Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Gustavo Curaqueo.
Composting is described as a sustainable alternative to organic waste reuse from the agricultural and household sectors. The organic matter degradation and stabilization product presents great variability due to the waste composition used.
- compost
- UV-Vis spectroscopy
- IR spectroscopy
- fluorescence spectroscopy
1. Introduction
The worldwide population growth is estimated at around 26% by 2050 [1]. This would imply an increase in food production of almost 70% through agricultural systems [2]. This scenario represents a challenge regarding soil nutrition, the capacity to support the intensification level and ecosystem pressure, and the increased organic wastes generated by the industrial and/or household sector [3]. Therefore, an increase in agricultural production could impact soil degradation due to the application of agrochemicals and intensive management [4]. Thus, from the crop’s perspective, the nutrient uptake from the soil system can become a limiting factor [5]. Hence, sustainable alternatives are required that are capable of addressing the recycling of nutrients while minimizing the environmental impact [6].
In this sense, composting is an economic and controlled method for handling and converting organic matter from waste. This is defined as an aerobic biological control process of the decomposition of organic matter in a stable amendment with high fertilization value [7]. This occurs as a natural process that can be enhanced by adding different types of organic or biological substrates that optimize the development of microbial communities and favor the degradation of organic matter [8]. This generates a product high in bioavailable nutrients, free of pathogenic microorganisms and phytotoxicity with potential agronomic use [9], which is considered a natural alternative to chemical fertilizers, involving the concept of the circular economy [10].
Furthermore, due to the variability and characteristics of the residues used in the composting process, different parameters are analyzed to determine the stability, maturity, and quality of the composted product in each bio-oxidative and maturity stage [11]. This includes various analytical methodologies such as chromatography techniques, spectroscopy, or microbiological sequencing associated with the metabolic response, which allow the evaluation of their physical-chemical and biochemical process [12,13][12][13]. However, using a single method to investigate the decomposition process is insufficient. Integrating spectroscopic techniques to complement traditional methods is recommended as a better way to characterize the humification degree and organic matter stability [14]. Thus, spectroscopy methods have been incorporated due to their multiple advantages, such as short analysis time, minimal sample quantity, non-destructive sample, environmentally friendly, and experimental reproducibility [15].
These analyses include UV-Vis spectroscopy to evaluate the organic matter humification degree [16]. Infra-red (IR) spectroscopy for the monitoring of functional groups associated with the transformation of organic substances [17]. Fluorescence spectroscopy for the analysis of fluorescent compounds such as amino acids, proteins, and humic and fulvic substances [18]. Nuclear magnetic resonance (NMR) spectroscopy is associated with the study of carbon structures to analyze the transformation of aliphatic compounds and aromaticity degree [19]. Thus, spectroscopy methods are established as comparative and descriptive methods that allow the complementation of results for monitoring the composting process and its different residues [15].
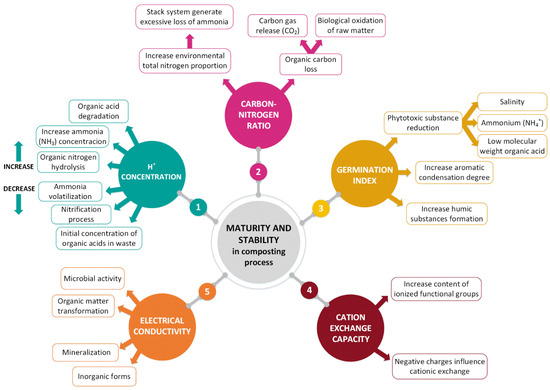
2. Composting Process
Composting has become an important alternative worldwide for the reduction and reuse of waste generated in the industrial and household sectors. Highlighted as a sustainable, economical, and controlled method for managing organic matter degradation [24][20] to increase eco-friendly practices and circular economy within productive processes [25][21]. It is defined as an aerobic biological process of organic matter decomposition to a stable form with a high content of humic and fulvic substances [21][22]. A transformation that occurs naturally and can be enhanced or accelerated by adding different organic waste types and inoculums that optimize microbial growth and favor substrate degradation [26,27][23][24]. On the other hand, the process effectiveness and product quality will be related to the development of each stage. First, the bio-oxidative stage consists of three phases (mesophilic, thermophilic, and cooling) differentiated by the temperature reached during the decomposition process of simple and complex organic matter [28][25]. Then, it ends with the maturity stage, where the reorganization and condensation of organic matter into stabilized compounds such as humic and fulvic substances is highlighted [29][26]. Thus, among the characteristics provided by mature compost to the soil matrix are its physical properties. It highlights that facilitating soil management, generating an adequate porosity that improves water retention and gas exchange, reduces the risks of erosion, improves the physicochemical characteristics of the soil, enriches the movement of nutrients, helps regulate soil temperature, and reduces water evaporation, which regulates soil moisture by maintaining a stable balance of microorganisms [7]. Optimal development of these properties requires monitoring some key parameters associated with maturity and stability parameters in the composting process (Figure 1). Factors such as pH and temperature are the most important characteristics during composting [26][23]. These parameters define the metabolic processes and the succession of microbial communities at each stage [35][27]. Initially, vegetable or food waste is characterized by lowering the pH in the pile due to the release of organic acids in the early stages of degradation [36][28]. Also, incorrect storage of these wastes can cause the accumulation of acidic substances due to fermentation processes. Consequently, low pH levels in the early stages of composting can inhibit the degradation of organic matter [37][29] and decrease the temperature of the pile, delaying the transition to the thermophilic stage.
Figure 1.
General scheme of physicochemical parameters associated with the composting process.
