You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Dequan Liu and Version 3 by Camila Xu.
Oncology vaccines represent a breakthrough in oncology, using the principles of immunotherapy to stimulate the body's immune system to fight cancer, providing a more targeted approach with fewer side effects than traditional treatments such as surgery, radiotherapy, and chemotherapy.
- TME
- cancer immunology
- oncology vaccines
- combination therapies
1. Introduction
According to a comprehensive epidemiological survey conducted in 2020, malignancy has been the leading cause of morbidity and mortality worldwide, with a shocking incidence of approximately 19,3 new cancers and nearly 10,1 deaths during the year [1][<>]. Health and health systems face enormous challenges. However, new hope for cancer treatment is in the form of oncology vaccines. Oncology vaccines represent an oncology breakthrough that uses the principles of immunotherapy to stimulate the body's immune system to fight cancer, providing a more targeted approach with fewer side effects than conventional treatments such as surgery, radiotherapy, and chemotherapy [2][<>].
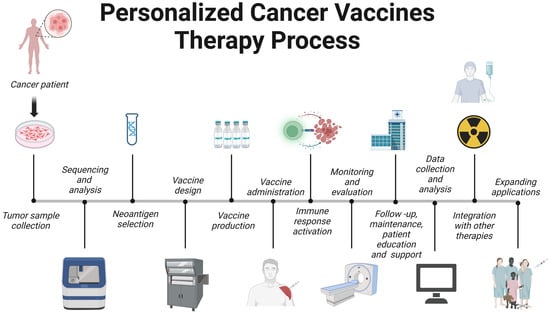
The evolution of cancer vaccines has unfolded over decades, with each era marked by key advances (Figure 1). The journey began with William Coley in the late 19th century, who developed Colley toxin after noticing that infections often cause tumors in cancer patients to shrink [3]. This approach harnesses the immune system to fight cancer – a concept further expanded by Lewis Thomas and Frank McFarlane Burnett in the mid-20th century, when they introduced the ideas of cancer immune editing and tumor immunity [4]. The dawn of a clinically approved cancer vaccine came in 2010 with the FDA approval of sipuleucel-T, the first therapeutic cancer vaccine against prostate cancer [5]. Subsequent eras continue to this day and are characterized by the development of modern cancer vaccines and checkpoint inhibitors, such as the combination therapy approved in 2021 for the treatment of lymphoma [6]. Currently, cancer vaccine development emphasizes personalization, and multiple strategies are being studied, including neoantigen vaccines, DNA/RNA vaccines, and viral vectors, aimed at tailoring treatments to individual patients' tumors.
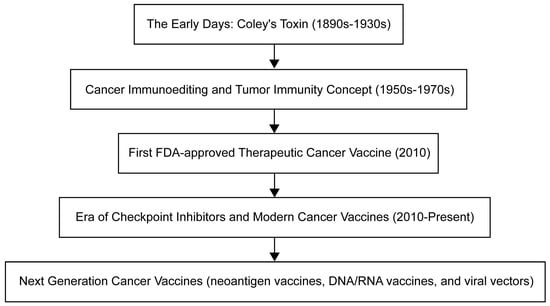
Figure 1. Timeline for oncology vaccine development.
Timeline for oncology vaccine development.
In-depth study of the mechanistic complexity in which the tumor microenvironment (TME) in which tumors exist and evolve plays a key role in tumor progression. As a result, TME has become a scientific research center in the field of anti-cancer therapeutic drug evolution [7]. As proof of contemporary medical innovation, tumor vaccines have been carefully designed to harness the innate and adaptive nature of the host immune system to target abnormal cells. These groundbreaking formulations strategically regulate TME, thereby wisely minimizing collateral damage to healthy tissue [8].
