You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Mohamed O. Radwan.
Nuclear receptors (NRs) constitute a superfamily of ligand-activated transcription factors with a paramount role in ubiquitous physiological functions such as metabolism, growth, and reproduction. Owing to their physiological role and druggability, NRs are deemed attractive and valid targets for medicinal chemists. Pentacyclic triterpenes (PTs) represent one of the most important phytochemical classes present in higher plants, where oleanolic acid (OA) is the most studied PTs representative owing to its multitude of biological activities against cancer, inflammation, diabetes, and liver injury.
- oleanolic acid
- nuclear receptors
- metabolic disorders
- NASH
- farnesoid X receptor
- liver X receptor
- peroxisome-proliferator activated receptors
1. Modulation of FXR
FXR is implicated in bile acid, lipid, and glucose homeostasis, and hepatic inflammation, regeneration, and fibrosis, and is widely distributed in organs such as the liver, kidney, intestinal tract, and adrenal gland [62,63,64,65][1][2][3][4]. Bile acids, the natural ligands of FXR, have been considered potential intestinal tumor promoters [66][5]. FXRα is an attractive drug target for the treatment of diverse metabolic diseases, including NASH, primary biliary cirrhosis (PBC), diabetes [67][6], and atherosclerosis [5,67,68,69,70][6][7][8][9][10] in addition to cancer [71][11]. Interaction with FXRα recruits coactivators such as SRC1 or corepressors such as nuclear receptor corepressor (NCoR1) [5][7]. Extensive research effort ended up with the discovery of some clinical FXR ligands, including obeticholic acid, the only approved FXR modulator to date for PBC therapy, and under clinical phase III for the treatment of NASH [72][12].
FXR expression is found to be decreased in human intestinal tumors due to the promotion of Wnt signaling, while the reactivation of FXR in a xenograft model via adenoviral infection induced cytotoxicity through the induction of apoptosis and inhibition of proinflammatory and antiapoptotic genes [59][13]. On the contrary, FXR activation promotes transforming growth factor β (TGF-β) induced epithelial-mesenchymal transition in hepatocellular carcinoma cells [73][14]. By virtue of its complicated role in cholesterol and bile acids homeostasis, both FXR agonists and antagonists may be therapeutically useful for the treatment of metabolic diseases [5][7].
The first report relating OA with FXR was disclosed by Liu and Wong in 2010 [74][15]. They supposed that OA health benefits are partially attributed to FXR modulation. Luciferase assay using hepatocellular carcinoma cells showed that OA competitively suppressed the activity of FXR-LBD induced by its endogenous activator chenodoxycholic acid (CDCA) without affecting the latter’s metabolism. OA not only bound to FXR-LBD and suppressed its activity in a dose-dependent manner, but also partially blocked the interaction with the coactivator SRC-3, as shown in a cell-free model. At 25 μM concentration of OA in HepG2, quantitative RT-PCR (RT-qPCR) showed that OA partially blocked CDCA induction of bile salt export pump (BSEP) and cytochrome P450 7A1 (CYP7A1) target genes but did not affect the expression level of another target gene, organic solute transporter (OST-β), and slightly enhanced SHP, suggesting that OA works as a gene selective modulator of FXR. OA did not significantly reduce LXRα and LXRβ activity induced by their known synthetic ligand TO901317 [74,75][15][16].
Another interesting work showcased the effect of OA on mice models with obstructive cholestasis by bile duct ligation (BDL) [76][17]. Basically, the histological examination of hepatocytes indicated that 20 mg/kg OA administration ameliorates BDL-induced liver injury. Furthermore, pretreatment with OA or ursodeoxycholic acid (UDCA), the only approved drug for treatment of PBC by Food and Drug Administration (FDA), in BDL mice lowered the level of alanine aminotransferase (ALT) by 59% and 41%, aspartate transaminase (AST) by 33% and 28%, alkaline phosphatase (ALP) by 44% and 39%, respectively. Furthermore, OA attenuated BDL-induced extrahepatic cholestasis in association with association with enhancement of urine bile acid output. Mechanistically, gene expression analysis proved that OA resulted in increased mRNA expression of bile acid efflux transporters MRP2, MRP3, and MRP4 [44][18], which is ascribed to OA-mediated accumulation of nuclear factor-erythroid 2-related factor (NRF2). A significant decrease in Bsep expression when OA was administrated to Sham mice was also further confirmed by RT-qPCR. The latter effect was confirmed to be due to OA antagonism of FXR through a dual luciferase reporter assay in HepG2 cells. In the latter assay, CDCA was used as a positive control and significantly enhanced the luciferase activity of the FXR reporter gene, which was opposed by co-treatment with OA in a dose-dependent manner. Taken together, Chen et al. concluded that OA’s protective role against BDL-induced extrahepatic cholestasis is ascribed to increasing basolateral bile acid export, probably via NRF2-mediated upregulation of MRP2, MRP3, and MRP4; meanwhile, decreased canalicular Bsep expression by OA, which is mediated by FXR antagonism, may also have a paramount role in attenuating bile duct injury [43,44,76][17][18][19].