In turn, organic matter is a key factor to consider during the composting process, a parameter related to the C/N ratio that controls the evolution of the microbial community and humification processes [5]. Optimal initial C/N values range from 20 to 30, which avoids N mineralization and immobilization processes [38][30]. Consequently, the decrease in organic matter content, CO2 formation, N ammonification processes, and volatilization as NH3 form generate a variation in the C/N ratio during the composting process [29][26].
On the other hand, electrical conductivity reflects the waste mineralization process, and this is associated with salt formation due to the organic matter transformation into inorganic matter by the action of microorganisms [39][31]. Moreover, cation exchange capacity increases due to the content of polar functional groups such as hydroxyl, carboxyl, and methoxy by the action of raw material biological oxidation [40][32].
Furthermore, Figure 2 summarizes the main products obtained by the degradation of microbial communities at each stage of the composting process. These results have been adapted from [21,42][22][33] in which different ways of formation of humic compounds are proposed considering the environmental variations. The synthesis of these substances is characterized by their particular resistance to degradation and their beneficial properties of agronomic interest in soil quality and against environmental phytotoxicity [21][22].
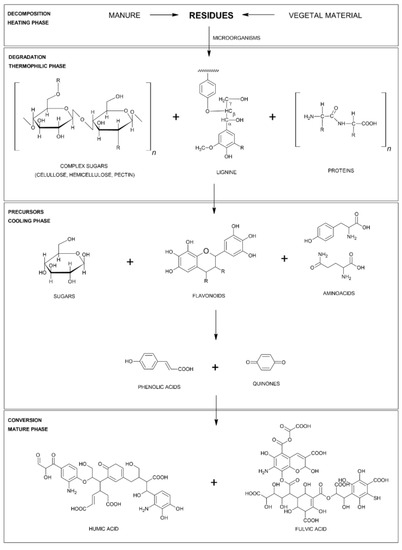

Figure 2. Transformation mechanism of organic matter in each stage of the composting process [21,42].
3. Ultraviolet-Visible Spectroscopy (UV-Vis)
UV-Vis spectroscopy in composting allows for the detection of the variation in the absorption bands during the process. These bands are related to the degradation of organic matter. They are interpreted as the change of absorption at a given wavelength by the effect of the variation of the electronic structures of the compounds [46]. The UV-Vis spectra have different absorption regions: 280 nm indicates the beginning of the transformation of aliphatic compounds and lignins, 465 nm indicates the beginning of the humification process by depolymerization of complex organic molecules, and 665 nm indicates process humification with high oxygen content and aromatic groups [47,48].
These parameters have also been interpreted as the relationship ratio between the regions described. The first one indicates the ratio between the beginning of the organic matter transformation process and the humification degree. It is expected that the latter will increase at the end of the process, and the transformation process will decrease over time [52]. Thus, research reports the relationship E250/E203 as a measure to evaluate the substitution of aromatic rings and the presence of aliphatic groups. Therefore, polar functional groups are found at higher rates in aromatic rings, such as hydroxyl, carbonyl, and carboxyl groups [53,54].
In turn, another parameter has been described to evaluate organic matter transformation. Specific ultraviolet absorbance (SUVA) is defined as absorbance at a given wavelength normalized by dissolved organic carbon concentration (DOC) [58]. Aromaticity degree can be associated with ranges between 250–280 nm, and SUVA
500) has been described. This index investigates the organic matter sources [74] and correlates with humic substances origin. A value less than 1.4 indicates terrestrially derived humic substances, and larger than 1.90 indicates microbially derived humic substances [75].
Transformation mechanism of organic matter in each stage of the composting process [22][33].
254 is directly related to the percentage of aromaticity of humic substances and molecular weight [59]. 3. Ultraviolet-Visible Spectroscopy (UV-Vis)
UV-Vis spectroscopy in composting allows for the detection of the variation in the absorption bands during the process. These bands are related to the degradation of organic matter. They are interpreted as the change of absorption at a given wavelength by the effect of the variation of the electronic structures of the compounds [34]. The UV-Vis spectra have different absorption regions: 280 nm indicates the beginning of the transformation of aliphatic compounds and lignins, 465 nm indicates the beginning of the humification process by depolymerization of complex organic molecules, and 665 nm indicates process humification with high oxygen content and aromatic groups [35][36].
These parameters have also been interpreted as the relationship ratio between the regions described. The first one indicates the ratio between the beginning of the organic matter transformation process and the humification degree. It is expected that the latter will increase at the end of the process, and the transformation process will decrease over time [37]. Thus, research reports the relationship E250/E203 as a measure to evaluate the substitution of aromatic rings and the presence of aliphatic groups. Therefore, polar functional groups are found at higher rates in aromatic rings, such as hydroxyl, carbonyl, and carboxyl groups [38][39].
In turn, another parameter has been described to evaluate organic matter transformation. Specific ultraviolet absorbance (SUVA) is defined as absorbance at a given wavelength normalized by dissolved organic carbon concentration (DOC) [40]. Aromaticity degree can be associated with ranges between 250–280 nm, and SUVA
4. Infrared Spectroscopy (IR)
IR spectroscopy is obtained from the molecular vibration generated by the different functional groups [61]. In the characterization of the composting process, peaks have been described that can contribute to monitoring the conversion stages of organic matter.IR spectroscopy is obtained from the molecular vibration generated by the different functional groups [42]. In the characterization of the composting process, peaks have been described that can contribute to monitoring the conversion stages of organic matter.
Table 2 describes the main functional groups attributed to these bands and their vibrations. The bands 2964 to 2930 cm1 describes the main functional groups attributed to these bands and their vibrations. The bands 2964 to 2930 cm
−1 describe aliphatic carbon groups attributed to the presence of fatty acids [62]. The constant presence of this signal may be due to the concentration of biodegradable plant material composed of lignins, cutins, and suberins [63]. describe aliphatic carbon groups attributed to the presence of fatty acids [43]. The constant presence of this signal may be due to the concentration of biodegradable plant material composed of lignins, cutins, and suberins [44].