The field of cancer immunotherapy is undergoing significant advances with the development of various oncology vaccines, each with its own unique advantages and challenges. Although peptide vaccines are effective in eliciting an immune response, adjuvants are often required [9]. DNA/RNA-based vaccines allow endogenous antigen generation but face barriers to delivery and expression [10][11][10,11]. Viral vector-based vaccines can stimulate a robust immune response, but are limited by safety concerns and pre-existing immunity [12]. While dendritic cell-based vaccines can induce robust responses, they require complex production processes and different patient responses [13]. Whole-cell-based vaccines address production and standardization issues while harnessing whole tumor cells to trigger an immune response [14]. A deep understanding of these vaccines and their limitations is critical for their effective deployment in cancer immunotherapy [15]. Current studies recognize the limited efficacy of oncology vaccines and are seeking to enhance therapeutic efficacy by combining them with other therapies such as immune checkpoint inhibitors, chemotherapy, radiotherapy, targeted therapy, and oncolytic viral therapy [16][17][18][19][16,17,18,19]. At the same time, the innovative field of personalized cancer vaccines is gaining traction, providing tailored immune responses to individual-specific tumors [8]. Despite some challenges, including the complexity of neoantigen identification and resource-intensive production, technological advances promise a faster and more affordable future for personalized cancer vaccines [20][21][22][23][20,21,22,23].
2. Tumor microenvironment
The tumor microenvironment (TME), which is critical to cancer onset and progression, is a headache for scientists. It is not only dynamic, but also very complex, shaping the heterogeneity of tumors. TME is a mixture of cellular and noncellular components (eg, cancer cells, stromal cells, immune cells, ECMs) and signaling molecules (eg, growth factors and cytokines) [24][25][24,25]. ECM is a tangled network of proteins, glycoproteins, and proteoglycans that provide structure and shape cell behavior through biochemical and biomechanical signals. When cancer alters the composition of the ECM, it can promote angiogenesis, immune evasion, and treatment resistance [26][27][26,27]. For example, as seen by overexpression of lysine oxidase (LOX), ECM hardness increases, promotes cell proliferation and survival through mechanisms such as FAK activation, and also enhances tumor aggressiveness [28]. Similarly, accumulation of hyaluronic acid is often associated with a poor prognosis, which can promote tumor growth and promote immune escape [29]. Alterations in matrix metalloproteinase (MMP) expression can remodel the ECM, favoring tumor invasion by releasing growth factors [30][31][30,31]. In addition, specific ECM proteins such as elastin, laminin, tenascin-C, and periosteum protein support tumor cell migration and survival when overexpressed [32]. Notably, changes in collagen orientation lead to a fibrous arrangement that provides a pathway for enhanced tumor cell migration, which often indicates a higher risk of metastasis [33]. ]。 ECM is also involved in the secretion of various growth factors such as transforming growth factor-β (TGF-β), interleukin-1β (IL-1β), IL-6, tumor necrosis factor-α (TNF-α), and vascular endothelial growth factor (VEGF), which are secreted by the individual cells of TME and can initiate tumor cell growth, survival, migration, angiogenesis, and epithelial-mesenchymal transformation (EMT). This is achieved by modulating its specific receptors and stimulus signaling pathways [34] [34] (Figure 2). This intricate interaction between ECM and tumor cells not only drives cancer progression, but also presents treatment challenges due to factors such as drug permeability barriers and activation of cell survival pathways.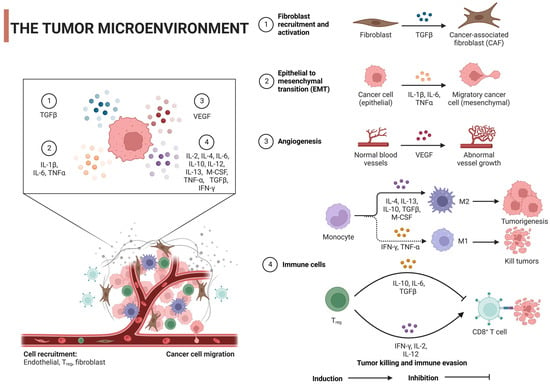
Figure 2. Regulatory mechanism of tumor microenvironment on tumor cells: TGF-β, transforming growth factor-β; IL, interleukin; VEGF, vascular endothelial growth factor; TNF-α, tumor necrosis factor α; IFN-γ, interferon γ; M-cerebrospinal fluid, macrophage colony-stimulating factor; Tregs, regulatory T cells. Created with BioRender.com.
