polymers offers several advantages for ICG, including improved stability, prevention of aggregation, and reduced fluorescence quenching. Polymeric carriers provide benefits during ICG delivery, such as enhanced deep tissue permeability, generation of singlet oxygen upon light exposure, and minimal autofluorescence. Additionally, polymers can extend the half-life of ICG, reduce its rapid degradation, and improve its biocompatibility, biodegradability, fluorescence intensity, physicochemical stability, target specificity, and pharmacokinetic properties. Different polymers have been utilized to encapsulate and deliver ICG, including poly (D,L-lactic-co-glycolic acid) (PLGA), polyethylene glycol (PEG), and poly (ε-caprolactone) (PCL). These polymers have been employed in various applications such as imaging, diagnostics, and therapeutics. However, each polymer has its advantages and limitations [21][16]. Lipid-polymer hybrid nanoparticles (LPNs) are core-shell structures composed of a polymer core and a lipid or lipid-PEG shell. LPNs combine the advantages of both polymer nanoparticles and liposomes [22][17], particularly in terms of physical stability and biocompatibility [23][18]. To enhance the antitumor efficacy of ICG, it is crucial to improve its accumulation in tumors by controlling the nanoparticle size. Zhao et al. developed ICG-PLGA-lecithin-PEG core-shell nanoparticles (INPs) with sizes of 39 nm, 68 nm, and 116 nm using a one-step nanoprecipitation method [25][19]. INPs at 39 nm demonstrated increased absorption into pancreatic cancer tumor cells and improved phototherapeutic effects in vitro when compared to bigger diameters (68 nm and 116 nm). DOX and ICG-loaded PLGA-lecithin-PEG nanoparticles (DINPs) were prepared by Zheng et al. using a single-step ultrasound method. Synergistic treatment with DINPs demonstrated improved apoptosis and cell death compared to chemotherapy or photothermal treatment alone [26][20]. In addition to polymeric nanoparticles and lipid-polymers, polymeric nanocapsules have been designed to encapsulate ICG. These nanocapsules consist of polyallylamine hydrochloride (PAH) chains and are covalently coated with PEG via a reductive amination reaction [27][21]. The encapsulation of ICG in these constructs prolongs its circulation time and retards hepatic accumulation, addressing the rapid elimination of ICG from the vascular system.3.3. Liposome-Coated ICG
Liposome-encapsulated ICG has been developed to address the limitations of ICG, such as lack of targeting, easy quenching, self-aggregation, and instability. Liposomes are lipid bilayer structures that can encapsulate hydrophobic compounds like ICG, providing stability and controlled release. Here are some key findings from studies using liposome-encapsulated ICG. Imaging-guided PTT of retinoblastoma (Rb). Liu et al. synthesized ICG encapsulated in lipid bilayer multifunctional liposomes (ILP) for the treatment of Rb [29][22]. ILP exhibited better tumor targeting and higher temperature elevation compared to free ICG when irradiated with near-infrared laser. ILP showed improved imaging and PTT outcomes for Rb. For synergistic PTT and PDT, in order to have combined anticancer effects, Dai et al. liposomally encapsulated ICG and the hypoxia-activated prodrug tirapamycin (TPZ). Photothermal effect of ICG, combined with photodynamic effect of released Ce6, resulted in cytotoxic singlet oxygen generation. The hypoxia caused by this process activated the antitumor activity of TPZ [34][23]. For photo-triggered decomposable delivery system, Gao et al. developed a nanoparticle system (Lipo@ICG@CuS) consisting of liposomes. The photothermal ability of CuS NPs caused temperature to rise in response to near-infrared laser irradiation, which led the liposomes′ structural integrity to break down and the release of ICG and CuS NPs. Synergistic PDT and PTT were made possible by this technique [35][24].3.4. ICG-Based Micelles Composites
Multifunctional pH/reduction dual-response drug delivery system: Zhang et al. developed a drug delivery system based on polymer micelles that responded to both pH and reduction stimuli. The micelles encapsulated the chemotherapeutic drug DOX and the photosensitizer ICG for NIR imaging and targeted chemotherapy-photothermal combination therapy [36][25]. The micelles remained stable during blood circulation, accumulated in tumors through enhanced permeation and retention (EPR) effects, and released the drugs in response to acid hydrolysis and high glutathione (GSH) concentrations. ICG enhanced cellular uptake and accelerated drug release under 808 nm light irradiation. Covalent conjugation for enhanced tumor accumulation: Covalent conjugation of ICG-NH2 to poly (ethylene glycol) -block-poly (2-methyl-2-carboxy-propenyl carbonate) (PEG-PCC) copolymers were reported to self-assemble into highly loaded micelles of ICG. This covalent action enhanced tumor accumulation of ICG in vivo, as demonstrated by NIR imaging. The micelles showed improved therapeutic efficacy when combined with NIR irradiation, leading to complete tumor regression [37][26]. Polymer micelles for combined chemotherapy and PTT: Yang et al. prepared polymer micelles loaded with the chemotherapeutic agent glyburic acid (GA) and coupled with ICG. Under 808 nm laser irradiation, ICG in the micelles enabled PTT and generation of reactive oxygen species, which cleaved the TK bond of the polymer, causing the micelles to break down and release GA. GA inhibited heat shock protein 90 (HSP90), enhancing the PTT efficiency of ICG. This combination of PTT and chemotherapy showed potential for complete tumor destruction [38][27].3.5. ICG/Gold Nanocomposites