The signal between 1590 to 1504 cm
−1 corresponds to the presence of amide II groups and the lignin and cellulose present in residues containing plant materials such as plants or wood, which are rich in this compound [64]. Another typical band of lignin is caused by the vibration of the aromatic (C=C) skeleton [65]. On the other hand, a band of protein origin can be found in nitrogen-rich composts associated with amide II groups [66]. corresponds to the presence of amide II groups and the lignin and cellulose present in residues containing plant materials such as plants or wood, which are rich in this compound [45]. Another typical band of lignin is caused by the vibration of the aromatic (C=C) skeleton [46]. On the other hand, a band of protein origin can be found in nitrogen-rich composts associated with amide II groups [47].
Table 21. Summary wavenumber described for the evaluation of the composting process.
| Wavenumber (cm−1) | Description | Reference |
|---|---|---|
| 3437–3263 | This occurs due to stretching vibration produced by OH groups of alcohols, phenols, and organic acids. | [28,52,53,57,66][25][37][38][47][48] |
| 2964–2930 | Bands corresponding to C-H stretching and asymmetric vibrations in aliphatic structures. | |
| 1652–1642 | Bands produced by stretching vibrations of C=C bonds in aromatic structures by ketone groups such as quinones and amide groups (C-N). | |
| 1590–1500 | Obtained due to the deformation and vibration stretching of amide II groups (N-H) and (C-N) of secondary amides, respectively. | |
| 1408 | Intensity assigned to the vibration asymmetric stretching of carboxyl groups (C-O). | |
| 1387 | Described by the deformation of phenolic OH groups and aromatic alcohols, by the asymmetric stretching of carboxyl ions (COO−) of disubstituted aromatic rings, and by the presence of inorganic nitrogen as nitrates. | |
| 1116–1003 | Characteristics of C-O bond stretching vibrations in structures such as polysaccharides, ethers, and secondary alcohols. |
5. Fluorescence Spectroscopy
Fluorescence spectroscopy is a nondestructive method for characterizing organic matter in relation to its important fluorescent compounds, which include humic-like, fulvic-like, and proteinaceous substances [69]. This method is based on the signal interpretation by light emission effect in the process of electronic de-excitation of conjugated systems [70]. It is used to evaluate the humification degree of organic matter in the composting process. Fluorescence in organic matter is related to the condensed aromatic rings presence of unsaturated aliphatic carbon chains [71]. Fluorescence bands at long wavelengths have been described by high molecular weight and complex structural compounds with a high conjugation degree, and fluorescence bands at shorter wavelengths have been described for simple structures and small degrees of conjugated chromophores due to a low degree of aromatic polycondensation [72]. Therefore, the stability of organic matter and the increase in its condensation degree is associated with a chemically stable structure with greater residence in the environment, which contributes to enhancing the structure and fertility of the soil [73].5.1. Emission and Excitation Spectra
Emission spectra are generated due to the sample's exposure to a specific excitation wavelength and are represented by one or more emission bands. The excitation wavelength for obtaining emission spectra in the composting process analysis is 254 nm. The results reported a fluorescence peak at 340 nm in the initial phase, changing to 430 nm during the process, indicating an increase in the aromatic group condensation due to the maturation of the compost [55]. Thus, the relationship between emission spectra at 450 and 500 nm with an excitation wavelength of 370 nm (FFluorescence spectroscopy is a nondestructive method for characterizing organic matter in relation to its important fluorescent compounds, which include humic-like, fulvic-like, and proteinaceous substances [49]. This method is based on the signal interpretation by light emission effect in the process of electronic de-excitation of conjugated systems [50]. It is used to evaluate the humification degree of organic matter in the composting process. Fluorescence in organic matter is related to the condensed aromatic rings presence of unsaturated aliphatic carbon chains [51]. Fluorescence bands at long wavelengths have been described by high molecular weight and complex structural compounds with a high conjugation degree, and fluorescence bands at shorter wavelengths have been described for simple structures and small degrees of conjugated chromophores due to a low degree of aromatic polycondensation [52]. Therefore, the stability of organic matter and the increase in its condensation degree is associated with a chemically stable structure with greater residence in the environment, which contributes to enhancing the structure and fertility of the soil [53].
4505.1. Emission and Excitation Spectra
Emission spectra are generated due to the sample's exposure to a specific excitation wavelength and are represented by one or more emission bands. The excitation wavelength for obtaining emission spectra in the composting process analysis is 254 nm. The results reported a fluorescence peak at 340 nm in the initial phase, changing to 430 nm during the process, indicating an increase in the aromatic group condensation due to the maturation of the compost [54]. Thus, the relationship between emission spectra at 450 and 500 nm with an excitation wavelength of 370 nm (F/F
Contrary to emission spectra, excitation spectra are determined using a fixed emission wavelength and the excitation scan at different wavelengths. Moreover, the results obtained can be assimilated to the UV-Vis absorption spectra, the information from which can be used to generate the emission spectra [78][57]. Studies [14] describe a composting of municipal solid waste, where they evaluate an excitation spectrum of 300 to 500 nm using an emission wavelength of 520 nm. The results reported four major peaks, of which only two were representative. One of these, around 436 nm, was attributed to aromatic rings bearing electron donor groups, and the second at 383 nm, possibly due to fluorophores originating from the polycondensation of carbonyl groups and lignin-derived phenolic structures [79][58]. These two peaks were associated through the fluorescence ratio determination (I436/I383) that showed an inverse correlation to the SUVA254 indicator and the emission at 351 nm, which are related to the abundance of aromatic carbons and the organic matter humification, respectively [80,81][59][60].
