Please note this is a comparison between Version 2 by Dequan Liu and Version 1 by Dequan Liu.
肿瘤疫苗代表了肿瘤学的突破,利用免疫治疗原理刺激人体免疫系统对抗癌症,与手术、放疗和化疗等传统治疗相比,提供了一种更有针对性的方法,副作用更少。Oncology vaccines represent a breakthrough in oncology, using the principles of immunotherapy to stimulate the body's immune system to fight cancer, providing a more targeted approach with fewer side effects than traditional treatments such as surgery, radiotherapy, and chemotherapy.
- TME
- cancer immunology
- oncology vaccines
- combination therapies
1. 简介Introduction
根据According to a comprehensive epidemiological survey conducted in 2020年进行的一项全面的流行病学调查,恶性肿瘤作为全球发病率和死亡率的主要诱因一直占据首位,令人震惊的是,该年内新发癌症发病率约为19万例,死亡近3万例, malignancy has been the leading cause of morbidity and mortality worldwide, with a shocking incidence of approximately 19,3 new cancers and nearly 10,1 deaths during the year [10<>]。卫生和医疗系统面临着巨大的挑战。然而,癌症治疗的新希望以肿瘤疫苗的形式出现。肿瘤疫苗代表了肿瘤学的突破,它利用免疫治疗原理刺激人体免疫系统对抗癌症,与手术、放疗和化疗等常规治疗相比,提供了一种更具针对性的方法,副作用更少. Health and health systems face enormous challenges. However, new hope for cancer treatment is in the form of oncology vaccines. Oncology vaccines represent an oncology breakthrough that uses the principles of immunotherapy to stimulate the body's immune system to fight cancer, providing a more targeted approach with fewer side effects than conventional treatments such as surgery, radiotherapy, and chemotherapy [1<>]。.
癌症疫苗的演变已经展开了几十年,每个时代都以关键的进步为标志(图The evolution of cancer vaccines has unfolded over decades, with each era marked by key advances (Figure 1)。这段旅程始于19世纪末的威廉·科利(). The journey began with William Coley),他在注意到感染经常导致癌症患者的肿瘤缩小后,产生了科利毒素 in the late 19th century, who developed Colley toxin after noticing that infections often cause tumors in cancer patients to shrink [3]。这种方法利用免疫系统来对抗癌症——刘易斯·托马斯和弗兰克·麦克法兰·伯内特在. This approach harnesses the immune system to fight cancer – a concept further expanded by Lewis Thomas and Frank McFarlane Burnett in the mid-20世纪中叶进一步扩展了这一概念,他们引入了癌症免疫编辑和肿瘤免疫的思想th century, when they introduced the ideas of cancer immune editing and tumor immunity [4]。临床批准的癌症疫苗的曙光于. The dawn of a clinically approved cancer vaccine came in 2010年到来,FDA批准了 with the FDA approval of sipuleucel-T,这是第一种针对前列腺癌的治疗性癌症疫苗, the first therapeutic cancer vaccine against prostate cancer [5]。随后的时代一直持续到今天,其特点是现代癌症疫苗和检查点抑制剂的开发,例如. Subsequent eras continue to this day and are characterized by the development of modern cancer vaccines and checkpoint inhibitors, such as the combination therapy approved in 2021年批准用于治疗淋巴瘤的联合疗法 for the treatment of lymphoma [6]。目前,癌症疫苗的开发强调个性化,正在研究多种策略,包括新抗原疫苗、. Currently, cancer vaccine development emphasizes personalization, and multiple strategies are being studied, including neoantigen vaccines, DNA/RNA疫苗和病毒载体,旨在针对个体患者的肿瘤量身定制治疗方法。 vaccines, and viral vectors, aimed at tailoring treatments to individual patients' tumors.
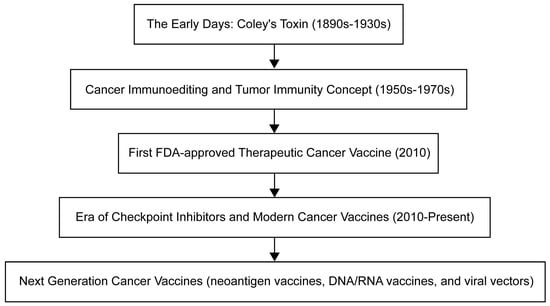
图Figure 1.肿瘤疫苗开发时间表。Timeline for oncology vaccine development.
深入研究机制复杂性,肿瘤在其中存在和进化的肿瘤微环境(In-depth study of the mechanistic complexity in which the tumor microenvironment (TME)在肿瘤进展中起着关键作用。因此,TME已成为抗癌治疗药物进化领域的科学研究中心) in which tumors exist and evolve plays a key role in tumor progression. As a result, TME has become a scientific research center in the field of anti-cancer therapeutic drug evolution [7]。作为当代医学创新的证明,肿瘤疫苗经过精心设计,以利用宿主免疫系统的先天和适应性来靶向异常细胞。这些开创性的配方战略性地调节. As proof of contemporary medical innovation, tumor vaccines have been carefully designed to harness the innate and adaptive nature of the host immune system to target abnormal cells. These groundbreaking formulations strategically regulate TME,从而明智地最大限度地减少对健康组织造成的附带破坏, thereby wisely minimizing collateral damage to healthy tissue [8]。.
随着各种肿瘤疫苗的开发,癌症免疫治疗领域正在经历重大进展,每种疫苗都有其独特的优势和挑战。肽疫苗虽然可有效引发免疫应答,但通常需要使用佐剂The field of cancer immunotherapy is undergoing significant advances with the development of various oncology vaccines, each with its own unique advantages and challenges. Although peptide vaccines are effective in eliciting an immune response, adjuvants are often required [9]。基于. DNA/RNA的疫苗允许抗原的内源性产生,但在递送和表达方面面临障碍-based vaccines allow endogenous antigen generation but face barriers to delivery and expression [10,11]。基于病毒载体的疫苗可以刺激强大的免疫应答,但受到安全问题和预先存在的免疫力的限制. Viral vector-based vaccines can stimulate a robust immune response, but are limited by safety concerns and pre-existing immunity [12]。虽然基于树突状细胞的疫苗可以诱导强大的反应,但它们需要复杂的生产过程和不同的患者反应. While dendritic cell-based vaccines can induce robust responses, they require complex production processes and different patient responses [13]。基于全细胞的疫苗在利用整个肿瘤细胞触发免疫反应的同时,努力解决生产和标准化问题. Whole-cell-based vaccines address production and standardization issues while harnessing whole tumor cells to trigger an immune response [14]。深入了解这些疫苗及其局限性对于其在癌症免疫治疗中的有效部署至关重要. A deep understanding of these vaccines and their limitations is critical for their effective deployment in cancer immunotherapy [15]。目前的研究认识到肿瘤疫苗的独立疗效有限,正在通过与其他治疗方法(如免疫检查点抑制剂、化疗、放疗、靶向治疗和溶瘤病毒疗法)联合使用来寻求增强治疗效果. Current studies recognize the limited efficacy of oncology vaccines and are seeking to enhance therapeutic efficacy by combining them with other therapies such as immune checkpoint inhibitors, chemotherapy, radiotherapy, targeted therapy, and oncolytic viral therapy [16,17,18,19]。同时,个性化癌症疫苗的创新领域正在获得牵引力,为个体特异性肿瘤提供量身定制的免疫反应. At the same time, the innovative field of personalized cancer vaccines is gaining traction, providing tailored immune responses to individual-specific tumors [8]。尽管存在一些挑战,包括新抗原鉴定的复杂性和资源密集型生产,但技术进步有望为个性化癌症疫苗带来更快、更实惠的未来. Despite some challenges, including the complexity of neoantigen identification and resource-intensive production, technological advances promise a faster and more affordable future for personalized cancer vaccines [20,21,22,23]。.