The higher temperature mediated by light exposure significantly accelerates the photodegradation of ICG in aqueous solution through thermal decomposition. Therefore, the stability and good biocompatibility of gold (Au)-based nanomaterials such as gold nanorods (Au NRs), gold nanoparticles (Au NPs), and hollow gold nanospheres (HAuNS) have been extensively studied for enhancing the stability of ICG [42,43][28][29]. Due to the deep light penetration of ICG in the near-infrared region, it can be used for the treatment of deeply buried tumors with minimal damage to normal tissues and excellent fluorescence imaging ability. Gold nanoshells (GNs) have been approved for clinical trials to evaluate their efficacy in patients with refractory and/or recurrent head and neck tumors. Hu et al. developed an interventional photothermal therapy (IPTT) to kill pancreatic cancer (PC) cells deep in the abdominal cavity. IPTT involves inserting a NIR fiber through an 18 G percutaneous transhepatic cholangiopancreatography (PTC) puncture needle, allowing access to deep PC tissue. They selected gold nanoshells coupled with anti-urokinase-type fibrinogen activator receptor (uPAR) antibody, polyethylene glycol, and indocyanine green (uIGNs) as the PTT reagents. CT and fluorescence imaging can be used for tumor visualization and to mediate PTT of PC. The first systematic comparison of the efficacy of clinical iodine-125 (125I) tissue interposition radiotherapy (IBT-125-I) and IPTT was performed in a human pancreatic cancer in situ transplantation tumor model. The results showed that the median survival rate of IPTT with one-time interventional complete ablation was improved by 25% compared to IBT-125-I [44][30].3.6. ICG/ Silica Nanocomposites
Core-shell nanoparticles composed of gold nanorod cores and silica shells have shown higher image contrast compared to “bare” gold nanorods in both photoacoustic imaging (PAI) and fluorescence imaging (FMI). These nanoparticles can be loaded with ICG to create bimodal imaging probes that overcome fluorophore quenching. The ICG-loaded gold nanorod–silica nanoparticles (Au@SiO2) exhibit low cytotoxicity and can maintain their photophysical properties in the near-infrared region. In one study by Kang et al., four types of nanoparticles containing encapsulated ICG were prepared and tested to enhance the PA signal. These nanoparticles included calcium silicate-encapsulated porous silicon nanoparticles (Ca-pSiNP-ICG composites), porous calcium silicate nanoparticles (CaS-ICG), microporous silica NPs sealed with calcium silicate (Ca-Silica-ICG), and liposomal NPs (Lip-ICG). It was found that the encapsulation of ICG in porous silicon, porous silica, or calcium silicate NPs significantly enhanced the PA response, with the porous silicon NPs (pSiNPs) showing the strongest enhancement [50][31]. These materials have low thermal conductivity and are biocompatible, making them suitable for in vivo applications.3.7. ICG-Based Multifunctional Composites
ICG is a biosafe photosensitizer capable of killing tumor cells by generating singlet oxygen and photothermal effects under near-infrared irradiation. However, it has limitations such as easy aggregation, rapid water degradation, and a short half-life. Combining ICG with NPs addresses these limitations effectively. ICG can be encapsulated not only with various organic nanomaterials (such as polymers, micelles, liposomes, dendrimers, and proteins) but also with inorganic nanomaterials (including magnetic, gold, mesoporous, calcium, and LDH-based materials) for drug delivery. Among these, colloidal mesoporous silica nanoparticles (MSNs) are one of the most mature and representative inorganic materials used in biology and medicine. They have transitioned from extensive in vitro studies to preliminary in vivo assays in small animal disease models. CaXs biomaterials, including calcium phosphate, calcium carbonate, calcium silicate, and calcium fluoride, have found wide biomedical applications due to their excellent biocompatibility and biodegradability. Among them, calcium carbonate is employed for controlled drug release, leveraging its degradation in acidic environments to prevent drug inactivation and minimize side effects. In conclusion, the hybridization of calcium-based biomaterials with inorganic/organic nanocarriers provides effective strategies for ICG molecular delivery. This approach holds promise for tumor imaging, drug tracing, and antitumor therapy.4. Imaging and Light Therapy of ICG Molecules