5.2. Synchronous-Scan Spectra
5.2. Synchronous-Scan Spectra
Synchronous-scan spectra are generated from the simultaneous detection of excitation and emission scans at a predetermined wavelength difference, favoring signal amplification in trace compounds [82][61]. In turn, compared with other fluorescence analyses, it is possible to obtain a unique and clear spectrum associated with specific functional groups and chemical structures [83][62]. Synchronous fluorescence represents the spectra summation of different fluorophores in dissolved organic matter and exhibits better resolution than excitation and emission spectra [14]. This analysis reports the presence of characteristic peaks for the composting process associated with organic matter biodegradation and biosynthesis of humic-like substances [28][25]. Furthermore, a Peak B was reported between 308–365 nm attributed to the presence of fulvic-like indicates the presence of polycyclic aromatics with three to four fused benzene rings and two to three conjugated systems in unsaturated aliphatic structures [84,85,86][63][64][65]. The identification of humic-like substances has been reported in Peak C 363–595 nm [14,28][14][25] related to the presence of polycyclic aromatic compounds with approximately 5–7 fused benzene rings tend to increase in intensity as the compost matures [85,87][64][66].5.3. Excitation-Emission Matrix
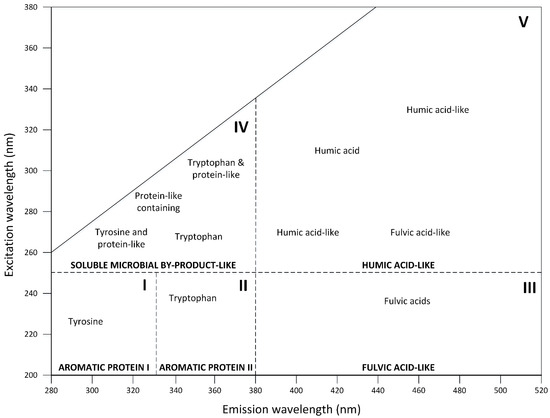
Excitation–emission matrix (EEM) spectra are obtained by subsequently scanning the emission spectra range by increasing the excitation wavelength. Figure 3 compiles the Excitation-Emission matrix results reported by [89][67]. These results are represented in a graph divided into five regions. The dissolved organic matter of each region represents particular structures, in the case of the composting process, which can increase or decrease the intensity of fluorescence as it moves towards its maturity stage [68]. These peaks have been mainly associated with organic compounds such as humic and fulvic acid-like amino acids, proteins, or phenolic structures [90][69].
Figure 3. Location of structures for each region in an Excitation-Emission Matrix (EEM) of organic matter (adapted from [89]).
Location of structures for each region in an Excitation-Emission Matrix (EEM) of organic matter (adapted from [67]).
In this sense, Regions I and II are related to amino acids derived from simple aromatic proteins such as tyrosine and tryptophan with peaks at shorter excitation-emission wavelengths (<250 nm/<350 nm); Region III is described by peaks at shorter excitation wavelengths and longer emission wavelengths (<250 nm/>380 nm) are related to fulvic substances; Region IV is associated with microbial by-product-like material as a result of microbial protein degradation, reporting peaks at intermediate excitation, and shorter emission wavelengths (250~280 nm/<380 nm); Region V includes a longer excitation range and longer emission wavelengths (>280 nm/>380 nm) and is directly associated with humic substances and may even contain part of fulvic substances fluorescence, depending on the intensity of these [14,68,91,92][14][68][70][71].
6. Nuclear Magnetic Resonance (13C NMR)
Nuclear magnetic resonance (NMR) is produced by the reorganization of nuclear spin due to the application of a magnetic field to the nuclei at the low-energy level, which will absorb electromagnetic energy and transit to the high-energy level. When the electromagnetic field disappears, the nucleus releases the absorbed energy, returning to its low-energy state in a process called relaxation. This energy is detected as radiofrequency detects the presence of carbon and its relationship with the functional groups [97][72].
During the composting process, chemical shifts have been described to monitor the organic matter decomposition and to evaluate structures stabilized over time. Figure 4 summarizes the main chemical shifts reported in the literature, corresponding to carbon monitoring as Alkyl-C (45-0 ppm), methoxyl C (60–45 ppm), aliphatic C substituted with O or N (110–60 ppm), aromatic C (110–145 ppm), phenolic C and aromatic ether substituted with O or N (160–145 ppm) and carbonyl C, carboxyl C, quinones, ester, and amides carbon (210–160 ppm) [19,52,98,99][19][37][73][74].
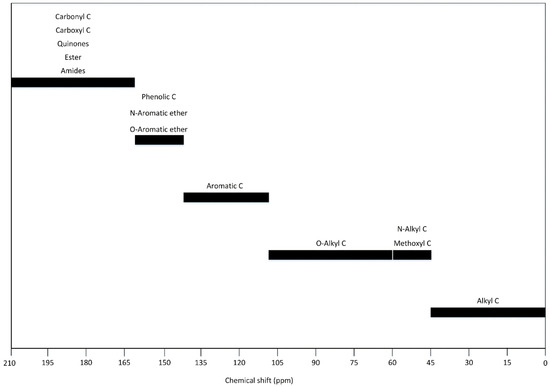

Figure 4. Signal distribution 13C NMR about the chemical shift described in the composting process [19,52].
7. Conclusions
In conclusion, the composting process has been established as a sustainable alternative within the circular economy for the reduction and treatment of organic waste. Also, it is a useful method for generating soil amendments capable of recycling nutrients from organic waste generated by the agricultural and household sectors. The stabilization of organic matter and correct degradation of complex structures allows the generation of an optimal product, minimizing the probability of environmental impact due to the persistence of phytopathogenic agents or the presence of toxic substances in concentrations that affect soil quality. Spectroscopy techniques such as UV-Vis, IR, Fluorescence, and 13C NMR allow monitoring of the different stages of the composting, showing the stabilization and degradation of organic matter in highly condensed compounds. Each technique establishes a relationship concerning the functional groups and elucidates the part of the structures that are formed as a result of the action of microorganisms on organic waste. Therefore, it is considered that these methodologies establish reliable and complementary quantitative indicators to establish the necessary quality criteria required by a compost.