2. 肿瘤微环境Tumor microenvironment
肿瘤微环境(The tumor microenvironment (TME)对癌症的发作和进展至关重要,是科学家头疼的问题。它不仅是动态的,而且非常复杂,塑造了肿瘤的异质性。TME是多种细胞和非细胞成分(如癌细胞、基质细胞、免疫细胞、ECM)和信号分子(如生长因子和细胞因子)的混合物), which is critical to cancer onset and progression, is a headache for scientists. It is not only dynamic, but also very complex, shaping the heterogeneity of tumors. TME is a mixture of cellular and noncellular components (eg, cancer cells, stromal cells, immune cells, ECMs) and signaling molecules (eg, growth factors and cytokines) [24,25]。. ECM是蛋白质,糖蛋白和蛋白聚糖的纠结网络,通过生化和生物力学信号提供结构并塑造细胞行为。当癌症改变ECM的组成时,它可以促进血管生成、免疫逃避和治疗抵抗 is a tangled network of proteins, glycoproteins, and proteoglycans that provide structure and shape cell behavior through biochemical and biomechanical signals. When cancer alters the composition of the ECM, it can promote angiogenesis, immune evasion, and treatment resistance [26,27]。例如,如赖氨氧化酶(. For example, as seen by overexpression of lysine oxidase (LOX)过表达所见,ECM硬度增加,通过FAK激活等机制促进细胞增殖和存活,同时也增强肿瘤侵袭性), ECM hardness increases, promotes cell proliferation and survival through mechanisms such as FAK activation, and also enhances tumor aggressiveness [28]。同样,透明质酸的积累通常与预后不良有关,可以促进肿瘤生长并促进免疫逃逸. Similarly, accumulation of hyaluronic acid is often associated with a poor prognosis, which can promote tumor growth and promote immune escape [29]。基质金属蛋白酶(. Alterations in matrix metalloproteinase (MMP)表达的改变可以重塑ECM,通过释放生长因子有利于肿瘤侵袭) expression can remodel the ECM, favoring tumor invasion by releasing growth factors [30,31]。此外,当过度表达时,弹性蛋白、层粘连蛋白、. In addition, specific ECM proteins such as elastin, laminin, tenascin-C和骨膜蛋白等特异性ECM蛋白支持肿瘤细胞迁移和存活, and periosteum protein support tumor cell migration and survival when overexpressed [32]。值得注意的是,胶原取向的变化导致纤维排列,为增强肿瘤细胞迁移提供了途径,这种排列通常表明转移的风险更高. Notably, changes in collagen orientation lead to a fibrous arrangement that provides a pathway for enhanced tumor cell migration, which often indicates a higher risk of metastasis [33]. ]。ECM还参与各种生长因子的分泌,如转化生长因子-β(TGF-β),白细胞介素-1β(IL-1β),IL-6,肿瘤坏死因子-α(TNF-α)和血管内皮生长因子(VEGF),这些因子由TME的各个细胞分泌,可以启动肿瘤细胞的生长,存活,迁移,血管生成和上皮-间充质转化(EMT)。这是通过调节其特异性受体和刺激信号通路来实现的 ECM is also involved in the secretion of various growth factors such as transforming growth factor-β (TGF-β), interleukin-1β (IL-1β), IL-6, tumor necrosis factor-α (TNF-α), and vascular endothelial growth factor (VEGF), which are secreted by the individual cells of TME and can initiate tumor cell growth, survival, migration, angiogenesis, and epithelial-mesenchymal transformation (EMT). This is achieved by modulating its specific receptors and stimulus signaling pathways [34](图 (Figure 2)。). This intricate interaction between ECM和肿瘤细胞之间这种错综复杂的相互作用不仅推动了癌症的进展,而且由于药物渗透屏障和细胞存活途径的激活等因素,也给治疗带来了挑战。 and tumor cells not only drives cancer progression, but also presents treatment challenges due to factors such as drug permeability barriers and activation of cell survival pathways.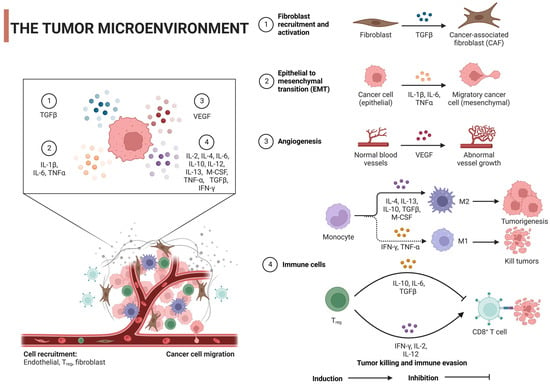
图Figure 2.肿瘤微环境对肿瘤细胞的调控机制:Regulatory mechanism of tumor microenvironment on tumor cells: TGF-β,转化生长因子, transforming growth factor-β;IL,白细胞介素;VEGF,血管内皮生长因子;TNF-α,肿瘤坏死因子α;IFN-γ,干扰素γ;M-脑脊液,巨噬细胞集落刺激因子;Tregs,调节性T细胞。用 IL, interleukin; VEGF, vascular endothelial growth factor; TNF-α, tumor necrosis factor α; IFN-γ, interferon γ; M-cerebrospinal fluid, macrophage colony-stimulating factor; Tregs, regulatory T cells. Created with BioRender.com 创建。.
3. 肿瘤疫苗Oncology vaccines
3.1. Cancer 癌症免疫学immunology
适应性免疫或获得性免疫是对病原体和异常细胞的高度特异性和持久的防御,主要由Adaptive immunity or acquired immunity is a highly specific and persistent defense against pathogens and abnormal cells, administered primarily by T和B淋巴细胞管理 and B lymphocytes [66]。这种类型的免疫以其免疫记忆而闻名,可以持久地抵御以前遇到过的东西,如病原体或抗原. This type of immunity is known for its immune memory, which provides long-lasting resistance to previously encountered things, such as pathogens or antigens [67]。研究适应性免疫的来龙去脉对于制造疫苗和靶向免疫疗法至关重要。这些突破为预防和治疗从感染和自身免疫性疾病到癌症等一系列疾病提供了巨大的潜力. Studying the ins and outs of adaptive immunity is critical to making vaccines and targeted immunotherapies. These breakthroughs offer great potential for the prevention and treatment of a range of diseases, from infections and autoimmune diseases to cancer [68,69,70]。. 在癌症的早期阶段,免疫系统参与一个称为免疫监视的过程,在那里它追捕并消灭异常细胞,阻止肿瘤形成In the early stages of cancer, the immune system is involved in a process called immune surveillance, where it hunts down and destroys abnormal cells, preventing tumor formation [71,72]。但狡猾的癌细胞使用不同的技巧来躲避免疫系统,帮助肿瘤生长和进展. But cunning cancer cells use different techniques to evade the immune system and help tumors grow and progression [73]。通过研究这些免疫逃避策略,科学家们提出了新的免疫疗法,如免疫检查点抑制剂(. By studying these immune evasion strategies, scientists have proposed new immunotherapies such as immune checkpoint inhibitors (ICIs)和过继细胞转移。这些创新疗法旨在提高身体对抗肿瘤的能力,并克服癌症躲避免疫系统的技能) and adoptive cell transfer. These innovative therapies are designed to improve the body's ability to fight tumors and overcome cancer's ability to evade the immune system [74]。. 免疫疗法,如Immunotherapies, such as ICI和嵌合抗原受体(CAR)T细胞疗法,通过使用免疫系统对抗癌细胞,完全改变了癌症的治疗 and chimeric antigen receptor (CAR) T-cell therapy, have completely changed the treatment of cancer by using the immune system to fight cancer cells [75]。但即使有这些惊人的进展,一些患者对免疫疗法的反应不佳,甚至可能对免疫疗法产生耐药性,这意味着需要更多地了解免疫系统和癌症如何相互作用才能学习. But even with these amazing advances, some patients respond poorly to immunotherapy and may even develop resistance to immunotherapy, which means that more needs to be learned about how the immune system and cancer interact [76]。获得这些知识将有助于创建新的免疫治疗策略,并找到预测性生物标志物,使患者结局更好. Gaining this knowledge will help create new immunotherapy strategies and identify predictive biomarkers that lead to better patient outcomes [77,78]。.3.2. Mechanism 作用机制of Action
癌症疫苗的基本功能在于它们动员先天性和适应性免疫应答的能力,以识别、对抗和根除肿瘤细胞The fundamental function of cancer vaccines lies in their ability to mobilize innate and adaptive immune responses to identify, fight, and eradicate tumor cells [20]。以下论述将全面分析其错综复杂的作用机制(图. The following discussion will provide a comprehensive analysis of its intricate mechanisms of action (Figure 3)。).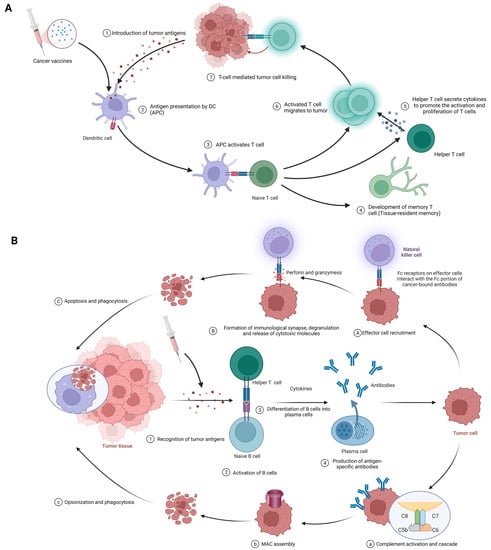
Figure 3. (A) Cellular immune response against cancer (step 1 to step 7); APCs, antigen-presenting cells; APC, antigen-presenting cell. (B) Humoral immunity response against cancer (step 1 to step 4). A–C; antibody-dependent cell-mediated cytotoxicity; a–c: complement-dependent cytotoxicity; MAC, membrane attack complex. Created with BioRender.com.