3. Oncology vaccines
3.1. Cancer immunology
Adaptive immunity or acquired immunity is a highly specific and persistent defense against pathogens and abnormal cells, administered primarily by T and B lymphocytes [64][66]. This type of immunity is known for its immune memory, which provides long-lasting resistance to previously encountered things, such as pathogens or antigens [65][67]. Studying the ins and outs of adaptive immunity is critical to making vaccines and targeted immunotherapies. These breakthroughs offer great potential for the prevention and treatment of a range of diseases, from infections and autoimmune diseases to cancer [66][67][68][68,69,70]. In the early stages of cancer, the immune system is involved in a process called immune surveillance, where it hunts down and destroys abnormal cells, preventing tumor formation [69][70][71,72]. But cunning cancer cells use different techniques to evade the immune system and help tumors grow and progression [71][73]. By studying these immune evasion strategies, scientists have proposed new immunotherapies such as immune checkpoint inhibitors (ICIs) and adoptive cell transfer. These innovative therapies are designed to improve the body's ability to fight tumors and overcome cancer's ability to evade the immune system [72][74]. Immunotherapies, such as ICI and chimeric antigen receptor (CAR) T-cell therapy, have completely changed the treatment of cancer by using the immune system to fight cancer cells [73][75]. But even with these amazing advances, some patients respond poorly to immunotherapy and may even develop resistance to immunotherapy, which means that more needs to be learned about how the immune system and cancer interact [74][76]. Gaining this knowledge will help create new immunotherapy strategies and identify predictive biomarkers that lead to better patient outcomes [75][76][77,78].3.2. Mechanism of Action
The fundamental function of cancer vaccines lies in their ability to mobilize innate and adaptive immune responses to identify, fight, and eradicate tumor cells [20]. The following discussion will provide a comprehensive analysis of its intricate mechanisms of action (Figure 3).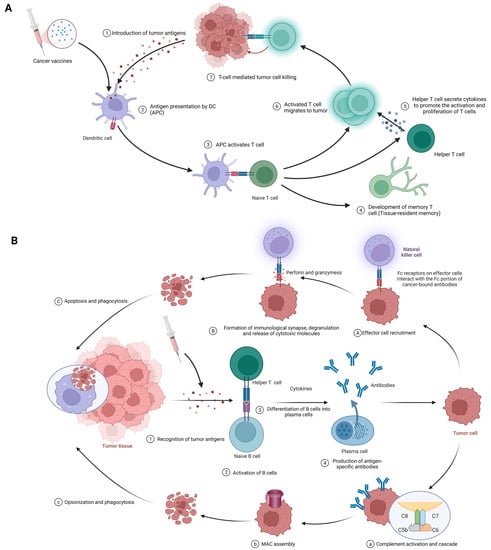
Figure 3. (A) Cellular immune response against cancer (step 1 to step 7); APCs, antigen-presenting cells; APC, antigen-presenting cell. (B) Humoral immunity response against cancer (step 1 to step 4). A–C; antibody-dependent cell-mediated cytotoxicity; a–c: complement-dependent cytotoxicity; MAC, membrane attack complex. Created with BioRender.com.