Signal distribution 13C NMR about the chemical shift described in the composting process [19][37].
7. Conclusions
In conclusion, the composting process has been established as a sustainable alternative within the circular economy for the reduction and treatment of organic waste. Also, it is a useful method for generating soil amendments capable of recycling nutrients from organic waste generated by the agricultural and household sectors. The stabilization of organic matter and correct degradation of complex structures allows the generation of an optimal product, minimizing the probability of environmental impact due to the persistence of phytopathogenic agents or the presence of toxic substances in concentrations that affect soil quality. Spectroscopy techniques such as UV-Vis, IR, Fluorescence, and 13C NMR allow monitoring of the different stages of the composting, showing the stabilization and degradation of organic matter in highly condensed compounds. Each technique establishes a relationship concerning the functional groups and elucidates the part of the structures that are formed as a result of the action of microorganisms on organic waste. Therefore, it is considered that these methodologies establish reliable and complementary quantitative indicators to establish the necessary quality criteria required by a compost.
References
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank Publications: Washington, DC, USA, 2018; ISBN 9781464813290.
- Hunter, M.C.; Smith, R.G.; Schipanski, M.E.; Atwood, L.W.; Mortensen, D.A. Agriculture in 2050: Recalibrating Targets for Sustainable Intensification. Bioscience 2017, 67, 386–391.
- Pergola, M.; Persiani, A.; Palese, A.M.; Di Meo, V.; Pastore, V.; D’Adamo, C.; Celano, G. Composting: The Way for a Sustainable Agriculture. Appl. Soil Ecol. 2018, 123, 744–750.
- Sharma, N.; Singhvi, R. Effects of Chemical Fertilizers and Pesticides on Human Health and Environment: A Review. Int. J. Agric. Environ. Biotechnol. 2017, 10, 675–680.
- Wang, R.; Yao, Z.; Lei, Y. Modeling of Soil Available Phosphorus Surplus in an Intensive Wheat–Maize Rotation Production Area of the North China Plain. Agric. Ecosyst. Environ. 2019, 269, 22–29.
- Wei, Y.; Li, J.; Shi, D.; Liu, G.; Zhao, Y.; Shimaoka, T. Environmental Challenges Impeding the Composting of Biodegradable Municipal Solid Waste: A Critical Review. Resour. Conserv. Recycl. 2017, 122, 51–65.
- Sánchez, Ó.J.; Ospina, D.A.; Montoya, S. Compost Supplementation with Nutrients and Microorganisms in Composting Process. Waste Manag. 2017, 69, 136–153.
- Nakasaki, K.; Hirai, H.; Mimoto, H.; Quyen, T.N.M.; Koyama, M.; Takeda, K. Succession of Microbial Community during Vigorous Organic Matter Degradation in the Primary Fermentation Stage of Food Waste Composting. Sci. Total Environ. 2019, 671, 1237–1244.
- Sanasam, S.D.; Talukdar, N.C. Quality Compost Production from Municipality Biowaste in Mix with Rice Straw, Cow Dung, and Earthworm Eisenia Fetida. Compost Sci. Util. 2017, 25, 141–151.
- Curaqueo, G.; Riquelme, P.; Carmona, E.; Pérez-San Martín, A.; González, A. Composting with Industrial and Domiciliary Ashes in Temuco, Chile. IOP Conf. Ser. Earth Environ. Sci. 2020, 503, 012026.
- Akratos, C.S.; Tekerlekopoulou, A.G.; Vasiliadou, I.A.; Vayenas, D.V. Cocomposting of Olive Mill Waste for the Production of Soil Amendments; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128053140.
- Torres-Climent, A.; Gomis, P.; Martín-Mata, J.; Bustamante, M.A.; Marhuenda-Egea, F.C.; Pérez-Murcia, M.D.; Pérez-Espinosa, A.; Paredes, C.; Moral, R. Chemical, Thermal and Spectroscopic Methods to Assess Biodegradation of Winery-Distillery Wastes during Composting. PLoS ONE 2015, 10, e0138925.
- Ma, C.; Hu, B.; Wei, M.-B.; Zhao, J.-H.; Zhang, H.-Z. Influence of Matured Compost Inoculation on Sewage Sludge Composting: Enzyme Activity, Bacterial and Fungal Community Succession. Bioresour. Technol. 2019, 294, 122165.
- He, X.; Xi, B.; Wei, Z.; Guo, X.; Li, M.; An, D.; Liu, H. Spectroscopic Characterization of Water Extractable Organic Matter during Composting of Municipal Solid Waste. Chemosphere 2011, 82, 541–548.
- Biyada, S.; Merzouki, M.; Elkarrach, K.; Benlemlih, M. Spectroscopic Characterization of Organic Matter Transformation during Composting of Textile Solid Waste Using UV–Visible Spectroscopy, Infrared Spectroscopy and X-Ray Diffraction (XRD). Microchem. J. 2020, 159, 105314.
- Guo, X.; Li, C.; Zhu, Q.; Huang, T.; Cai, Y.; Li, N.; Liu, J.; Tan, X. Characterization of Dissolved Organic Matter from Biogas Residue Composting Using Spectroscopic Techniques. Waste Manag. 2018, 78, 301–309.
- Aranganathan, L.; Rajasree, S.R.R.; Suman, T.Y.; Remya, R.R.; Gayathri, S.; Jayaseelan, C.; Karthih, M.G.; Gobalakrishnan, M. Comparison of Molecular Characteristics of Type A Humic Acids Derived from Fish Waste and Sugarcane Bagasse Co-Compost Influenced by Various Alkaline Extraction Protocols. Microchem. J. 2019, 149, 104038.