3.2.1. Cellular Immunity
The procedure of eliciting a cellular immune response against cancer, exemplified by the use of cancer vaccines, is complex and sequential. It commences with the delivery of tumor antigens and concludes with the activation of humoral immunity. The following is a detailed breakdown of each phase in this process (Figure 3A). Introduction of tumor antigens: With cancer vaccines, referring to tumor antigens being introduced to antigen-presenting cells (APCs) like dendritic cells. Options include whole tumor cells, peptides, proteins, DNA, mRNA, or even dendritic cells loaded with tumor antigens or packing tumor-derived genetic material. Tumor vaccines play a role in this step. This crucial first step gets the ball rolling in the immune response against cancer [18,79]. Antigen processing and presentation: APCs capture, process, and present tumor-derived peptides on their surface. Dendritic cells efficiently cross-present exogenous antigens to both MHC class I and II molecules, activating both CD8+ cytotoxic T lymphocytes (CTLs) and CD4+ helper T cells [18,80]. Activation of T cells: Presentation of tumor antigens by APCs activates and expands antigen-specific T cells. T-cell activation requires antigen recognition via the T-cell receptor (TCR) and costimulatory signals provided by the interaction between costimulatory molecules on APCs and their receptors on T cells [81,82]. CTLs and helper T cells in action: CTLs directly kill tumor cells by recognizing and binding to MHC class I molecules presenting tumor antigens, while helper T cells produce cytokines that support CTLs’ activation, proliferation, and differentiation. CD4+ helper T cells also provide help to B cells, facilitating antibody production and enhancing the function of APCs and CTLs [2,42]. 刺激体液免疫:癌症疫苗诱导Stimulation of humoral immunity: Cancer vaccines induce B细胞产生抗原特异性抗体。这些抗体靶向并消除肿瘤细胞 cells to produce antigen-specific antibodies. These antibodies target and eliminate tumor cells [15,83]。.3.2.2. 体液免疫Humoral immunity
该程序从识别肿瘤抗原开始,最终实现由抗体介导的效应功能。以下是此过程中每个阶段的详细信息。(图The procedure begins with the identification of tumor antigens and ultimately achieves effector function mediated by antibodies. Here are the details of each stage in this process. (Figure 3B)。B). 肿瘤抗原的识别:与细胞免疫一样,肿瘤细胞具有免疫系统可以识别的肿瘤相关抗原(Recognition of tumor antigens: Like cellular immunity, tumor cells have tumor-associated antigens (TAAs)和肿瘤特异性抗原(TSA)s) and tumor-specific antigens (TSAs) that the immune system can recognize [84]。. Activation of B细胞的活化:B细胞通过其B细胞受体(BCR)检测和识别肿瘤抗原。加入一些来自辅助性T细胞的共刺激信号,B细胞被激活以增殖和分化 cells: B cells detect and recognize tumor antigens through their B cell receptor (BCR). With the addition of some co-stimulatory signals from helper T cells, B cells are activated to proliferate and differentiate [72]。. B细胞分化为浆细胞:激活后, cells differentiate into plasma cells: Upon activation, B细胞转变为浆细胞,专门用于产生大量针对已识别肿瘤抗原的特异性抗体 cells transform into plasma cells, which are specifically designed to produce large amounts of specific antibodies against recognized tumor antigens [84]。. 抗原特异性抗体的产生:浆细胞产生抗原特异性抗体,这些抗体通过血流并附着在癌细胞上的肿瘤抗原上Antigen-specific antibody production: Plasma cells produce antigen-specific antibodies that pass through the bloodstream and attach to tumor antigens on cancer cells [85]。. 抗体的效应功能:一旦结合,抗体就会对肿瘤细胞采取各种策略,例如:Effector function of antibodies: Once bound, antibodies employ various strategies against tumor cells, such as:-
抗体依赖性细胞介导的细胞毒性(Antibody-dependent cell-mediated cytotoxicity (ADCC)(图3 A(1) (Figure 3A(1-3)): In the first phase, effector cell recruitment: Fc receptor-3)):在第一阶段,效应细胞募集:携带Fcarrying immune cells, suc受体的免疫细胞,如自然杀伤(h as natural killer (NK)细胞、巨噬细胞和中性粒细胞,通过其Fc受体与与恶性细胞结合的抗体的Fc片段之间的相互作用被吸引到肿瘤部位) cells, macrophages, and neutrophils, are attracted to the tumor site through interactions between their Fc receptors and Fc fragments of antibodies bound to malignant cells [86,87].在下一阶段,免疫突触的形成、脱颗粒和细胞毒性分子的释放:当效应细胞上的 In the next stage, the formation of immune synapses, degranulation, and the release of cytotoxic molecules: Immune synapses are formed when the Fc受体与癌症结合抗体的Fc部分相互作用时,就会形成免疫突触,从而能够靶向释放细胞毒性分子 receptor on effector cells interacts with the Fc portion of the cancer-binding antibody, enabling the targeted release of cytotoxic molecules [88]。效应细胞脱颗粒随之而来,最终通过穿孔素和颗粒酶消除癌细胞. Effector cell degranulation ensues, eventually eliminating cancer cells by perforin and granzyme [89]。最后,吞噬作用:细胞凋亡后,巨噬细胞吞噬癌性碎片,促进清除并防止有害细胞成分的传播. Finally, phagocytosis: After apoptosis, macrophages engulf cancerous debris, facilitating clearance and preventing the spread of harmful cellular components [90]。.
-
补体依赖性细胞毒性(CDC)(图Complement-dependent cytotoxicity (CDC) (Figure 3B(B(1-4)):在第一阶段,补体活化和级联反应:结合抗体的)): In the first phase, complement activation and cascade reaction: the Fc部分与C1q(补体成分1,q亚组分)结合,激发经典补体途径和C3转化酶复合物的形成 moiety of the binding antibody binds to C1q (complement component 1,q subcomponent), stimulating the classical complement pathway and the formation of C3 convertase complexes [91,92]。在下一阶段,膜攻击复合物(. In the next stage, membrane attack complex (MAC)组装:补体蛋白切割和活化产生MAC,包括C5b,C6,C7,C8和C9) assembly: complement protein cleavage and activation produces MAC, including C5b, C6, C7, C8, and C9 [93]。之后,细胞裂解:. After that, cell lysis: MAC穿孔癌细胞膜,导致不受控制的离子和水运动,细胞裂解和死亡 perforates the cancer cell membrane, resulting in uncontrolled ion and water movement, cell lysis, and death [93]。最后,调理和吞噬作用:当补体激活促进调理作用时,癌细胞被吞噬细胞靶向破坏. Finally, opsonics and phagocytosis: when complement activation promotes opsonship, cancer cells are targeted for destruction by phagocytes [92]。.