4.1. Imaging and Light Therapy for Tumors
ICG is used in PTT and PDT due to its ability to absorb light energy and convert it into heat or generate singlet oxygen. However, there are challenges associated with using ICG in these therapies. One challenge is the exacerbation of tumor hypoxia caused by the tumor cell′s hypoxic microenvironment after PDT with ICG under light irradiation. This can hinder the effectiveness of tumor eradication. Another challenge is the limited immunogenicity of immunogenic cell death (ICD) induced by ROS generated through endoplasmic reticulum (ER) stress [56,57][32][33]. When ICG enters the cellular internalization, it mainly distributes in the cytoplasm, limiting its impact on ICD. To address these challenges, a dual ER-targeting strategy has been proposed by Li et al. [31][34]. The strategy combines PDT, PTT and immunotherapy using a nanosystem called FAL-ICGHAuNS (endoplasmic reticulum-targeted pardaxin-modified indocyanine green-fixed hollow gold nanospheres) and FAL-Hb lipo (oxygen-carrying hemoglobin liposomes). This strategy aims to reverse hypoxia in tumors. The FAL peptide-mediated hemoglobin targeting the ER replenishes oxygen under PDT hypoxic conditions and induces strong ER stress and calcium reticulum protein (CRT) exposure on the cell surface under NIR irradiation. This exposure triggers “eat me” signals that allow dendritic cells (DCs) to mature and present antigens to cytotoxic cells, leading to immunogenic cell death. To enhance ICG′s phototoxicity, it can be combined with another photosensitizer molecule. Zinc phthalocyanine (ZNPC), known for its admirable ROS production capacity, is considered a suitable candidate for combination with ICG [58][35]. In clinical applications, ICG’s rapid clearance by the liver can limit its utility. To overcome this limitation, Du et al. developed ICG-PEG45, which is the first renal tubule-secreted NIR fluorophore. ICG-PEG45 selectively accumulates in renal cancer tissues with low expression of P-glycoprotein (P-gP) efflux transport protein [60[36][37],61], enabling highly specific fluorescence detection [60][36]. Due to the rapid liver clearance of ICG molecules, their short retention time in tumors, and limited penetration depth, liposomal IJA has been investigated. In aqueous solution, ICG primarily exists as monomers and H-dimers, exhibiting absorption peaks at approximately 780 nm and 715 nm, respectively. Upon water bath heating at 65 °C, these peaks diminish, while a new absorption peak near 895 nm emerges, indicating the formation of J-aggregates. The J-aggregates are characterized by narrow absorption spectra (10–20 nm) and significant red shifts (~100 nm) due to the π-π stacking and electrostatic interaction arrangement of ICG molecules [63][38].4.2. Antibacterial Phototherapy
Antibiotics have been extensively utilized as the primary treatment method for bacterial infections. However, the emergence of antibiotic-resistant bacteria poses a challenge to traditional antibiotic therapy, making it difficult to completely eradicate bacteria and effectively manage bacterial infectious diseases. aPDT has emerged as a promising therapeutic approach, offering potential solutions to the issues of surgical invasiveness and antibiotic resistance [68,69,70][39][40][41]. By utilizing photosensitizers (PSs), aPDT can induce bacterial necrosis and apoptosis by generating large quantities of ROS upon exposure to light. These ROS can damage bacterial cell membranes and DNA [68,69,70][39][40][41]. Various PSs, including methylene blue, curcumin, dihydroporphyrin (Ce6), and porphyrins [71,72,73,74,75,76][42][43][44][45][46][47], can be activated by ultraviolet (UV) or visible light. However, there are two challenges that need to be addressed: the depth of infectious diseases within tissues and the limited tissue penetration ability of UV and visible light, which impose high requirements on the photosensitizers used in PTT. NIR light offers several advantages, such as deeper tissue penetration, improved biosafety, and minimal background interference. As a result, NIR-responsive PSs have gained significant attention. ICG can generate abundant ROS when exposed to 808 nm NIR light, making it a promising candidate for NIR antimicrobial therapy. Despite the advancements in aPDT, two challenges hinder its clinical application. Despite the significant progress in aPDT, two challenges need to be addressed before its clinical application. Firstly, according to the mechanism of aPDT, photosensitizers can produce abundant singlet oxygen when oxygen is present and appropriate light is applied. However, bacterial infections at deep sites often have an oxygen-deficient microenvironment, limiting the effectiveness of oxygen-dependent