3.2.1. Cellular Immunity
The procedure of eliciting a cellular immune response against cancer, exemplified by the use of cancer vaccines, is complex and sequential. It commences with the delivery of tumor antigens and concludes with the activation of humoral immunity. The following is a detailed breakdown of each phase in this process (Figure 3A). Introduction of tumor antigens: With cancer vaccines, referring to tumor antigens being introduced to antigen-presenting cells (APCs) like dendritic cells. Options include whole tumor cells, peptides, proteins, DNA, mRNA, or even dendritic cells loaded with tumor antigens or packing tumor-derived genetic material. Tumor vaccines play a role in this step. This crucial first step gets the ball rolling in the immune response against cancer [18][77][18,79]. Antigen processing and presentation: APCs capture, process, and present tumor-derived peptides on their surface. Dendritic cells efficiently cross-present exogenous antigens to both MHC class I and II molecules, activating both CD8+ cytotoxic T lymphocytes (CTLs) and CD4+ helper T cells [18][78][18,80]. Activation of T cells: Presentation of tumor antigens by APCs activates and expands antigen-specific T cells. T-cell activation requires antigen recognition via the T-cell receptor (TCR) and costimulatory signals provided by the interaction between costimulatory molecules on APCs and their receptors on T cells [79][80][81,82]. CTLs and helper T cells in action: CTLs directly kill tumor cells by recognizing and binding to MHC class I molecules presenting tumor antigens, while helper T cells produce cytokines that support CTLs’ activation, proliferation, and differentiation. CD4+ helper T cells also provide help to B cells, facilitating antibody production and enhancing the function of APCs and CTLs [2][40][2,42]. Stimulation of humoral immunity: Cancer vaccines induce B cells to produce antigen-specific antibodies. These antibodies target and eliminate tumor cells [15][81][15,83].3.2.2. Humoral immunity
The procedure begins with the identification of tumor antigens and ultimately achieves effector function mediated by antibodies. Here are the details of each stage in this process. (Figure 3B). Recognition of tumor antigens: Like cellular immunity, tumor cells have tumor-associated antigens (TAAs) and tumor-specific antigens (TSAs) that the immune system can recognize [82][84]. Activation of B cells: B cells detect and recognize tumor antigens through their B cell receptor (BCR). With the addition of some co-stimulatory signals from helper T cells, B cells are activated to proliferate and differentiate [70][72]. B cells differentiate into plasma cells: Upon activation, B cells transform into plasma cells, which are specifically designed to produce large amounts of specific antibodies against recognized tumor antigens [82][84]. Antigen-specific antibody production: Plasma cells produce antigen-specific antibodies that pass through the bloodstream and attach to tumor antigens on cancer cells [83][85]. Effector function of antibodies: Once bound, antibodies employ various strategies against tumor cells, such as:-
Antibody-dependent cell-mediated cytotoxicity (ADCC) (Figure 3A(1-3)): In the first phase, effector cell recruitment: Fc receptor-carrying immune cells, such as natural killer (NK) cells, macrophages, and neutrophils, are attracted to the tumor site through interactions between their Fc receptors and Fc fragments of antibodies bound to malignant cells [84][85][86,87]. In the next stage, the formation of immune synapses, degranulation, and the release of cytotoxic molecules: Immune synapses are formed when the Fc receptor on effector cells interacts with the Fc portion of the cancer-binding antibody, enabling the targeted release of cytotoxic molecules [88]. In thEffe next stage, the formation of immune synapses,ctor cell degranulation, and the release of cytotoxic molecules: Immune synapses are formed when the Fc receptor on effector cells interacts with the Fc portion of the cancer-binding antibody, enabling the targeted release of cytotoxic molecules ensues, eventually eliminating cancer cells by perforin and granzyme [86][89]. EFinally, phagocytosis: Affector cell degranulation ensues, eventually eliminter apoptosis, macrophages engulf cancerous debris, facilitating cancer cells by perforin and granzymelearance and preventing the spread of harmful cellular components [87][90]. Finally, phagocytosis: After apoptosis, macrophages engulf cancerous debris, facilitating clearance and preventing the spread of harmful cellular components [88].
-
Complement-dependent cytotoxicity (CDC) (Figure 3B(1-4)): In the first phase, complement activation and cascade reaction: the Fc moiety of the binding antibody binds to C1q (complement component 1,q subcomponent), stimulating the classical complement pathway and the formation of C3 convertase complexes [89][90][91,92]. In the next stage, membrane attack complex (MAC) assembly: complement protein cleavage and activation produces MAC, including C5b, C6, C7, C8, and C9 [93]. InAfter the next stage,at, cell lysis: MAC perforates the cancer cell membrane attack complex (MAC) assembly: complement protein cleavage and activation produces MAC, including C5b, C6, C7, C8, and C9, resulting in uncontrolled ion and water movement, cell lysis, and death [91][93]. After thFinat, cell lysis: MAC perforates thelly, opsonics and phagocytosis: when complement activation promotes opsonship, cancer cell membrane, resulting in uncontrolled ion and water movement, cell lysis, and death [91]s are targeted for destruction by phagocytes [92]. Finally, opsonics and phagocytosis: when complement activation promotes opsonship, cancer cells are targeted for destruction by phagocytes [90].
-
Neutralization of growth factors and inhibition of signaling pathways: Antibodies impede tumor cell proliferation, promote growth factors, and block signaling pathways that are critical for cancer cell survival and invasion [15].