-
生长因子的中和和信号通路的抑制:抗体阻碍肿瘤细胞增殖促进生长因子,并阻碍对癌细胞存活和侵袭至关重要的信号通路Neutralization of growth factors and inhibition of signaling pathways: Antibodies impede tumor cell proliferation, promote growth factors, and block signaling pathways that are critical for cancer cell survival and invasion [15]。.
第Chapter 4章 癌症疫苗的种类和特点: Types and characteristics of cancer vaccines
4.1. 肽疫苗Peptide vaccines
肽疫苗代表了一种潜在的癌症免疫治疗方法,该方法采用源自肿瘤特异性或肿瘤相关抗原(Peptide vaccines represent a potential cancer immunotherapy approach that uses short amino acid sequences derived from tumor-specific or tumor-associated antigens (TAAs)的短氨基酸序列来引起针对恶性细胞的靶向免疫反应s) to elicit a targeted immune response against malignant cells [94]。. TAAs(包括分化抗原、过表达抗原、癌/睾丸抗原和突变抗原)是多种免疫治疗技术(如癌症疫苗、过继性T细胞疗法和免疫检查点抑制剂)的可行靶点, including differentiated antigens, overexpressed antigens, cancer/testis antigens, and mutant antigens, are viable targets for a variety of immunotherapy techniques, such as cancer vaccines, adoptive T cell therapies, and immune checkpoint inhibitors [95,96]。与全蛋白或减毒活疫苗相比,肽疫苗具有多种优点,包括易于合成、特异性和良好的安全性,因为肽疫苗引发自身免疫应答的可能性较低. Peptide vaccines offer several advantages over whole protein or live attenuated vaccines, including ease of synthesis, specificity, and good safety because peptide vaccines are less likely to elicit an autoimmune response [97,98]。然而,它们遇到了免疫原性欠佳、体内降解迅速、. However, they have encountered limitations such as poor immunogenicity, rapid degradation in vivo, weak CD4+T细胞反应弱以及与免疫逃避和肿瘤诱导的免疫抑制相关的挑战等局限性 T cell response, and challenges associated with immune evasion and tumor-induced immunosuppression [97]。为了克服这些局限性,策略包括加入佐剂、优化肽序列、利用载体增强稳定性和免疫原性,以及将肽疫苗与其他免疫疗法联合使用. To overcome these limitations, strategies include the addition of adjuvants, optimization of peptide sequences, enhancement of stability and immunogenicity with vectors, and combination of peptide vaccines with other immunotherapies [94]。. 目前,相对成熟的多肽疫苗包括Currently, relatively mature peptide vaccines include Nelipepimut-S( (NeuVax)、), CIMAvax-EGF和基于MUC1的多肽疫苗。 and MUC1-based peptide vaccines. Nelipepimut-S,也称为, also known as NeuVax,是一种靶向表达, is a peptide vaccine targeting HER2/neu的癌细胞的肽疫苗,主要针对不适合标准HER2治疗的早期HER1 2+和2+乳腺癌患者-expressing cancer cells primarily in patients with early-stage HER2 1+ and 2+ breast cancer who are not candidates for standard HER2 therapy [99]。它将. It binds the E75 peptide of HER75/neu的E2肽与2/neu to GM-CSF结合,作为增强免疫应答的佐剂 as an adjuvant to enhance the immune response [100]。虽然其范围可能涵盖其他. Although its scope may cover other HER2/neu癌症,如卵巢癌和胃癌,但其临床开发遇到了障碍 cancers, such as ovarian and gastric cancers, clinical development has encountered obstacles [101]。最近的. Recent phase III.期试验结果表明, trial results suggest that Nelipepimut-S在乳腺癌患者中仍表现出良好的疗效和耐受性 continues to exhibit good efficacy and tolerability in breast cancer patients [102]。同样,靶向非小细胞肺癌(N. SCLC)表皮生长因子的Cimilarly, CIMAvax-EGF将重组EGF与蛋白质载体合并。它已显示出延长晚期肺癌患者生命的希望,已完成III.期试验, which targets non-small cell lung cancer (NSCLC) epidermal growth factor, merges recombinant EGF with protein carriers. It has shown promise for prolonging the lives of patients with advanced lung cancer and has completed phase III trials [103,104]。此外,基于. In addition, MUC1的肽疫苗侧重于乳腺癌和胰腺癌等癌症中异常表达的糖蛋白-based peptide vaccines focus on abnormally expressed glycoproteins in cancers such as breast and pancreatic cancer [105]。尽管一些药物已进入. Although some agents have entered phase I.期和II.期试验,且安全性和免疫指标良好,但引发强有力的临床反应仍然很复杂,因此需要探索联合治疗 and II trials with good safety and immunologic indicators, eliciting a robust clinical response remains complex, and combination therapies need to be explored [106,107]。总的来说,这些疫苗代表了尖端的癌症治疗方法,每种方法都有其独特的靶点和开发阶段。. Collectively, these vaccines represent cutting-edge cancer treatments, each with its own unique targets and stages of development.4.2. 基于 DNA/RNA 的疫苗-based vaccines
基于DNA/RNA的肿瘤疫苗的工作原理是将编码肿瘤抗原的遗传物质输送到宿主细胞。这种方法利用病毒载体、脂质纳米颗粒或裸核酸等各种载体,刺激免疫系统识别和破坏癌细胞,从而产生适应性免疫反应-based tumor vaccines work by delivering genetic material encoding tumor antigens to host cells. This approach utilizes a variety of vectors, such as viral vectors, lipid nanoparticles, or naked nucleic acids, to stimulate the immune system to recognize and destroy cancer cells, resulting in an adaptive immune response [108]。与传统方法相比,这些疫苗具有多种优势,包括安全性、易于制造、强大的免疫反应诱导和对修饰的适应性。它们还提供了个性化的可能性,以满足每个患者独特的肿瘤特征. These vaccines offer several advantages over traditional methods, including safety, ease of manufacture, robust immune response induction, and adaptability to modifications. They also offer the possibility of individualization to meet each patient's unique tumor profile [109,110]。尽管它们很有希望,但诸如将. Although they are promising, challenges such as efficient delivery and uptake of DNA/RNA有效递送和摄取到细胞中、自身免疫反应的风险以及个性化成本和时间的限制等挑战构成了重大障碍 into cells, the risks of autoimmune responses, and the limitations of personalized costs and time pose significant barriers [111,112,113]。. 然而,最近的进展令人鼓舞,包括临床试验中的几种疫苗和技术进步提高了疫苗递送的有效性However, recent advances are encouraging, including several vaccines and technological advances in clinical trials that have improved the effectiveness of vaccine delivery [114]。该领域期待利用基因组测序、生物信息学和纳米技术的进步来克服当前的局限性,渴望将这些强效疫苗与其他免疫治疗策略相结合,以全面根除癌症. The field expects to use advances in genome sequencing, bioinformatics, and nanotechnology to overcome current limitations, eager to combine these potent vaccines with other immunotherapy strategies to achieve total cancer eradication [115,116]。其中,脂质纳米颗粒(. Among them, technologies such as lipid nanoparticles (LNP)等技术在s) are pioneering in mRNA COVID-19疫苗中具有开创性意义,正在被应用于癌症疫苗开发,以促进肿瘤特异性抗原的递送 vaccines and are being applied to cancer vaccine development to facilitate the delivery of tumor-specific antigens [117,118]。