aPDT. Secondly, aPDT solely targets bacteria and cannot eliminate bacterial-induced inflammation, which can cause damage to normal tissues. Therefore, novel aPDT strategies that address pro-oxygenation and anti-inflammation are greatly needed. Significantly, MoS2/ICG/Ag exhibited remarkable inhibition of S. aureus biofilm formation and effectively eradicated bacteria residing deep within the biofilm. Furthermore, in vivo studies demonstrated the successful treatment of S. aureus biofilm-infected wounds using MoS2/ICG/Ag with minimal toxicity. Another study conducted by Xiao et al. focused on the rational integration of copper peroxide (CP) and indocyanine green into polydopamine NPs to create a multifunctional hybrid nanoplatform called PDA/CP/ICG [85][48]. This nanoplatform aimed to alleviate hypoxia within the microenvironment, thereby enhancing reactive oxygen species generation for efficient biofilm elimination.5. ICG for Angiography, Surgery, and Organ Reconstruction
Under physiological conditions, upon intravenous injection, ICG molecules can undergo aggregation and polymerization when interacting with plasma proteins or lipoproteins. This process causes the main peak of the absorption spectrum to red-shift to around 805 nm or 810 nm, resulting in a relatively stable spectrum. It is worth noting that the fluorescence yield of ICG polymers is generally weaker compared to free or protein-bound ICG monomers. These properties make ICG molecules suitable for clinical applications as angiographic contrast agents, intraoperative visualization tools for tumor localization and resection, lymphatic imaging, and reducing the risk of false-positive resections. Near-infrared fluorescence (NIRF) imaging has shown promise in providing noninvasive in vivo imaging of human and animal lymphatic vessels [86][49]. NIRF imaging enables direct visualization of lymphatic vessels, and intracutaneous injection of ICG allows for the in vivo visualization of lymphatic transport, the NIR fluorescence imaging of healthy and affected limbs is shown in Figure 112A,B [86][49]. This technique can be utilized for early diagnosis of lymphedema, assessment of lymphatic function, and evaluating the response to lymphedema treatment (refer to Figure 112).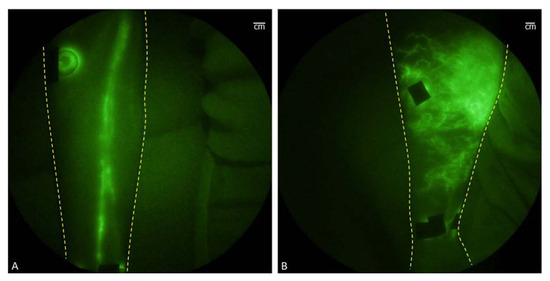
5.1. Angiography
5.2. Surgery
Sentinel lymph node biopsy (SLNB) plays a crucial role in determining cancer spread and staging. Traditional tracers used for SLNB, such as blue dyes, nuclear proteins, nanocarbon, and radioactive technetium-99-labeled colloids or complexes, have limitations in terms of real-time monitoring and biosafety, potentially causing tissue damage. ICG offers several advantages as a tracer for SLNB. It exhibits strong tissue penetration, high sensitivity, and non-invasiveness, with absorption and emission wavelengths around 765 nm and 820 nm, respectively. ICG is particularly suitable for real-time dynamic monitoring of SLN tracers and surgical navigation optical imaging due to its low molecular weight, non-toxicity, and rapid clearance through the liver without toxic accumulation in the body.5.3. Organ Reconstruction
Intraurethral injection of ICG combined with NIR light visualization offers a real-time imaging method for visualizing the ureter [100][51]. This technique involves inserting the tip of a 6-F ureteral catheter into the ureteral orifice. Prior to undergoing robotic-assisted laparoscopic sacrovaginal fixation, a solution containing 25 milligrams of ICG dissolved in 10 mL of sterile water is injected through the open catheter. ICG binds reversibly to proteins on the ureteral cortex, leading to the staining of the ureteral lining. When excited by an NIR laser, the ICG molecules emit a green fluorescence, enabling the identification and localization of the ureter. One of the main advantages of this technique is that it only requires the insertion of the ureteral catheter’s tip [101][52].6. Conclusions
ICG has the benefits of a good safety profile and near-infrared absorption, but it also has certain drawbacks, such as instability in aqueous solution, quick clearance in plasma, and limited uptake in living things. However, nanotechnology has become a potentially effective response to these problems. ICGs can be enclosed in nanomaterials, which has a number of benefits. They can extend ICG’s stay in circulation in the body and shield it from degradation and elimination. This is made possible by the improved EPR effect, which enables passive accumulation of nanomaterials at tumor or other disease locations. ICG’s chemical and photothermal stability is increased by nanomaterials, which also enhances the performance of ICG. Nanoplatforms that include ICG have a lot of promise for the clinical treatment of tumors and bacterial infectious illnesses.References
- De Paiva, A.D.C.M.; da Costa Ferreira, M.; da Fonseca, A.D.S. Photodynamic therapy for the treatment of bacterial keratitis. Photodiagn. Photodyn. Ther. 2022, 37, 102717.