Chapter 4.: Types and Ccharacteristics of Ccancer Vvaccines
4.1. Peptide Vaccines
4.1. Peptide vaccines
Peptide vaccines represent a potential cancer immunotherapy approach that uses short amino acid sequences derived from tumor-specific or tumor-associated antigens (TAAs) to elicit a targeted immune response against malignant cells [92][94]. TAAs, including differentiated antigens, overexpressed antigens, cancer/testis antigens, and mutant antigens, are viable targets for a variety of immunotherapy techniques, such as cancer vaccines, adoptive T cell therapies, and immune checkpoint inhibitors [93][94][95,96]. Peptide vaccines offer several advantages over whole protein or live attenuated vaccines, including ease of synthesis, specificity, and good safety because peptide vaccines are less likely to elicit an autoimmune response [95][96][97,98]. However, they have encountered limitations such as poor immunogenicity, rapid degradation in vivo, weak CD4+ T cell response, and challenges associated with immune evasion and tumor-induced immunosuppression [95][97]. To overcome these limitations, strategies include the addition of adjuvants, optimization of peptide sequences, enhancement of stability and immunogenicity with vectors, and combination of peptide vaccines with other immunotherapies [92][94]. Currently, relatively mature peptide vaccines include Nelipepimut-S (NeuVax), CIMAvax-EGF and MUC1-based peptide vaccines. Nelipepimut-S, also known as NeuVax, is a peptide vaccine targeting HER2/neu-expressing cancer cells primarily in patients with early-stage HER2 1+ and 2+ breast cancer who are not candidates for standard HER2 therapy [97][99]. It binds the E75 peptide of HER2/neu to GM-CSF as an adjuvant to enhance the immune response [98][100]. Although its scope may cover other HER2/neu cancers, such as ovarian and gastric cancers, clinical development has encountered obstacles [99][101]. Recent phase III trial results suggest that Nelipepimut-S continues to exhibit good efficacy and tolerability in breast cancer patients [100][102]. Similarly, CIMAvax-EGF, which targets non-small cell lung cancer (NSCLC) epidermal growth factor, merges recombinant EGF with protein carriers. It has shown promise for prolonging the lives of patients with advanced lung cancer and has completed phase III trials [101][102][103,104]. In addition, MUC1-based peptide vaccines focus on abnormally expressed glycoproteins in cancers such as breast and pancreatic cancer [103][105]. Although some agents have entered phase I and II trials with good safety and immunologic indicators, eliciting a robust clinical response remains complex, and combination therapies need to be explored [104][105][106,107]. Collectively, these vaccines represent cutting-edge cancer treatments, each with its own unique targets and stages of development.4.2. DNA/RNA-Based Vaccines
4.2. DNA/RNA-based vaccines
DNA/RNA-based tumor vaccines work by delivering genetic material encoding tumor antigens to host cells. This approach utilizes a variety of vectors, such as viral vectors, lipid nanoparticles, or naked nucleic acids, to stimulate the immune system to recognize and destroy cancer cells, resulting in an adaptive immune response [106][108]. These vaccines offer several advantages over traditional methods, including safety, ease of manufacture, robust immune response induction, and adaptability to modifications. They also offer the possibility of individualization to meet each patient's unique tumor profile [107][108][109,110]. Although they are promising, challenges such as efficient delivery and uptake of DNA/RNA into cells, the risks of autoimmune responses, and the limitations of personalized costs and time pose significant barriers [109][110][111][111,112,113]. However, recent advances are encouraging, including several vaccines and technological advances in clinical trials that have improved the effectiveness of vaccine delivery [112][114]. The field expects to use advances in genome sequencing, bioinformatics, and nanotechnology to overcome current limitations, eager to combine these potent vaccines with other immunotherapy strategies to achieve total cancer eradication [113][114][115,116]. Among them, technologies such as lipid nanoparticles (LNPs) are pioneering in mRNA COVID-19 vaccines and are being applied to cancer vaccine development to facilitate the delivery of tumor-specific antigens [115][116][117,118]. Electroporation and viral vectors (eg, adenovirus) enhance DNA/RNA uptake, while nonviral nanovectors and microneedle patches are designed to enhance this delivery without inducing a strong