电穿孔和病毒载体(如腺病毒)可增强. Electroporation and viral vectors (eg, adenovirus) enhance DNA/RNA的摄取,而非病毒纳米载体和微针贴片旨在增强这种递送,而不会诱导强烈的抗载体反应 uptake, while nonviral nanovectors and microneedle patches are designed to enhance this delivery without inducing a strong anti-vector response [119,120]。为了降低自身免疫风险,研究人员强调肿瘤特异性抗原选择、降低交叉反应性的序列优化以及瞬时表达技术,例如. To reduce autoimmune risk, researchers emphasize tumor-specific antigen selection, sequence optimization to reduce cross-reactivity, and transient expression techniques such as those inherent to mRNA疫苗固有的技术 vaccines [121,122]。此外,正在利用为靶向递送量身定制的破耐受性佐剂和纳米颗粒来微调免疫应答,最大限度地提高抗肿瘤功效,同时最大限度地减少对健康组织的附带损害. In addition, tolerable adjuvants and nanoparticles tailored for targeted delivery are being utilized to fine-tune the immune response, maximizing antitumor efficacy while minimizing collateral damage to healthy tissue [121,123]。总的来说,这些创新强调了癌症疫苗设计不断发展的格局,平衡了有效的肿瘤靶向与患者安全。. Taken together, these innovations underscore the evolving landscape of cancer vaccine design, balancing effective tumor targeting with patient safety. CV9104是一种靶向前列腺癌的基于mRNA的癌症疫苗 is an mRNA-based cancer vaccine targeting prostate cancer [124]。其进展达到了转移性去势抵抗性前列腺癌(. It progressed to a phase II trial of metastatic castration-resistant prostate cancer (mCRPC)的II.期试验) [124]。这种疫苗代表了. This vaccine represents an innovative application of mRNA在肿瘤学中的创新应用。 in oncology.4.3. 基于病毒载体的疫苗Viral vector-based vaccines
基于病毒载体的肿瘤疫苗是癌症免疫治疗中一个有希望的发展Viral vector-based oncology vaccines are a promising development in cancer immunotherapy [11]。这些疫苗利用病毒的先天能力浸润宿主细胞并有效递送肿瘤抗原,引发强烈的靶向免疫应答. These vaccines use the innate ability of the virus to infiltrate host cells and efficiently deliver tumor antigens, eliciting a strong targeted immune response [125,126,127]。这种方法促使宿主细胞在感染后产生肿瘤特异性或相关抗原,导致这些抗原被显示给. This approach encourages host cells to produce tumor-specific or relevant antigens after infection, resulting in these antigens being exposed to T细胞,随后启动对肿瘤细胞的强力防御 cells and subsequently initiating a strong defense against tumor cells [128]。这些疫苗的显着优势之一是它们能够触发有效的细胞和体液免疫反应。它们还可以被设计成表达多种肿瘤抗原,扩大其范围并增强免疫应答的效力. One of the significant advantages of these vaccines is their ability to trigger effective cellular and humoral immune responses. They can also be designed to express multiple tumor antigens, expanding their range and enhancing the potency of the immune response [129,130]。尽管有这些好处,但仍需要解决某些挑战,例如先前存在的病毒载体免疫力的影响以及与大规模生产相关的后勤问题. Despite these benefits, certain challenges need to be addressed, such as the impact of pre-existing viral vector immunity and the logistical issues associated with mass production [131,132]。. 在这些挑战中,预先存在的对病毒载体的免疫对其在肿瘤疫苗中的使用构成了重大挑战,因为免疫系统可能会在载体产生治疗作用之前中和载体Among these challenges, pre-existing immunity to viral vectors poses significant challenges for their use in tumor vaccines, as the immune system may neutralize the vector before the vector has a therapeutic effect [133]. ]。为了解决这个问题,研究人员正在探索一系列策略:使用人类暴露有限的稀有或新型病毒载体,假分型改变病毒包膜蛋白,采用不同载体的异源初免-增强策略,对病毒衣壳进行遗传修饰以降低可识别性,与免疫调节剂共同给药以暂时抑制某些免疫反应,选择非静脉内递送途径,如肿瘤内给药以避免高抗体浓度,并调整剂量,要么使用高载体剂量来克服中和作用,要么重复给予低剂量以逃避免疫检测 To address this, researchers are exploring a range of strategies: using rare or novel viral vectors with limited human exposure, pseudotyping to alter viral envelope proteins, adopting heterologous prime-augmentation strategies with different vectors, genetic modification of viral capsids to reduce recognizability, co-administration with immunomodulators to temporarily suppress certain immune responses, choosing non-intravenous delivery routes such as intratumoral administration to avoid high antibody concentrations, and adjusting the dose, or using high vector doses to overcome neutralization, Low doses are either repeated to evade immunodetection [134,135,136,137]。此外,正在探索佐剂,以将免疫应答的焦点从载体转移到递送的肿瘤抗原. In addition, adjuvants are being explored to shift the focus of the immune response from the vehicle to the delivered tumor antigen [138,139]。这些多方面的方法旨在优化基于病毒载体的肿瘤疫苗在面对预先存在的免疫力时的功效。. These multifaceted approaches aim to optimize the efficacy of viral vector-based oncology vaccines in the face of pre-existing immunity. OncoVEXGM-CSF或 or T-VEC(T-VEC)是一种溶瘤性HSV-1疫苗,针对肿瘤选择性和 (T-VEC) is an oncolytic HSV-1 vaccine modified for tumor selectivity and GM-CSF产生进行了修饰,主要针对黑色素瘤 production, primarily against melanoma [140]。在一项成功的. Following a successful phase III.期试验后,该药获得了FDA对不可切除的复发性黑色素瘤的批准 trial, the drug received FDA approval for unresectable recurrent melanoma [140]。. CG0070是另一种基于腺病毒的疫苗,用于在Rb通路缺陷的癌细胞中选择性复制,并靶向膀胱癌 is another adenovirus-based vaccine for selective replication in cancer cells with defects in the Rb pathway and targeting bladder cancer [141]。. LV305是一种基于慢病毒的疫苗,将 is a lentivirus-based vaccine that delivers the NY-ESO-1抗原基因递送至树突状细胞,靶向表达 antigen gene to dendritic cells to target NY-ESO-1的癌症,如黑色素瘤和肉瘤-expressing cancers such as melanoma and sarcoma [142]。. JX-594或 or Pexa-Vec是一种基于牛痘病毒的疫苗,经过修饰以表达 is a vaccinia virus-based vaccine modified to express GM-CSF并选择性靶向具有高胸苷激酶活性的癌细胞,并且已经进行了多项试验,包括一项针对肝细胞癌的III.期试验 and selectively target cancer cells with high thymidine kinase activity, and several trials have been conducted, including a phase III trial for hepatocellular carcinoma [143,144,145]。这些代表了肿瘤学中病毒疗法和免疫疗法的创新交叉点。. These represent innovative intersections of viral therapy and immunotherapy in oncology.4.4. Dendritic 基于树突状细胞的疫苗cell-based vaccines