- Li, X.; Huang, W.; Zheng, X.; Chang, S.; Liu, C.; Cheng, Q.; Zhu, S. Synergistic in Vitro Effects of Indocyanine Green and Ethylenediamine Tetraacetate-Mediated Antimicrobial Photodynamic Therapy Combined with Antibiotics for Resistant Bacterial Biofilms in Diabetic Foot Infection. Photodiagn. Photodyn. Ther. 2019, 25, 300–308.
- Guo, W.; Ren, Y.; Chen, Z.; Shen, G.; Lu, Y.; Zhou, H.; Li, Z.; Li, Z.; Lu, X.; Li, G.; et al. Targeted Magnetic Resonance Imag-ing/Near-Infrared Dual-Modal Imaging and Ferroptosis/Starvation Therapy of Gastric Cancer with Peritoneal Me-tastasis. Adv. Funct. Mater. 2023, 33, 22139.
- Saxena, V.; Sadoqi, M.; Shao, J. Degradation Kinetics of Indocyanine Green in Aqueous Solution. J. Pharm. Sci. 2003, 92, 2090–2097.
- Higbee-Dempsey, E.; Amirshaghaghi, A.; Case, M.J.; Miller, J.; Busch, T.M.; Tsourkas, A. Indocyanine Green–Coated Gold Nanoclusters for Photoacoustic Imaging and Photothermal Therapy. Adv. Therap. 2019, 2, 1900088.
- Toriumi, N.; Asano, N.; Ikeno, T.; Muranaka, A.; Hanaoka, K.; Urano, Y.; Uchiyama, M. Design of Photostable, Activatable Near-Infrared Photoacoustic Probes Using Tautomeric Benziphthalocyanine as a Platform. Angew. Chem. Int. Ed. 2019, 58, 7788–7791.
- Song, W.; Tang, Z.; Zhang, D.; Burton, N.; Driessen, W.; Chen, X. Comprehensive Studies of Pharmacokinetics and Biodistribution of Indocyanine Green and Liposomal Indocyanine Green by Multispectral Optoacoustic Tomography. RSC Adv. 2015, 5, 3807–3813.
- Wang, H.; Li, X.; Tse, B.W.-C.; Yang, H.; Thorling, C.A.; Liu, Y.; Touraud, M.; Chouane, J.B.; Liu, X.; Roberts, M.S.; et al. Indocyanine Green-Incorporating Nanoparticles for Cancer Theranostics. Theranostics 2018, 8, 1227–1242.
- Landsman, M.L.J.; Kwant, G.; Mordon Mook, J.K.; Zijlstra, G.W. Light-absorbing properties, stability, and spectral stabilization of indocyanine green. J. Appl. Physiol. 1976, 40, 575–583.
- Cheung, C.C.L.; Ma, G.; Karatasos, K.; Seitsonen, J.; Ruokolainen, J.; Koffi, C.-R.; Hassan, H.A.F.M.; Al-Jamal, W.T. Liposome-Templated Indocyanine Green J- Aggregates for In Vivo Near Infrared Imaging and Stable Photothermal Heating. Nanotheranostics 2020, 4, 91–106.
- Sun, C.-Y.; Qin, C.; Wang, X.-L.; Su, Z.-M. Metal-Organic Frameworks as Potential Drug Delivery Systems. Expert. Opin. Drug Deliv. 2013, 10, 89–101.
- Rao, C.; Liao, D.; Pan, Y.; Zhong, Y.; Zhang, W.; Ouyang, Q.; Nezamzadeh-Ejhieh, A.; Liu, J. Novel Formulations of Metal-Organic Frameworks for Controlled Drug Delivery. Expert. Opin. Drug Deliv. 2022, 19, 1183–1202.