anti-vector response [117][118][119,120]. To reduce autoimmune risk, researchers emphasize tumor-specific antigen selection, sequence optimization to reduce cross-reactivity, and transient expression techniques such as those inherent to mRNA vaccines [119][120][121,122]. In addition, tolerable adjuvants and nanoparticles tailored for targeted delivery are being utilized to fine-tune the immune response, maximizing antitumor efficacy while minimizing collateral damage to healthy tissue [119][121][121,123]. Taken together, these innovations underscore the evolving landscape of cancer vaccine design, balancing effective tumor targeting with patient safety. CV9104 is an mRNA-based cancer vaccine targeting prostate cancer [122][124]. It progressed to a phase II trial of metastatic castration-resistant prostate cancer (mCRPC) [122][124]. This vaccine represents an innovative application of mRNA in oncology.4.3. Viral Vector-Based Vaccines
4.3. Viral vector-based vaccines
Viral vector-based oncology vaccines are a promising development in cancer immunotherapy [11]. These vaccines use the innate ability of the virus to infiltrate host cells and efficiently deliver tumor antigens, eliciting a strong targeted immune response [123][124][125][125,126,127]. This approach encourages host cells to produce tumor-specific or relevant antigens after infection, resulting in these antigens being exposed to T cells and subsequently initiating a strong defense against tumor cells [126][128]. One of the significant advantages of these vaccines is their ability to trigger effective cellular and humoral immune responses. They can also be designed to express multiple tumor antigens, expanding their range and enhancing the potency of the immune response [127][128][129,130]. Despite these benefits, certain challenges need to be addressed, such as the impact of pre-existing viral vector immunity and the logistical issues associated with mass production [129][130][131,132]. Among these challenges, pre-existing immunity to viral vectors poses significant challenges for their use in tumor vaccines, as the immune system may neutralize the vector before the vector has a therapeutic effect [131][133]. ]。 To address this, researchers are exploring a range of strategies: using rare or novel viral vectors with limited human exposure, pseudotyping to alter viral envelope proteins, adopting heterologous prime-augmentation strategies with different vectors, genetic modification of viral capsids to reduce recognizability, co-administration with immunomodulators to temporarily suppress certain immune responses, choosing non-intravenous delivery routes such as intratumoral administration to avoid high antibody concentrations, and adjusting the dose, or using high vector doses to overcome neutralization, Low doses are either repeated to evade immunodetection [132][133][134][135][134,135,136,137]. In addition, adjuvants are being explored to shift the focus of the immune response from the vehicle to the delivered tumor antigen [136][137][138,139]. These multifaceted approaches aim to optimize the efficacy of viral vector-based oncology vaccines in the face of pre-existing immunity. OncoVEXGM-CSF or T-VEC (T-VEC) is an oncolytic HSV-1 vaccine modified for tumor selectivity and GM-CSF production, primarily against melanoma [138][140]. Following a successful phase III trial, the drug received FDA approval for unresectable recurrent melanoma [138][140]. CG0070 is another adenovirus-based vaccine for selective replication in cancer cells with defects in the Rb pathway and targeting bladder cancer [139][141]. LV305 is a lentivirus-based vaccine that delivers the NY-ESO-1 antigen gene to dendritic cells to target NY-ESO-1-expressing cancers such as melanoma and sarcoma [140][142]. JX-594 or Pexa-Vec is a vaccinia virus-based vaccine modified to express GM-CSF and selectively target cancer cells with high thymidine kinase activity, and several trials have been conducted, including a phase III trial for hepatocellular carcinoma [141][142][143][143,144,145]. These represent innovative intersections of viral therapy and immunotherapy in oncology.4.4. Dendritic Cell-Based Vaccines
4.4. Dendritic cell-based vaccines