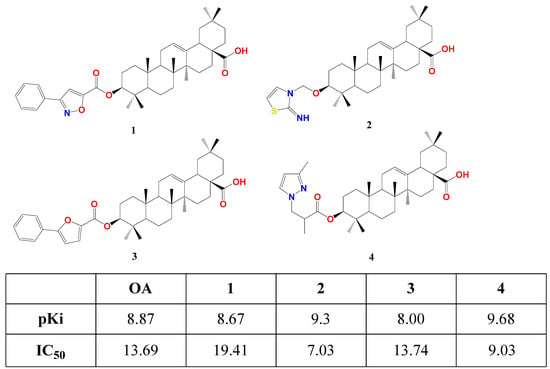
Fang’s research group succeeded in affording the first semi-synthetic OA derivatives as FXR antagonists in 2017 [77][20]. They designed and synthesized four OA derivatives through the transformation of its 3β-OH, considering the docking score of the candidates versus fexaramine as a control. They used the crystal structure of FXR as a model (PDB code: 1OSH), utilized Autodock 4.2 software in computational work, and synthesized the four top-scored compounds 1–4. These esters were afforded through reacting free OA or protected benzyl OA ester with the appropriate activated acid, followed by catalytic hydrogenolysis for deprotection. In human embryonic kidney 293T cells, compounds 1–4 suppressed FXR transactivation in a concentration-dependent manner with respective IC50 19.41, 7.03, 13.74, and 9.03 μM as settled by a dual-luciferase reporter assay. This is in agreement with their predicted docking energy values pKi (Figure 31). Seemingly, 2 and 4 have similar placement in FXR-LBD, forming two T-shaped π-π stacking with Trp458 in helix 11. This crucial interaction is missing in the case of OA, 1, and 3 [77][20].
Intriguingly, Pan et al. revealed that OA is likely to work as an FXR agonist. They studied the OA effect in human umbilical vein endothelial cells HUVECs atherosclerosis model by treating them with oxidized low-density lipoprotein (ox-LDL, 100 μg/mL) for 24 h [78][21]. Basically, OA interrupted the ox-LDL-induced cell apoptosis in the HUVECs. Luciferase reporter assay revealed that the FXR relative luciferase activity was significantly higher in the case of OA treatment in a dose-dependent manner, leading to angiotensin (Ang)-(1–7) upregulation, which, in turn, perturbs the development of atherosclerosis. The results were further validated in New Zealand white (NZW) rabbits as an atherosclerosis animal model. The atherosclerosis assessment at the abdominal aorta and thoracic aorta of the animal models was performed by histopathological analysis in the presence of OA and simvastatin as a positive control. Both OA and simvastatin inhibited the development of atherosclerosis by minimizing the aortic lesion area size and enhancing the collagen content. Although this study is in discrepancy with previous studies that reported FXR antagonism by OA, it introduced an insight into the therapeutic potential of the latter for the treatment of atherosclerosis [78][21]. It is worth repeating that the NRs modulation is tissue-specific; therefore, it is quite normal to find different pharmacodynamics in different tissues.
Another study explored the underlying mechanism of OA in alleviating alpha-naphthol isothiocyanate (ANIT)-induced cholestatic liver damage in rats instead of BDL [79,80][22][23]. As anticipated, OA decreased hepatocyte necrosis and reduced inflammatory cell infiltration in a similar way to UDCA. In rat hepatocytes, OA significantly restored glutathione levels of rat primary hepatocytes reduced by ANIT. This is by reversing the high serum levels of ALP, ALT, AST, total bile acid and (TBA), total bilirubin (TBiL), and gamma-glutamyl transferase (γ-GT) levels in the ANIT-induced model as shown by RT-qPCR. This is attributed to the restoration of FXR and Nrf2 mRNA and protein levels, which were reduced in ANIT. Consequently, treatment with OA decreased the expression of Cyp7a1 mRNA and protein in rats and restored Bsep levels [81][24].
The same research group conducted an extensive mechanistic study [82][25]. They compared OA protective effect on ANIT-induced cholestatic liver injury in wild-type and Nrf2 gene knockdown rats and demonstrated that the effect was much weaker in the latter case. This highlights the important role of OA in stimulating the Nrf2 pathway. Likewise, the protective effect of OA against ANIT-induced cholestatic liver injury was relatively weaker in FXR knockdown than in type rats. This demonstrates that the protective effects of OA on ANIT-induced injury and its regulatory role of the bile acids homeostasis gene are mainly ascribed to the simultaneous activation of NRF2 and FXR dual signaling pathways. The authors found a correlation between NRF2 and FXR signaling [82][25].