- Zhang, S.; Chen, Z.; Wen, Q.; Ma, J.; He, Z. Assessment of Maturity during Co-Composting of Penicillin Mycelial Dreg via Fluorescence Excitation-Emission Matrix Spectra: Characteristics of Chemical and Fluorescent Parameters of Water-Extractable Organic Matter. Chemosphere 2016, 155, 358–366.
- Cao, Y.; Wang, J.; Huang, H.; Sun, E.; Butterly, C.; Xu, Y.; He, H.; Zhang, J.; Chang, Z. Spectroscopic Evidence for Hyperthermophilic Pretreatment Intensifying Humification during Pig Manure and Rice Straw Composting. Bioresour. Technol. 2019, 294, 122131.
- Idrovo-Novillo, J.; Gavilanes-Terán, I.; Bustamante, M.A.; Paredes, C. Composting as a Method to Recycle Renewable Plant Resources Back to the Ornamental Plant Industry: Agronomic and Economic Assessment of Composts. Process Saf. Environ. Prot. 2018, 116, 388–395.
- Chiarelotto, M.; Restrepo, J.C.P.S.; Lorin, H.E.F.; Damaceno, F.M. Composting Organic Waste from the Broiler Production Chain: A Perspective for the Circular Economy. J. Clean. Prod. 2021, 329, 129717.
- Guo, X.-X.; Liu, H.-T.; Wu, S.-B. Humic Substances Developed during Organic Waste Composting: Formation Mechanisms, Structural Properties, and Agronomic Functions. Sci. Total Environ. 2019, 662, 501–510.
- Cáceres, R.; Malińska, K.; Marfà, O. Nitrification within Composting: A Review. Waste Manag. 2018, 72, 119–137.
- Zhang, T.; Wu, X.; Shaheen, S.M.; Rinklebe, J.; Bolan, N.S.; Ali, E.F.; Li, G.; Tsang, D.C.W. Effects of Microorganism-Mediated Inoculants on Humification Processes and Phosphorus Dynamics during the Aerobic Composting of Swine Manure. J. Hazard. Mater. 2021, 416, 125738.
- Song, C.; Li, M.; Xi, B.; Wei, Z.; Zhao, Y.; Jia, X.; Qi, H.; Zhu, C. Characterisation of Dissolved Organic Matter Extracted from the Bio-Oxidative Phase of Co-Composting of Biogas Residues and Livestock Manure Using Spectroscopic Techniques. Int. Biodeterior. Biodegrad. 2015, 103, 38–50.
- Wu, S.; Shen, Z.; Yang, C.; Zhou, Y.; Li, X.; Zeng, G.; Ai, S.; He, H. Effects of C/N Ratio and Bulking Agent on Speciation of Zn and Cu and Enzymatic Activity during Pig Manure Composting. Int. Biodeterior. Biodegrad. 2017, 119, 429–436.
- Zhao, X.; Wei, Y.; Zhang, F.; Tan, W.; Fan, Y.; Xi, B. How Do Fungal Communities and Their Interaction with Bacterial Communities Influence Dissolved Organic Matter on the Stability and Safety of Sludge Compost? Environ. Sci. Pollut. Res. 2019, 26, 4141–4146.
- Cheung, H.N.B.; Huang, G.H.; Yu, H. Microbial-Growth Inhibition during Composting of Food Waste: Effects of Organic Acids. Bioresour. Technol. 2010, 101, 5925–5934.
- Nakasaki, K.; Hirai, H. Temperature Control Strategy to Enhance the Activity of Yeast Inoculated into Compost Raw Material for Accelerated Composting. Waste Manag. 2017, 65, 29–36.
- Truong, T.H.H.; Marschner, P. Respiration, Available N and Microbial Biomass N in Soil Amended with Mixes of Organic Materials Differing in C/N Ratio and Decomposition Stage. Geoderma 2018, 319, 167–174.
- Awasthi, M.K.; Wong, J.W.C.; Kumar, S.; Awasthi, S.K.; Wang, Q.; Wang, M.; Ren, X.; Zhao, J.; Chen, H.; Zhang, Z. Biodegradation of Food Waste Using Microbial Cultures Producing Thermostable A-Amylase and Cellulase under Different PH and Temperature. Bioresour. Technol. 2018, 248, 160–170.
- Sharma, A.; Weindorf, D.C.; Wang, D.; Chakraborty, S. Characterizing Soils via Portable X-Ray Fluorescence Spectrometer: 4. Cation Exchange Capacity (CEC). Geoderma 2015, 239, 130–134.
- Hart, K.M. Assessing the Role of Soil Chemoautotrophs in Carbon Cycling: An Investigation into Isotopically Labelled Soil Microorganisms. Ph.D. Thesis, Dublin City University, Dublin, Ireland, 2011.
- Passos, M.L.C.; Sarraguça, M.C.; Saraiva, M.L.M.F.S.; Rao, T.P.; Biju, V.M. Spectrophotometry|Organic Compounds. Encycl. Anal. Sci. 2018, 9, 236–243.
- Sellami, F.; Hachicha, S.; Chtourou, M.; Medhioub, K.; Ammar, E. Maturity Assessment of Composted Olive Mill Wastes Using UV Spectra and Humification Parameters. Bioresour. Technol. 2008, 99, 6900–6907.
- Tahiri, A.; Richel, A.; Destain, J.; Druart, P.; Thonart, P.; Ongena, M. Comprehensive Comparison of the Chemical and Structural Characterization of Landfill Leachate and Leonardite Humic Fractions. Anal. Bioanal. Chem. 2016, 408, 1917–1928.