- Kang, W.; Tian, Y.; Zhao, Y.; Yin, X.; Teng, Z. Applications of Nanocomposites Based on Zeolitic Imidazolate Framework-8 in Photodynamic and Synergistic Anti-Tumor Therapy. RSC Adv. 2022, 12, 16927–16941.
- Fang, C.; Cen, D.; Wang, Y.; Wu, Y.; Cai, X.; Li, X.; Han, G. ZnS@ZIF-8 Core-Shell Nanoparticles Incorporated with ICG and TPZ to Enable H2S-Amplified Synergistic Therapy. Theranostics 2020, 10, 7671–7682.
- Sun, X.; He, G.; Xiong, C.; Wang, C.; Lian, X.; Hu, L.; Li, Z.; Dalgarno, S.J.; Yang, Y.-W.; Tian, J. One-Pot Fabrication of Hollow Porphyrinic MOF Nanoparticles with Ultrahigh Drug Loading toward Controlled Delivery and Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2021, 13, 3679–3693.
- Han, Y.-H.; Kankala, R.K.; Wang, S.-B.; Chen, A.-Z. Leveraging Engineering of Indocyanine Green-Encapsulated Polymeric Nanocomposites for Biomedical Applications. Nanomaterials 2018, 8, 360.
- Chopra, A. Folic Acid-Indocyanine Green-Poly(d,l-Lactide-Coglycolide)-Lipid Nanoparticles. In Molecular Imaging and Contrast Agent Database (MICAD); National Center for Biotechnology Information (US): Bethesda, MD, USA, 2004.
- Hadinoto, K.; Sundaresan, A.; Cheow, W.S. Lipid-Polymer Hybrid Nanoparticles as a New Generation Therapeutic Delivery Platform: A Review. Eur. J. Pharm. Biopharm. 2013, 85 Pt A, 427–443.
- Zhao, P.; Zheng, M.; Yue, C.; Luo, Z.; Gong, P.; Gao, G.; Sheng, Z.; Zheng, C.; Cai, L. Improving Drug Accumulation and Photothermal Efficacy in Tumor Depending on Size of ICG Loaded Lipid-Polymer Nanoparticles. Biomaterials 2014, 35, 6037–6046.
- Zheng, M.; Yue, C.; Ma, Y.; Gong, P.; Zhao, P.; Zheng, C.; Sheng, Z.; Zhang, P.; Wang, Z.; Cai, L. Single-Step Assembly of DOX/ICG Loaded Lipid--Polymer Nanoparticles for Highly Effective Chemo-Photothermal Combination Therapy. ACS Nano 2013, 7, 2056–2067.
- Bahmani, B.; Lytle, C.Y.; Walker, A.M.; Gupta, S.; Vullev, V.I.; Anvari, B. Effects of Nanoencapsulation and PEGylation on Biodistribution of Indocyanine Green in Healthy Mice: Quantitative Fluorescence Imaging and Analysis of Organs. Int. J. Nanomed. 2013, 8, 1609–1620.
- Liu, Y.; Han, Y.; Chen, S.; Liu, J.; Wang, D.; Huang, Y. Liposome-Based Multifunctional Nanoplatform as Effective Therapeutics for the Treatment of Retinoblastoma. Acta Pharm. Sin. B 2022, 12, 2731–2739.
- Dai, Y.; Su, J.; Wu, K.; Ma, W.; Wang, B.; Li, M.; Sun, P.; Shen, Q.; Wang, Q.; Fan, Q. Multifunctional Thermosensitive Liposomes Based on Natural Phase-Change Material: Near-Infrared Light-Triggered Drug Release and Multimodal Imaging-Guided Cancer Combination Therapy. ACS Appl. Mater. Interfaces 2019, 11, 10540–10553.
- Gao, F.; Jiang, L.; Zhang, J.; Chang, Y.; Gao, W.; Ding, L.; Ma, G.; Ma, X.; Guo, Y. Near-Infrared Light-Responsive Nanosystem with Prolonged Circulation and Enhanced Penetration for Increased Photothermal and Photodynamic Therapy. ACS Mater. Lett. 2023, 5, 1–10.
- Zhang, L.; Qin, Y.; Zhang, Z.; Fan, F.; Huang, C.; Lu, L.; Wang, H.; Jin, X.; Zhao, H.; Kong, D.; et al. Dual PH/Reduction-Responsive Hybrid Polymeric Micelles for Targeted Chemo-Photothermal Combination Therapy. Acta Biomater. 2018, 75, 371–385.