By virtue of the cellular context effect of NRs modulators, Fallon et al. studied the effect of hederagenin and OA on FXR in human colonic epithelial cells T84 in comparison to the GW4064, a synthetic FXR agonist. Surprisingly, they found that both hederagenin and OA compounds do not have direct agonistic FXR activity in this model. Having said that, they induced the overexpression of FXR mRNA and protein and upregulated GW4064-induced FXR signaling. This opens the way for the potential application of OA in colon cancer and secretory diarrheas [58][26].
The same research group of Fang reported a class of 12β-oxygenated oleanolic alkyl esters with FXR modulation properties [83][27]. In brief, OA 28-COOH was protected by benzylation, and 3-OH was protected by etherification with t-butylmethylsilyl (TBS). Olefin of the double-protected derivative was oxidized by meta-chloroperoxybenzoic acid (m-CPBA), affording a 12-oxo derivative. The latter was reduced by sodium borohydride (NaBH4) to a mixture of 12β- and 12α-hydroxyl derivatives in ca. 2:1 ratio. The 12β-OH compound was reacted with the corresponding carboxylic acids in the presence of N,N’-diisopropylcarbodiimide (DIC) or 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI) and 4-dimethylaminopyridine (DMAP), followed by deprotection of TBS and Bn groups with BF3.Et2O and Pd/C hydrogenolysis, respectively, to furnish a series of 12-OA alkyl esters. Various aliphatic and aromatic substituents were used with different polarities.
In HEK293T cells, screening of the synthesized series at 10 μM showed that compound 5 with an acetopropionyl group and 6 with a 4-acetobutanoyl group of the 12-O-alkanoic acid esters are pronounced FXR antagonists opposing the CDCA effect without observable cytotoxicity at 10 µM (Figure 42). Compounds with carboxy-, amino-, or phenyl terminal groups demonstrated less activity. Owing to its prominent antagonistic activity at 10 and 1 µM, the IC50 of compound 6 was calculated to be 0.10 µM. Its binding mode and pharmacophore placement into FXR-LBD is comparable to the FXR antagonist, ivermectin, as calculated by docking simulation [51][28]. To assess its selectivity, the authors tested compound 6 for the action on an array of NRs, including RXRα, RXRβ, RXRγ, PPARα, PPARβ, PPARγ, LXRα, LXRβ, PXR, and another bile acid receptor GPBAR compared to guggulsterone. While the latter antagonizes almost all tested NRs, compound 6 demonstrated an outstanding antagonism (>90%) against FXR and inconsiderable antagonism (10–20%) against LXRα and PPARα.
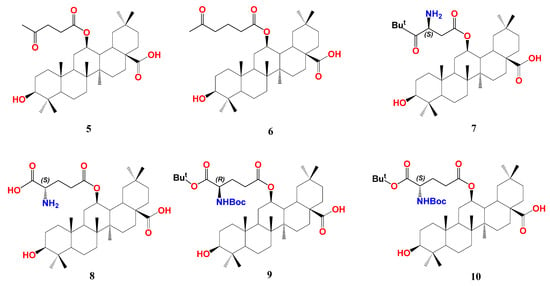
Transcription of FXR downstream genes was then evaluated in HepG2 cells. An amount of 10 μM of 6 efficiently reversed the induction of small heterodimeric partner (SHP) and BSEP by 50 μM of the endogenous agonist CDCA without significant effect on sterol regulatory element-binding protein-1c (SREBP-1c and CYP7A1). This is controversial due to the discrepancy with the effect of the parent OA, which suppresses CYP7A1 expression [74][15]. Intriguingly, in the absence of CDCA, compound 6 clearly hindered SHP, BSEP, and CYP7A1 at 10 μM but did not affect the expression of SREBP-1c, revealing a unique FXR downstream regulation.
Upon exploring the possible effect of 6 on FXR-controlled genes that regulate blood glucose level and gluconeogenesis [85][30], it was found to suppress mRNA levels of phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) in the presence or absence of CDCA. In addition, in KKay mice, treatment with 6 significantly decreased fasting and non-fasting blood glucose levels. Concomitantly, 6 improved glucose tolerance and insulin sensitivity and lowered HbA1c levels [83][27].