- Zittel, R.; da Silva, C.P.; Domingues, C.E.; Seremeta, D.C.H.; Estrada, R.A.; de Campos, S.X. Composting of Smuggled Cigarettes Tobacco and Industrial Sewage Sludge in Reactors: Physicochemical, Phytotoxic and Spectroscopic Study. Waste Manag. 2018, 79, 537–544.
- He, X.-S.; Xi, B.-D.; Jiang, Y.-H.; He, L.-S.; Li, D.; Pan, H.-W.; Bai, S.-G. Structural Transformation Study of Water-Extractable Organic Matter during the Industrial Composting of Cattle Manure. Microchem. J. 2013, 106, 160–166.
- Morán Vieyra, F.E.; Palazzi, V.I.; Sanchez de Pinto, M.I.; Borsarelli, C.D. Combined UV–Vis Absorbance and Fluorescence Properties of Extracted Humic Substances-like for Characterization of Composting Evolution of Domestic Solid Wastes. Geoderma 2009, 151, 61–67.
- Abid, W.; Mahmoud, I.B.; Masmoudi, S.; Triki, M.A.; Mounier, S.; Ammar, E. Physico-Chemical and Spectroscopic Quality Assessment of Compost from Date Palm (Phoenix Dactylifera L.) Waste Valorization. J. Environ. Manag. 2020, 264, 110492.
- Nguyen, H.V.-M.; Lee, H.-S.; Lee, S.-Y.; Hur, J.; Shin, H.-S. Changes in Structural Characteristics of Humic and Fulvic Acids under Chlorination and Their Association with Trihalomethanes and Haloacetic Acids Formation. Sci. Total Environ. 2021, 790, 148142.
- Soobhany, N.; Gunasee, S.; Rago, Y.P.; Joyram, H.; Raghoo, P.; Mohee, R.; Garg, V.K. Spectroscopic, Thermogravimetric and Structural Characterization Analyses for Comparing Municipal Solid Waste Composts and Vermicomposts Stability and Maturity. Bioresour. Technol. 2017, 236, 11–19.
- Fialho, L.L.; da Silva, W.T.L.; Milori, D.M.B.P.; Simões, M.L.; Martin-Neto, L. Characterization of Organic Matter from Composting of Different Residues by Physicochemical and Spectroscopic Methods. Bioresour. Technol. 2010, 101, 1927–1934.
- Marhuenda-Egea, F.C.; Martínez-Sabater, E.; Jordá, J.; Sánchez-Sánchez, A.; Moral, R.; Bustamante, M.A.; Paredes, C.; Pérez-Murcia, M.D. Evaluation of the Aerobic Composting Process of Winery and Distillery Residues by Thermal Methods. Thermochim Acta 2007, 454, 135–143.
- Smidt, E.; Lechner, P.; Schwanninger, M.; Haberhauer, G.; Gerzabek, M.H. Characterization of Waste Organic Matter by FT-IR Spectroscopy: Application in Waste Science. Appl. Spectrosc. 2002, 56, 1170–1175.
- El Fels, L.; Zamama, M.; Hafidi, M. Advantages and Limitations of Using FTIR Spectroscopy for Assessing the Maturity of Sewage Sludge and Olive Oil Waste Co-Composts. In Biodegradation and Bioremediation of Polluted Systems–New Advances and Technologies; InTech: New York, NY, USA, 2015.
- Grube, M.; Lin, J.G.; Lee, P.H.; Kokorevicha, S. Evaluation of Sewage Sludge-Based Compost by FT-IR Spectroscopy. Geoderma 2006, 130, 324–333.
- Li, S.; Li, D.; Li, J.; Li, G.; Zhang, B. Evaluation of Humic Substances during Co-Composting of Sewage Sludge and Corn Stalk under Different Aeration Rates. Bioresour. Technol. 2017, 245, 1299–1302.
- Chang, Y.-T.; Lee, C.-H.; Hsieh, C.-Y.; Chen, T.-C.; Jien, S.-H. Using Fluorescence Spectroscopy to Assess Compost Maturity Degree during Composting. Agronomy 2023, 13, 1870.
- Chirayil, C.J.; Abraham, J.; Mishra, R.K.; George, S.C.; Thomas, S. Instrumental Techniques for the Characterization of Nanoparticles; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 3, ISBN 9780323461450.
- Wei, Z.; Zhao, X.; Zhu, C.; Xi, B.; Zhao, Y.; Yu, X. Assessment of Humification Degree of Dissolved Organic Matter from Different Composts Using Fluorescence Spectroscopy Technology. Chemosphere 2014, 95, 261–267.
- He, X.-S.; Xi, B.-D.; Jiang, Y.-H.; Li, M.-X.; Yu, H.-B.; An, D.; Yang, Y.; Liu, H.-L. Elemental and Spectroscopic Methods with Chemometric Analysis for Characterizing Composition and Transformation of Dissolved Organic Matter during Chicken Manure Composting. Environ. Technol. 2012, 33, 2033–2039.
- Dos Santos, L.M.; Simões, M.L.; de Melo, W.J.; Martin-Neto, L.; Pereira-Filho, E.R. Application of Chemometric Methods in the Evaluation of Chemical and Spectroscopic Data on Organic Matter from Oxisols in Sewage Sludge Applications. Geoderma 2010, 155, 121–127.
- Wang, Q.; Awasthi, M.K.; Zhao, J.; Ren, X.; Wang, M.; Li, R.; Wang, Z.; Zhang, Z. Utilization of Medical Stone to Improve the Composition and Quality of Dissolved Organic Matter in Composted Pig Manure. J. Clean. Prod. 2018, 197, 472–478.
- Liu, D.; Yu, H.; Yang, F.; Liu, L.; Gao, H.; Cui, B. Characterizing Humic Substances from Native Halophyte Soils by Fluorescence Spectroscopy Combined with Parallel Factor Analysis and Canonical Correlation Analysis. Sustainability 2020, 12, 9787.