- Mundra, V.; Peng, Y.; Rana, S.; Natarajan, A.; Mahato, R.I. Micellar Formulation of Indocyanine Green for Phototherapy of Melanoma. J. Control. Release 2015, 220, 130–140.
- Yang, L.; Hou, X.; Zhang, Y.; Wang, D.; Liu, J.; Huang, F.; Liu, J. NIR-Activated Self-Sensitized Polymeric Micelles for Enhanced Cancer Chemo-Photothermal Therapy. J. Control. Release 2021, 339, 114–129.
- Kuo, W.-S.; Chang, Y.-T.; Cho, K.-C.; Chiu, K.-C.; Lien, C.-H.; Yeh, C.-S.; Chen, S.-J. Gold Nanomaterials Conjugated with Indocyanine Green for Dual-Modality Photodynamic and Photothermal Therapy. Biomaterials 2012, 33, 3270–3278.
- Li, W.; Zhang, H.; Guo, X.; Wang, Z.; Kong, F.; Luo, L.; Li, Q.; Zhu, C.; Yang, J.; Lou, Y.; et al. Gold Nanospheres-Stabilized Indocyanine Green as a Synchronous Photodynamic-Photothermal Therapy Platform That Inhibits Tumor Growth and Metastasis. ACS Appl. Mater. Interfaces 2017, 9, 3354–3367.
- Hu, Y.; Chi, C.; Wang, L.; Liang, P.; Liu, F.; Shang, W.; Wang, W.; Zhang, F.; Li, S.; Shen, H.; et al. A Comparative Study of Clinical Intervention and Interventional Photothermal Therapy for Pancreatic Cancer. Adv. Mater. 2017, 29, 170048.
- Kang, J.; Wang, J.; Hariri, A.; Kim, D.; Han, Y.; Park, J.-H.; Zuidema, J.M.; Jokerst, J.V.; Sailor, M.J. Enhanced Performance of a Molecular Photoacoustic Imaging Agent by Encapsulation in Mesoporous Silicon Nanoparticles. Adv. Mater. 2018, 30, e1800512.
- Deng, H.; Zhou, Z.; Yang, W.; Lin, L.-S.; Wang, S.; Niu, G.; Song, J.; Chen, X. Endoplasmic Reticulum Targeting to Amplify Immunogenic Cell Death for Cancer Immunotherapy. Nano Lett. 2020, 20, 1928–1933.
- Ma, H.; Lu, Y.; Huang, Z.; Long, S.; Cao, J.; Zhang, Z.; Zhou, X.; Shi, C.; Sun, W.; Du, J.; et al. ER-Targeting Cyanine Dye as an NIR Photoinducer to Efficiently Trigger Photoimmunogenic Cancer Cell Death. J. Am. Chem. Soc. 2022, 144, 3477–3486.
- Li, W.; Yang, J.; Luo, L.; Jiang, M.; Qin, B.; Yin, H.; Zhu, C.; Yuan, X.; Zhang, J.; Luo, Z.; et al. Targeting Photodynamic and Photothermal Therapy to the Endoplasmic Reticulum Enhances Immunogenic Cancer Cell Death. Nat. Commun. 2019, 10, 3349.
- Dai, L.; Yu, Y.; Luo, Z.; Li, M.; Chen, W.; Shen, X.; Chen, F.; Sun, Q.; Zhang, Q.; Gu, H.; et al. Photosensitizer Enhanced Disassembly of Amphiphilic Micelle for ROS-Response Targeted Tumor Therapy in Vivo. Biomaterials 2016, 104, 1–17.
- Du, B.; Chong, Y.; Jiang, X.; Yu, M.; Lo, U.-G.; Dang, A.; Chen, Y.-A.; Li, S.; Hernandez, E.; Lin, J.C.; et al. Hyperfluorescence Imaging of Kidney Cancer Enabled by Renal Secretion Pathway Dependent Efflux Transport. Angew. Chem. Int. Ed. Engl. 2021, 60, 351–359.
- Naito, S.; Sakamoto, N.; Kotoh, S.; Goto, K.; Matsumoto, T.; Kumazawa, J. Expression of P-Glycoprotein and Multidrug Resistance in Renal Cell Carcinoma. Eur. Urol. 1993, 24, 156–160.
- Liu, R.; Tang, J.; Xu, Y.; Zhou, Y.; Dai, Z. Nano-Sized Indocyanine Green J-Aggregate as a One-Component Theranostic Agent. Nanotheranostics 2017, 1, 430–439.