树突状细胞(Dendritic cells (DC)通过连接先天免疫系统和适应性免疫系统来介导免疫应答,并且在抗原呈递和随后的T细胞活化中至关重要,因此发现它们与癌症免疫治疗策略具有实质性相关性s) mediate immune responses by connecting the innate and adaptive immune systems and are critical in antigen presentation and subsequent T cell activation, so they have been found to be substantially relevant to cancer immunotherapy strategies [18,146]。这源于基于. This stems from a DC-based cancer vaccine that utilizes DC的癌症疫苗,该疫苗利用装有肿瘤相关抗原(TAA)的DC来促使针对癌细胞产生强大的免疫应答s loaded with tumor-associated antigens (TAAs) to promote a robust immune response against cancer cells [147]。用. Methods for loading these DCs with TAA加载这些DC的方法多种多样,从使用肿瘤裂解物和合成肽到编码肿瘤抗原的mRNA are varied, from the use of tumor lysates and synthetic peptides to mRNAs encoding tumor antigens [148]。临床试验强调了这些基于. Clinical trials highlight the promise of these DC的疫苗在各种癌症中的前景。尽管存在这种令人兴奋的潜力,但挑战仍然存在,包括DC疫苗生产中的技术困难、疫苗效力变化、免疫抑制肿瘤微环境以及缺乏用于患者选择的可靠生物标志物-based vaccines in a variety of cancers. Despite this exciting potential, challenges remain, including technical difficulties in DC vaccine production, changes in vaccine efficacy, immunosuppressive tumor microenvironments, and lack of reliable biomarkers for patient selection [149]。然而,基于新抗原的疫苗等个体化癌症免疫疗法的最新进展为基于. However, recent advances in personalized cancer immunotherapies, such as neoantigen-based vaccines, offer promising opportunities for DC的疫苗提供了有希望的机会,将这些疫苗与其他治疗方法相结合可能会增强其疗效-based vaccines that may be enhanced in combination with other therapeutics [8]。在最近的一项研究中,研究人员引入了一种使用叠氮糖的代谢聚糖标记技术来增强. In a recent study, researchers introduced a metabolic glycan labeling technique using azigo to enhance the DC疫苗 vaccine [150]。该方法不仅可以促进. This method promotes not only DC活化和抗原呈递,还可以促进细胞因子的有效偶联 activation and antigen presentation, but also efficient conjugation of cytokines [150]。此外,它有望在各种肿瘤中广泛应用,为调节. In addition, it is expected to be widely used in various tumors, provide a platform for regulating the interaction between DC与其他免疫细胞之间的相互作用提供平台,并增强树突状细胞疫苗的抗肿瘤功效。s and other immune cells, and enhance the anti-tumor efficacy of dendritic cell vaccines. 杰出的代表包括Prominent representatives include Provenge和 and DCVax-L。. Provenge( (sipuleucel-T)是FDA批准的用于晚期前列腺癌的自体细胞免疫疗法) is an FDA-approved autologous cell immunotherapy for advanced prostate cancer [5]。它使用暴露于融合蛋白. It uses peripheral blood mononuclear cells (PBMCs) of patients exposed to the fusion protein PA2024的患者外周血单核细胞(PBMC),该融合蛋白PA151将来自前列腺癌细胞的抗原与免疫激活剂, which binds antigens from prostate cancer cells to the immune activator GM-CSF结合,引发针对表达抗原的前列腺癌细胞的免疫反应[152,153,154,155,156]。另一方面,, eliciting an immune response against antigen-expressing prostate cancer cells [151, 152, 153, 154, 155]. DCVax-L是一种针对多形性胶质母细胞瘤(GBM)的自体树突状细胞疫苗, on the other hand, is an autologous dendritic cell vaccine against glioblastoma multiforme (GBM) [156]。该疫苗是通过从患者自身的肿瘤组织中加载肿瘤裂解物来制备患者的树突状细胞,使免疫系统能够识别和攻击相应的癌细胞. The vaccine prepares the patient's dendritic cells by loading tumor lysates from the patient's own tumor tissue, enabling the immune system to recognize and attack the corresponding cancer cells[156]。两种疫苗都利用树突状细胞靶向癌症,但它们的临床历程和疾病靶点不同(表. Both vaccines use dendritic cells to target cancer, but their clinical history and disease targets differ (Table <>)。).4.5. 全细胞疫苗Whole cell vaccines
基于全细胞的疫苗通过结合大量肿瘤相关抗原来刺激有效的免疫反应,为癌症免疫治疗提供了一种全面的方法。从机制上讲,这些疫苗利用辐照的肿瘤细胞(自体或同种异体)使免疫系统暴露于肿瘤的全部抗原库Whole-cell-based vaccines provide a comprehensive approach to cancer immunotherapy by binding a large number of tumor-associated antigens to stimulate an efficient immune response. Mechanistically, these vaccines utilize irradiated tumor cells (autologous or allogeneic) to expose the immune system to the full antigen pool of the tumor [157],从而诱导针对一系列肿瘤抗原的特异性和多价免疫应答, thereby inducing a specific and multivalent immune response against a range of tumor antigens [158]。该策略提供了广谱的已知和未知肿瘤抗原,避免了抗原丢失或下调(肿瘤采用的典型逃逸机制. This strategy provides a broad spectrum of known and unknown tumor antigens, avoids antigen loss or down-regulation (the typical escape mechanism employed by tumors [157]),并且无需为每位患者识别特定抗原,这可能既耗时又昂贵), and eliminates the need to identify specific antigens for each patient, which can be time-consuming and expensive [159]。但是,存在局限性。自体全细胞疫苗的生产可能是劳动密集型和个性化的,因此需要从每个患者身上分离和培养肿瘤细胞. However, there are limitations. The production of autologous whole cell vaccines can be labor-intensive and individualized, so tumor cells need to be isolated and cultured from each patient [159]。鉴于免疫抑制肿瘤微环境会限制疫苗效力,这些疫苗通常需要与佐剂或免疫调节剂共同给药以提高其免疫原性. Given that the immunosuppressive tumor microenvironment limits vaccine efficacy, these vaccines often need to be co-administered with adjuvants or immunomodulators to improve their immunogenicity [8,159]。疫苗制剂中自身抗原诱导的潜在自身免疫也令人担忧. Potential autoantigen-induced autoimmunity in vaccine formulations is also of concern [158]。因此,虽然基于全细胞的疫苗为癌症免疫治疗提供了一种有前途的方法,但需要进一步优化和完善这些策略以应对这些挑战和局限性。. Therefore, while whole-cell-based vaccines offer a promising approach to cancer immunotherapy, these strategies need to be further optimized and refined to address these challenges and limitations. Representatives include GVAX, Canvaxin, and Oncophage. GVAX is a whole-cell tumor vaccine, utilizing tumor cells genetically modified to secrete GM-CSF (an immune stimulant), and has been explored for cancers like pancreatic and prostate cancers, with mixed outcomes in later-phase trials [160,161]. Canvaxin, aimed at melanoma, combines irradiated autologous and allogeneic melanoma cells with the BCG adjuvant, but it failed to show significant survival benefits in a phase III trial for advanced melanoma [115,162]. Oncophage (Vitespen) is derived from patient-specific tumor heat shock proteins (HSPs) and primarily targets renal-cell carcinoma and melanoma [163,164]. It completed phase III trials with mixed results but secured approval in Russia for the treatment of kidney cancer [165]. While these vaccines showcase varied cancer immunotherapy strategies, each has faced challenges in late-stage clinical evaluations. (Table 1)Table 1. Below is a tabular list of various tumor vaccines in the last decade.