- Xie, Z.; Guan, W. Research on Fluorescence Spectroscopy Characteristics of Dissolved Organic Matter of Landfill Leachate in the Rear Part of Three Gorges Reservoir. J. Spectrosc. 2015, 2015, 9787.
- Zhang, H.; Huang, Y.; Hu, S.; Huang, Q.; Wei, C.; Zhang, W.; Kang, L.; Huang, Z.; Hao, A. Fluorescent Probes for “off–on” Sensitive and Selective Detection of Mercury Ions and l-Cysteine Based on Graphitic Carbon Nitride Nanosheets. J. Mater. Chem. C 2015, 3, 2093–2100.
- Provenzano, M.R.; D’Orazio, V.; Jerzykiewicz, M.; Senesi, N. Fluorescence Behaviour of Zn and Ni Complexes of Humic Acids from Different Sources. Chemosphere 2004, 55, 885–892.
- Marhuenda-Egea, F.C.; Martínez-Sabater, E.; Jordá, J.; Moral, R.; Bustamante, M.A.; Paredes, C.; Pérez-Murcia, M.D. Dissolved Organic Matter Fractions Formed during Composting of Winery and Distillery Residues: Evaluation of the Process by Fluorescence Excitation-Emission Matrix. Chemosphere 2007, 68, 301–309.
- Shao, Z.-H.; He, P.-J.; Zhang, D.-Q.; Shao, L.-M. Characterization of Water-Extractable Organic Matter during the Biostabilization of Municipal Solid Waste. J. Hazard. Mater. 2009, 164, 1191–1197.
- Sunuwar, S.; Manzanares, C.E. Excitation, Emission, and Synchronous Fluorescence for Astrochemical Applications: Experiments and Computer Simulations of Synchronous Spectra of Polycyclic Aromatic Hydrocarbons and Their Mixtures. Icarus 2021, 370, 114689.
- Lu, Z.; Lu, L.; Bi, J.; Li, Y.; Zhang, H. Study on Spectral Characteristics of Fulvic Acid in the Process of Mixed Composting of Bioleach Deep Dehydrated Sludge and Rice Straw. J. Phys. Conf. Ser. 2021, 2021, 012094.
- Guo, X.; He, X.; Zhang, H.; Deng, Y.; Chen, L.; Jiang, J. Characterization of Dissolved Organic Matter Extracted from Fermentation Effluent of Swine Manure Slurry Using Spectroscopic Techniques and Parallel Factor Analysis (PARAFAC). Microchem. J. 2012, 102, 115–122.
- Peuravuori, J.; Koivikko, R.; Pihlaja, K. Characterization, Differentiation and Classification of Aquatic Humic Matter Separated with Different Sorbents: Synchronous Scanning Fluorescence Spectroscopy. Water Res. 2002, 36, 4552–4562.
- Yang, Y.; Du, W.; Cui, Z.; Zhao, T.; Wang, X.; Lv, J. Spectroscopic Characteristics of Dissolved Organic Matter during Pig Manure Composting with Bean Dregs and Biochar Amendments. Microchem. J. 2020, 158, 105226.
- Santín, C.; González-Pérez, M.; Otero, X.L.; Vidal-Torrado, P.; Macías, F.; Álvarez, M.Á. Characterization of Humic Substances in Salt Marsh Soils under Sea Rush (Juncus Maritimus). Estuar. Coast Shelf Sci. 2008, 79, 541–548.
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence Excitation−Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter. Environ. Sci. Technol. 2003, 37, 5701–5710.
- Wu, X.; Wang, J.; Shen, L.; Wu, X.; Amanze, C.; Zeng, W. Effect of Bamboo Sphere Amendment on the Organic Matter Decomposition and Humification of Food Waste Composting. Waste Manag. 2021, 133, 19–27.
- Song, F.; Wu, F.; Feng, W.; Tang, Z.; Giesy, J.P.; Guo, F.; Shi, D.; Liu, X.; Qin, N.; Xing, B.; et al. Fluorescence Regional Integration and Differential Fluorescence Spectroscopy for Analysis of Structural Characteristics and Proton Binding Properties of Fulvic Acid Sub-Fractions. J. Environ. Sci. 2018, 74, 116–125.
- Huang, J.; Han, L.; Huang, G. Characterization of Digestate Composting Stability Using Fluorescence EEM Spectroscopy Combining with PARAFAC. Waste Manag. Res. J. A Sustain. Circ. Econ. 2019, 37, 486–494.
- Duan, H.; Ji, M.; Chen, A.; Zhang, B.; Shi, J.; Liu, L.; Li, X.; Sun, J. Evaluating the Impact of Rice Husk on Successions of Bacterial and Fungal Communities during Cow Manure Composting. Environ. Technol. Innov. 2021, 24, 102084.
- Xu, L.; Li, Q.; Myers, M.; Chen, Q.; Li, X. Application of Nuclear Magnetic Resonance Technology to Carbon Capture, Utilization and Storage: A Review. J. Rock Mech. Geotech. Eng. 2019, 11, 892–908.
- Jindo, K.; Sonoki, T.; Matsumoto, K.; Canellas, L.; Roig, A.; Sanchez-Monedero, M.A. Influence of Biochar Addition on the Humic Substances of Composting Manures. Waste Manag. 2016, 49, 545–552.
- Piccolo, A.; Spaccini, R.; De Martino, A.; Scognamiglio, F.; di Meo, V. Soil Washing with Solutions of Humic Substances from Manure Compost Removes Heavy Metal Contaminants as a Function of Humic Molecular Composition. Chemosphere 2019, 225, 150–156.
More
 Encyclopedia
Encyclopedia