- Zhang, R.; Li, Y.; Zhou, M.; Wang, C.; Feng, P.; Miao, W.; Huang, H. Photodynamic Chitosan Nano-Assembly as a Potent Alternative Candidate for Combating Antibiotic-Resistant Bacteria. ACS Appl. Mater. Interfaces 2019, 11, 26711–26721.
- Gnanasekar, S.; Kasi, G.; He, X.; Zhang, K.; Xu, L.; Kang, E.-T. Recent Advances in Engineered Polymeric Materials for Efficient Photodynamic Inactivation of Bacterial Pathogens. Bioact. Mater. 2023, 21, 157–174.
- Li, Z.; Lu, S.; Liu, W.; Dai, T.; Ke, J.; Li, X.; Li, R.; Zhang, Y.; Chen, Z.; Chen, X. Synergistic Lysozyme-Photodynamic Therapy Against Resistant Bacteria Based on an Intelligent Upconversion Nanoplatform. Angew. Chem. Int. Ed. Engl. 2021, 60, 19201–19206.
- Casu, C.; Orrù, G.; Scano, A. Curcumin/H2O2 Photodynamically Activated: An Antimicrobial Time-Response Assessment against an MDR Strain of Candida Albicans. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 8841–8851.
- Detty, M.R.; Gibson, S.L.; Wagner, S.J. Current Clinical and Preclinical Photosensitizers for Use in Photodynamic Therapy. J. Med. Chem. 2004, 47, 3897–3915.
- Schmitt, J.; Heitz, V.; Sour, A.; Bolze, F.; Ftouni, H.; Nicoud, J.-F.; Flamigni, L.; Ventura, B. Diketopyrrolopyrrole-Porphyrin Conjugates with High Two-Photon Absorption and Singlet Oxygen Generation for Two-Photon Photodynamic Therapy. Angew. Chem. Int. Ed. Engl. 2015, 54, 169–173.
- Sahu, A.; Choi, W.I.; Lee, J.H.; Tae, G. Graphene Oxide Mediated Delivery of Methylene Blue for Combined Photodynamic and Photothermal Therapy. Biomaterials 2013, 34, 6239–6248.
- Yue, L.; Zheng, M.; Wang, M.; Khan, I.M.; Ding, X.; Zhang, Y.; Wang, Z. Water-Soluble Chlorin E6-Hydroxypropyl Chitosan as a High-Efficiency Photoantimicrobial Agent against Staphylococcus Aureus. Int. J. Biol. Macromol. 2022, 208, 669–677.
- Sun, S.; Chen, J.; Jiang, K.; Tang, Z.; Wang, Y.; Li, Z.; Liu, C.; Wu, A.; Lin, H. Ce6-Modified Carbon Dots for Multimodal-Imaging-Guided and Single-NIR-Laser-Triggered Photothermal/Photodynamic Synergistic Cancer Therapy by Reduced Irradiation Power. ACS Appl. Mater. Interfaces 2019, 11, 5791–5803.
- Xiao, J.; Hai, L.; Yang, K.; Luo, Y.; Wang, Z.; Li, J.; Ou, C.; Wang, L.; Deng, L.; He, D. Self-Enhanced ROS Generation by Responsive Co-Delivery of H2O2 and O2 Based on a Versatile Composite Biomaterial for Hypoxia-Irrelevant Multimodal Antibiofilm Therapy. Chem. Eng. J. 2023, 465, 142958.
- Shaitelman, S.F.; Cromwell, K.D.; Rasmussen, J.C.; Stout, N.L.; Armer, J.M.; Lasinski, B.B.; Cormier, J.N. Recent Progress in the Treatment and Prevention of Cancer-Related Lymphedema: Lymphedema Treatment and Prevention. CA Cancer J. Clin. 2015, 65, 55–81.
- Guyer, D.R.; Yannuzzi, L.A.; Slakter, J.S.; Sorenson, J.A.; Hope-Ross, M.; Orlock, D.R. Digital Indocyanine-Green Videoangiography of Occult Choroidal Neovascularization. Ophthalmology 1994, 101, 1727–1735; discussion 1735–1737.
- Siddighi, S.; Yune, J.J.; Hardesty, J. Indocyanine Green for Intraoperative Localization of Ureter. Am. J. Obstet. Gynecol. 2014, 211, 436.e1–436.e2.
- Lee, Z.; Moore, B.; Giusto, L.; Eun, D.D. Use of Indocyanine Green during Robot-Assisted Ureteral Reconstructions. Eur. Urol. 2015, 67, 291–298.