| Types of Tumor Vaccines | Strengths | Weaknesses | Examples | Mechanisms of Action | Effects | Limitations | References |
|---|---|---|---|---|---|---|---|
| Peptide vaccines |
|
|
Nelipepimut-S (NeuVax) | HER2-derived peptide vaccine | Activation of T-cell response | Limited overall survival improvement | [166] |
| CIMAvax-EGF | EGF-based peptide vaccine | Inhibition of EGF signaling | No direct tumor targeting | [103] | |||
| MUC1-based peptide vaccine | Targeting MUC1 tumor-associated antigens | Enhanced immune response | Heterogeneous patient response | [167] | |||
| DNA/RNA-based vaccines |
|
|
CV9104 (CureVac) | Uses mRNA to encode six antigens overexpressed in prostate cancer | Induced antigen-specific immune responses in early clinical trials | Efficacy in late-stage trials yet to be established; possibility of inducing autoimmune responses | [168] |
| Viral-vector-based vaccines |
|
|
Adenovirus-based vaccines (OncoVEXGM-CSF, CG0070) | Adenoviruses are modified to express a tumor-specific antigen or an immunomodulatory molecule; these stimulate an immune response against the tumor | Effective in stimulating an immune response against the tumor | Immune response to the viral vector can limit repeat dosing | [169] |
| Lentivirus-based vaccines (LV305) | Lentiviruses are engineered to deliver tumor-specific antigens to dendritic cells to stimulate a T-cell response | Successful in initiating T-cell responses | Safety concerns over integration into the host genome | [23] | |||
| Vaccinia-virus-based vaccines (JX-594) |
Vaccinia viruses are genetically engineered to express a tumor antigen and/or immunostimulatory molecule; they can directly lyse cancer cells | Showed antitumor activity and were well tolerated in clinical trials | Immune response to the viral vector can limit its effectiveness | [170] | |||
| Dendritic-cell-based vaccines |
|
|
Provenge (Sipuleucel-T) | The patient’s own dendritic cells are exposed to a fusion protein (prostatic acid phosphatase linked to an immune cell stimulating factor) | Extended overall survival in metastatic castration-resistant prostate cancer | Limited clinical benefits, high cost, and complex manufacturing process | [5] |
| DCVax-L | Autologous dendritic cells are pulsed with tumor lysate | Prolonged progression-free survival in glioblastoma multiforme (GBM) patients | Not FDA-approved; requires personalized manufacturing | [171] | |||
| Whole-cell-based vaccines |
|
|
GVAX | Utilizes autologous/allogeneic tumor cells that have been genetically modified to secrete the immune-stimulating cytokine GM-CSF | Demonstrated a significant immune response against cancer, studied in various types of cancer, including pancreatic and prostate cancers | Production can be labor-intensive and personalized; often requires co-administration with adjuvants or other immunomodulatory agents to enhance their immunogenicity | [159,172] |
| Canvaxin | Allogeneic melanoma cells mixed with Bacillus Calmette–Guérin (BCG) to stimulate immune response | Intended for melanoma treatment, but development discontinued due to insufficient effectiveness | Limited efficacy; potential for BCG-related side effects | [173] | |||
| Oncophage (Vitespen) | Uses heat shock proteins (gp96) derived from the patient’s tumor as an autologous vaccine | Showed efficacy in extending disease-free survival in certain patients with kidney cancer and melanoma | Not universally effective; personalized manufacturing can be labor-intensive | [174,175] |
The preceding table encapsulates seminal instances of assorted classifications of cancer vaccines, encompassing peptide-based, DNA/RNA-based, viral-vector-based, dendritic-cell-based, and whole-cell-based vaccines. Each paradigm is delineated in exhaustive detail, supplemented by pertinent bibliographical citations for subsequent scholarly inquiry.
4.6. Another Cancer Vaccine Therapy: In Situ Cancer Vaccines
In situ cancer vaccines represent a therapeutic approach where the tumor inside a patient’s body is directly targeted to serve as its own vaccine [63]. Rather than extracting tumor cells for external processing and reintroduction, in situ vaccines stimulate the immune system by damaging the tumor in its native environment [63]. As the tumor cells die, they release antigens, which are then recognized by the immune system. Often, this is achieved by injecting immune-stimulating agents or oncolytic viruses into the tumor [176,177]. This not only aims to destroy the immediate tumor but also primes the immune system to recognize and combat tumor cells elsewhere in the body [176]. In situ cancer vaccines have shown promise in preliminary studies.4.7. Influencing Factors of Tumor Vaccines
Boosting the power of cancer vaccines is a top priority for researchers, who are diving deep into adjuvants and combination therapies to ramp up immune responses, outsmart tumor immune evasion, and prevent cancers from coming back [178,179]. The efficacy of cancer vaccines hinges on several factors, including picking the right antigens, choosing the adjuvants wisely, and using the best delivery systems [2]. Antigen selection is of paramount significance. The antigens should be specific to the tumor, or associated with it, so that the immune response zeroes in on cancer cells without harming healthy tissues [180]. Plus, the chosen antigens need to be highly immunogenic and able to stimulate both CD8+ cytotoxic T cells and CD4+ helper T cells for a strong, long-lasting attack against tumors [15]. Adjuvants help by making cancer vaccines more immunogenic. They stimulate the innate immune system, encourage antigen uptake by APCs, and help activate and expand antigen-specific T cells [181,182]. There are different types of adjuvants, like alum, toll-like receptor (TLR) agonists, and cytokines, each with unique mechanisms of action and varying effectiveness [183,184]. The latest research shows that TLR agonists, such as TLR9 and TLR7/8 agonists, have shown promise by bolstering antigen-presenting cells and intensifying immune responses [185]. Researchers have developed a nanosystem that can inhibit a process called MerTK-mediated efferocytosis. This inhibition leads to the release of immunogenic contents into the tumor microenvironment, potentially boosting the body’s natural defenses against the tumor [186]. Similarly, STING agonists enhance dendritic cell activity, boosting T-cell responses against tumors [187,188]. Oncolytic viruses, while serving as direct antitumor agents, also act as adjuvants by releasing tumor antigens within an inflammatory milieu [132]. These recent breakthroughs encapsulate the dynamic progression in adjuvant research, aiming to optimize the immune system’s potency against tumors.5. Combination Therapies
Combining cancer vaccines with other therapies has emerged as a promising strategy to enhance the overall therapeutic efficacy and overcome the limitations of single-agent treatments [189]. Cancer vaccines, which aim to stimulate a patient’s immune system to recognize and attack tumor cells, may benefit from being combined with other immunotherapies, such as immune checkpoint inhibitors, to boost immune responses and counteract immunosuppressive mechanisms within the tumor microenvironment (TME) [190]. Several types of combination therapies involving cancer vaccines and other treatment modalities have been explored in recent years, such as combining cancer vaccines with chemotherapy, targeted therapies, and radiation therapy [189]. These combination approaches hold significant promise for optimizing cancer treatment outcomes and providing more effective, personalized therapy options for patients [74].5.1. Cancer Vaccine + Immune Checkpoint Inhibitors
These vaccines can be combined with other treatments to enhance their effectiveness. Immune checkpoint inhibitors like pembrolizumab (Keytruda), nivolumab (Opdivo), and ipilimumab (Yervoy) disable immune checkpoints, thereby unleashing a more potent attack on cancer cells [191,192,193,194]. This combination hopes to enhance recognition of cancer cells (via the vaccine) and amplify the immune response (via the checkpoint inhibitors) [5,178]. This combined approach has shown promise in preclinical models and early clinical trials by generating tumor-specific T cells and preventing their exhaustion [195]. The mechanism behind these effects is that cancer vaccines aim to boost T cells’ recognition of tumor antigens, but this immune response can be dampened by the tumor’s evasion mechanisms [196]. Enter immune checkpoint inhibitors, which block inhibitory checkpoints (PD-1 and CTLA-4) on T cells, essentially “releasing the brakes” and amplifying their antitumor activity [197]. By combining cancer vaccines, which enhance the number of tumor-recognizing T cells, with checkpoint inhibitors that ensure that these T cells are not suppressed, there is a synergistic boost in the antitumor immune response. Preliminary studies suggest that this combination augments tumor attack, potentially leading to improved patient outcomes [198,199,200,201].5.2. Cancer Vaccine + Chemotherapy