Dendritic cells (DCs) mediate immune responses by connecting the innate and adaptive immune systems and are critical in antigen presentation and subsequent T cell activation, so they have been found to be substantially relevant to cancer immunotherapy strategies [18][144][18,146]. This stems from a DC-based cancer vaccine that utilizes DCs loaded with tumor-associated antigens (TAAs) to promote a robust immune response against cancer cells [145][147]. Methods for loading these DCs with TAA are varied, from the use of tumor lysates and synthetic peptides to mRNAs encoding tumor antigens [146][148]. Clinical trials highlight the promise of these DC-based vaccines in a variety of cancers. Despite this exciting potential, challenges remain, including technical difficulties in DC vaccine production, changes in vaccine efficacy, immunosuppressive tumor microenvironments, and lack of reliable biomarkers for patient selection [147][149]. However, recent advances in personalized cancer immunotherapies, such as neoantigen-based vaccines, offer promising opportunities for DC-based vaccines that may be enhanced in combination with other therapeutics [8]. In a recent study, researchers introduced a metabolic glycan labeling technique using azigo to enhance the DC vaccine [148][150]. This method promotes not only DC activation and antigen presentation, but also efficient conjugation of cytokines [148][150]. In addition, it is expected to be widely used in various tumors, provide a platform for regulating the interaction between DCs and other immune cells, and enhance the anti-tumor efficacy of dendritic cell vaccines. Prominent representatives include Provenge and DCVax-L. Provenge (sipuleucel-T) is an FDA-approved autologous cell immunotherapy for advanced prostate cancer [5]. It uses peripheral blood mononuclear cells (PBMCs) of patients exposed to the fusion protein PA2024, which binds antigens from prostate cancer cells to the immune activator GM-CSF, eliciting an immune response against antigen-expressing prostate cancer cells [149][150][151][152][153][151, 152, 153, 154, 155]. DCVax-L, on the other hand, is an autologous dendritic cell vaccine against glioblastoma multiforme (GBM) [154][156]. The vaccine prepares the patient's dendritic cells by loading tumor lysates from the patient's own tumor tissue, enabling the immune system to recognize and attack the corresponding cancer cells [154][156]. Both vaccines use dendritic cells to target cancer, but their clinical history and disease targets differ (Table 1<>).4.5. Whole Cell Vaccines
4.5. Whole cell vaccines
Whole-cell-based vaccines provide a comprehensive approach to cancer immunotherapy by binding a large number of tumor-associated antigens to stimulate an efficient immune response. Mechanistically, these vaccines utilize irradiated tumor cells (autologous or allogeneic) to expose the immune system to the full antigen pool of the tumor [155][157], thereby inducing a specific and multivalent immune response against a range of tumor antigens [156][158]. This strategy provides a broad spectrum of known and unknown tumor antigens, avoids antigen loss or down-regulation (the typical escape mechanism employed by tumors [155][157]), and eliminates the need to identify specific antigens for each patient, which can be time-consuming and expensive [157][159]. However, there are limitations. The production of autologous whole cell vaccines can be labor-intensive and individualized, so tumor cells need to be isolated and cultured from each patient [157][159]. Given that the immunosuppressive tumor microenvironment limits vaccine efficacy, these vaccines often need to be co-administered with adjuvants or immunomodulators to improve their immunogenicity [8][157][8,159]. Potential autoantigen-induced autoimmunity in vaccine formulations is also of concern [156][158]. Therefore, while whole-cell-based vaccines offer a promising approach to cancer immunotherapy, these strategies need to be further optimized and refined to address these challenges and limitations. Representatives include GVAX, Canvaxin, and Oncophage. GVAX is a whole-cell tumor vaccine, utilizing tumor cells genetically modified to secrete GM-CSF (an immune stimulant), and has been explored for cancers like pancreatic and prostate cancers, with mixed outcomes in later-phase trials [158][159][160,161]. Canvaxin, aimed at melanoma, combines irradiated autologous and allogeneic melanoma cells with the BCG adjuvant, but it failed to show significant survival benefits in a phase III trial for advanced melanoma [113][160][115,162]. Oncophage (Vitespen) is derived from patient-specific tumor heat shock proteins (HSPs) and primarily targets renal-cell carcinoma and melanoma [161][162][163,164]. It completed phase III trials with mixed results but secured approval in Russia for the treatment of kidney cancer [163][165]. While these vaccines showcase varied cancer immunotherapy strategies, each has faced challenges in late-stage clinical evaluations. (Table 1)Table 1.