Chemotherapy is a destructive force against cancer cells, hindering their growth and division, but may also inadvertently harm rapidly dividing normal cells such as those in bone marrow, the digestive tract, and skin [202]. The potential synergy between cancer vaccines and chemotherapy arises from some chemotherapeutic agents inducing immunogenic cell death, increasing the visibility of dying cancer cells to the immune system and potentially enhancing the efficacy of cancer vaccines [203]. Several chemotherapeutic agents have been identified to potentially enhance the efficacy of cancer vaccines due to their immunomodulatory effects. For instance, cyclophosphamide and temozolomide can deplete immune-suppressing regulatory T cells (Tregs), creating a more receptive tumor environment for vaccine action [204]. Docetaxel, used for cancers like breast and prostate cancers, can bolster antigen presentation, thereby enhancing immune recognition of tumor cells [205]. Gemcitabine targets and reduces myeloid-derived suppressor cells (MDSCs) [206]. When combined with cancer vaccines, these agents can modify the tumor environment, diminish immune suppression, or amplify the immune response against tumors, although the choice of combination depends on multiple factors, including cancer type and patient health [203]. However, there are substantial challenges to this approach. Determining the optimal timing and dosage of chemotherapy in relation to cancer vaccines remains a complex task [159]. The side effects of both chemotherapy and cancer vaccines, including chemotherapy’s often severe systemic side effects such as fatigue, infection, hair loss, and nausea, are a significant concern [207]. Furthermore, the treatment’s responsiveness is limited, as not all cancer types respond well to chemotherapy or cancer vaccines, with variability in individual patient responses adding to the complexity of treatment plans [208,209]. Additionally, the complexity of the tumor microenvironment, which can evolve various mechanisms to resist or evade treatment, may limit the effectiveness of these combined therapies [210].5.3. Cancer Vaccine + Radiotherapy
Radiotherapy employs high-energy particles or waves, such as X-rays, gamma rays, electron beams, or protons, to annihilate or damage cancer cells. This radiation induces small breaks in the DNA inside cells, inhibiting their growth and division, and eventually leading to their death [211]. When combined with tumor vaccines, these treatments might produce a synergistic effect, with radiotherapy potentially leading to the release of cancer cell antigens and stimulating the immune system, thereby enhancing the effectiveness of cancer vaccines [212,213,214]. Research indicates that radiotherapy exerts both cytotoxic and immunomodulatory effects on the tumor microenvironment. Beyond directly damaging tumor cells, RT induces immunogenic cell death, leading to the release of damage-associated molecular patterns (DAMPs) [215]. These DAMPs serve as “danger signals”, enhancing dendritic cell function and fostering antitumor immune responses. Concurrently, radiotherapy damages the tumor vasculature, increasing its permeability due to direct effects on endothelial cells and the upregulated release of VEGF from irradiated tumor cells [215,216]. This can lead to both transient improvements in oxygen and nutrient delivery and enhanced immune cell infiltration into the tumor. However, this combination approach has its limitations. Not all patients or cancer types respond well to either radiotherapy or cancer vaccines, making the efficacy of this approach unclear in a broad population [217]. The optimal timing and dosage of radiotherapy relative to cancer vaccines are not well understood, posing a risk of radiotherapy killing immune cells stimulated by the vaccine, and thereby reducing the effectiveness of the treatment [218]. Both treatments can cause side effects, such as skin changes, fatigue, and other symptoms for radiotherapy, and usually mild but possibly flu-like symptoms for cancer vaccines [63], and some tumors may develop resistance to radiotherapy, which could limit the effectiveness of this combined approach [219].5.4. Cancer Vaccine + Targeted Therapy
Compared to cancer vaccines, targeted therapies obstruct specific proteins or processes that aid in cancer growth and progression, offering a more cancer-cell-selective approach compared to traditional chemotherapy and resulting in fewer side effects. Notable targeted therapies include small-molecule inhibitors, like Gleevec (imatinib), and monoclonal antibodies, like Herceptin (trastuzumab) [220,221,222]. When utilized in combination, targeted therapies aim to inhibit cancer cells’ proliferation and survival, rendering the cancer cells more susceptible to the immune response provoked by the cancer vaccine. Studies have revealed the potential of this combination, with targeted therapies able to modulate the tumor microenvironment, thereby possibly enhancing the effectiveness of the vaccine-stimulated immune response [223] and helping to prevent or delay resistance to targeted therapies [2]. However, challenges and limitations remain, including the development of resistance to targeted therapies over time [224], potential side effects ranging from mild skin rashes or diarrhea to severe liver toxicity or heart problems [225], limited responsiveness in certain cancer types or patients [208], and the complex and not fully understood interaction effects between cancer vaccines and targeted therapies, which could potentially interfere with the vaccine-stimulated immune response [16].5.5. Cancer Vaccine + Oncolytic Virotherapy
Oncolytic virotherapy constitutes a novel paradigm in the therapeutic approach towards malignant neoplasms, exhibiting a mechanism of action that distinguishes it from traditional tumor vaccines. It capitalizes on the unique capabilities of selected or genetically engineered viruses, which are orchestrated to specifically target and eradicate neoplastic cells [19,226]. Upon administration, these oncolytic viruses infiltrate the patient’s system, subjugating cancerous cells and commandeering their biological machinery for viral replication, consequently leading to cell lysis [227,228]. This lysogenic cycle not only facilitates direct oncolysis but also liberates tumor-specific antigens, providing a catalyst for the patient’s immune system to mount an anticancer response—an underpinning that is shared with the concept of tumor vaccines [132]. This dual-action mechanism that harmonizes direct cellular destruction with immune activation embodies a promising pathway in the realm of cancer therapy. The dual-action mechanism encompasses direct tumor cell lysis, releasing tumor-associated antigens, and the unveiling of damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs). These elements activate both the innate and adaptive arms of the immune system, enhancing antitumor responses. As newly assembled viral entities continue their onslaught against other malignant cells, a self-propagating cycle is established. Recent advancements in this burgeoning field have entailed the exploration of diverse oncolytic virus models, such as the Parapoxvirus ovis model, known to induce an immunogenic form of cell death termed pyroptosis [229]. Scientific investigations have also scrutinized the immunostimulatory effects of these bioengineered viruses and combinational therapeutic strategies that kindle pyroptosis, consequently fostering potent antitumor activity [230,231,232]. These engineered viruses have some potential in the delivery of antitumor drugs [233]. Pioneering therapeutic strategies, such as the KISIMA/VSV-GP heterologous prime–boost methodology and the development of adenovirus-based tumor vaccines, have further emphasized the potential of oncolytic virotherapy as a formidable armament in the arsenal of cancer immunotherapy [234]. Cancer vaccines and oncolytic virotherapy offer a potential synergistic approach for cancer treatment. Cancer vaccines introduce cancer-specific antigens into the body, training the immune system to recognize and attack cells displaying these antigens [5]. Concurrently, oncolytic virotherapy uses engineered viruses that selectively infect and eliminate cancer cells, subsequently releasing tumor antigens and new viral particles that can infect nearby cancer cells, thereby stimulating an immune response [19]. In combination, the cancer vaccine’s potential enhancement of the immune response, coupled with the direct cellular damage from the oncolytic virus, could increase therapeutic effectiveness. Initial studies suggest that this combination can result in a more robust and long-lasting immune response against tumors, even potentially overcoming some immune evasion tactics employed by cancer cells [235]. Nevertheless, this approach is not without limitations. The immune system could respond to the oncolytic virus, reducing its cancer-killing effectiveness [236]. Additionally, delivering both oncolytic viruses and cancer vaccines to the tumor site, particularly in solid tumors, is challenging [237]. The diverse nature of cancers and variability in patient responses can limit the overall responsiveness of this combined therapy [238]. Finally, safety is a significant concern, as both oncolytic virotherapy and cancer vaccines can cause side effects, with the former potentially leading to severe or life-threatening reactions in rare instances [239]. Recent advancements will change this domain. Viruses are now engineered for heightened tumor specificity, some are armed with therapeutic genes to turn tumors into producers of anticancer agents, and combinations with treatments like immune checkpoint inhibitors are showing synergistic effects [240,241]. Moreover, refined genetic engineering techniques have improved the safety profiles of these oncolytic viruses, making them more amenable for therapeutic applications [242,243]. This means that they have reduced virulence in non-target tissues and minimized side effects.6. Personalized Cancer Vaccines
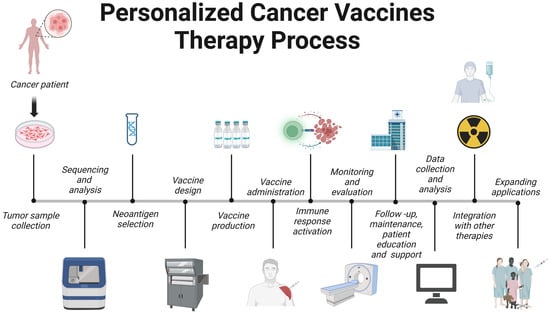
Personalized cancer vaccine therapy is an innovative approach that tailors cancer treatment to a patient’s unique tumor profile. The process can be outlined as follows (Figure 4).
Figure 4. Therapeutic pipeline for personalized vaccines. Created with BioRender.com.
 Encyclopedia
Encyclopedia