Below is a tabular list of various tumor vaccines in the last decade.
| Types of Tumor Vaccines | Strengths | Weaknesses | Examples | Mechanisms of Action | Effects | Limitations | References |
|---|---|---|---|---|---|---|---|
| Peptide vaccines |
|
|
Nelipepimut-S (NeuVax) | HER2-derived peptide vaccine | Activation of T-cell response | Limited overall survival improvement | [164][166] |
| CIMAvax-EGF | EGF-based peptide vaccine | Inhibition of EGF signaling | No direct tumor targeting | [101][103] | |||
| MUC1-based peptide vaccine | Targeting MUC1 tumor-associated antigens | Enhanced immune response | Heterogeneous patient response | [165][167] | |||
| DNA/RNA-based vaccines |
|
|
CV9104 (CureVac) | Uses mRNA to encode six antigens overexpressed in prostate cancer | Induced antigen-specific immune responses in early clinical trials | Efficacy in late-stage trials yet to be established; possibility of inducing autoimmune responses | [166][168] |
| Viral-vector-based vaccines |
|
|
Adenovirus-based vaccines (OncoVEXGM-CSF, CG0070) | Adenoviruses are modified to express a tumor-specific antigen or an immunomodulatory molecule; these stimulate an immune response against the tumor | Effective in stimulating an immune response against the tumor | Immune response to the viral vector can limit repeat dosing | [167][169] |
| Lentivirus-based vaccines (LV305) | Lentiviruses are engineered to deliver tumor-specific antigens to dendritic cells to stimulate a T-cell response | Successful in initiating T-cell responses | Safety concerns over integration into the host genome | [23] | |||
| Vaccinia-virus-based vaccines (JX-594) |
Vaccinia viruses are genetically engineered to express a tumor antigen and/or immunostimulatory molecule; they can directly lyse cancer cells | Showed antitumor activity and were well tolerated in clinical trials | Immune response to the viral vector can limit its effectiveness | [168][170] | |||
| Dendritic-cell-based vaccines |
|
|
Provenge (Sipuleucel-T) | The patient’s own dendritic cells are exposed to a fusion protein (prostatic acid phosphatase linked to an immune cell stimulating factor) | Extended overall survival in metastatic castration-resistant prostate cancer | Limited clinical benefits, high cost, and complex manufacturing process | [5] |
| DCVax-L | Autologous dendritic cells are pulsed with tumor lysate | Prolonged progression-free survival in glioblastoma multiforme (GBM) patients | Not FDA-approved; requires personalized manufacturing | [169][171] | |||
| Whole-cell-based vaccines |
|
|
GVAX | Utilizes autologous/allogeneic tumor cells that have been genetically modified to secrete the immune-stimulating cytokine GM-CSF | Demonstrated a significant immune response against cancer, studied in various types of cancer, including pancreatic and prostate cancers | Production can be labor-intensive and personalized; often requires co-administration with adjuvants or other immunomodulatory agents to enhance their immunogenicity | [157][170][159,172] |
| Canvaxin | Allogeneic melanoma cells mixed with Bacillus Calmette–Guérin (BCG) to stimulate immune response | Intended for melanoma treatment, but development discontinued due to insufficient effectiveness | Limited efficacy; potential for BCG-related side effects | [171][173] | |||
| Oncophage (Vitespen) | Uses heat shock proteins (gp96) derived from the patient’s tumor as an autologous vaccine | Showed efficacy in extending disease-free survival in certain patients with kidney cancer and melanoma | Not universally effective; personalized manufacturing can be labor-intensive | [172][173][174,175] |
The preceding table encapsulates seminal instances of assorted classifications of cancer vaccines, encompassing peptide-based, DNA/RNA-based, viral-vector-based, dendritic-cell-based, and whole-cell-based vaccines. Each paradigm is delineated in exhaustive detail, supplemented by pertinent bibliographical citations for subsequent scholarly inquiry.
The preceding table encapsulates seminal instances of assorted classifications of cancer vaccines, encompassing peptide-based, DNA/RNA-based, viral-vector-based, dendritic-cell-based, and whole-cell-based vaccines. Each paradigm is delineated in exhaustive detail, supplemented by pertinent bibliographical citations for subsequent scholarly inquiry.