In continuation of their work, Fang’s group designed and synthesized more OA hybrids with 12β-O-β-aspartyl or 12β-O-γ-glutamyl moiety trying to enhance the affinity of compound 6 towards FXR-LBD as shown for compounds 7–10 [83,84][27][29]. The design considered the presence of unoccupied hydrophobic space around the compound 6 terminal chain, thus aimed at using more branched chains. The authors adopted similar synthetic procedures for OA 12β-OH esterification that were used for the synthesis of compound 6, as mentioned above. Among the new series, compound 10 with S-γ-glutamyl moiety possesses the highest antagonism to FXR with IC50 0.44 µM in HEK293T cells. Using the R-γ-glutamyl chain lowered the activity (9, IC50 1.95 µM). OA-bearing S-aspartyl chain, compound 7, is slightly less active than 10 with IC50 0.95 µM. Protection of the terminal amino- group by tert-butyloxycarbonyl (Boc) and the terminal carboxyl with tert-butyl (t-Bu) group is crucial for activity; for example, compound 8 with both free terminal groups dramatically lost activity. In general, OA derivatives bearing (S) configuration side chains outperform those bearing (R) configuration ones, and glutamyl derivatives outperform aspartyl derivatives. In a similar fashion to compound 6, 10 showed favorable selectivity against FXR with much less effect on LXRα and PPARα and no effect on other tested metabolic NRs. Consequently, 10 significantly inhibited the expression of PEPCK and G6Pase at 1 and 10 µM concentrations. In hepatic stellate cell line LX-2, compound 10 lowered mRNA expressions of liver fibrosis marker genes, including collagen type I α-1, actin-α-2, transforming growth factor β-1, connective tissue growth factor, and integrin α-V, whereas guggulsterone lowered expression of only collagen type I α-1, actin-α-2.
In a bile duct ligation (BDL) rat model, the oral administration of compound 10 for two weeks effectively decreased the levels of AST, ALT, TBA, and TBiL, which means less liver fibrosis. Liver histopathology using hematoxylin and eosin (H&E) staining showed that compound 10 intake alleviated bile duct hyperplasia and parenchymal necrosis that accompany BDL. The collagen-specific Sirius red staining showed less collagen accumulation in the compound 10 treated group. These in vitro data of reducing hepatic fibrosis markers were further validated in vivo. Interestingly, in a NASH mice model, the titled compound reduced intrahepatic steatosis and hepatic lobular inflammation, indicating less liver fat accumulation. This is accompanied by a reduction in mRNA expression of fibrosis marker genes. Collectively, compound 10 is an OA derivative and a promising FXR modulator with NASH and diabetes therapeutic potential [84][29].
Collectively, OA reprograms the liver to protect against hepatotoxic chemicals, but its intake should be with care since its high doses are reported to be hepatotoxic and can develop cholestatic liver injury [41,86,87][31][32][33]. It is worth noting that the knockdown of FXR ameliorated OA-induced cholestatic liver injury [86][32]. Such paradoxical hepatoprotection and hepatotoxicity are common for natural herbs. In conclusion, OA dose is the one to differentiate between its role as a remedy and a poison [88][34].
2. Modulation of PPARs
PPARs comprise three different subtypes, α, β, and γ, that orchestrate lipid homeostasis and insulin sensitivity, making them attractive targets for controlling metabolic syndrome and diabetes [89,90,91,92][35][36][37][38]. PPARα reduces the formation of triglycerides; however, PPARβ controls serum lipid profile and insulin sensitivity [93][39]. PPARγ has a significant role in controlling insulin sensitivity and adipogenesis [94][40]. Fibrates and thiazolidinedione are two classes of PPARs modulators approved for hyperlipidemia and diabetes therapy, respectively [19][41]. Fibrates such as pemafibrate modulate PPARα, whereas thiazolidinediones such as rosiglitazone upregulate PPARγ [95][42].
In 2005, Huang et al. revealed that OA is a concentration-dependent PPARα activator through luciferase reporter assay in human embryonic kidney 293 cells, unlike ursolic acid and gallic acid. The OA-induced activation of PPARα was demolished upon adding a selective PPARα antagonist MK-886 [96][43]. The anti-hyperlipidemia effect of Pomegranate flower extract was ascribed mainly to the presence of OA [96][43]. Additionally, OA is reported to be a weak activator of PPARγ, which has an in-part role in the antidiabetic activity of Salvia officinalis extract [97,98][44][45]. Such results contradict the reported selective modulation of FXR by OA [74][15].
The generation of a pharmacophore model of PPARγ partial agonists using the Chinese natural product database led to the identification of OA as a PPARγ modulator [99][46]. Chios mastic gum (CMG) is therapeutically beneficial in managing diabetes [100][47], hyperlipidemia, insulin resistance [101][48], and diet-induced NASH [102][49]. Combining regular physical exercise with CMG intake for six months highly enhanced those health benefits in young Japanese men [101][48]. Those health benefits were attributed to the presence of a high amount of OA in CMG and its modulation of PPARγ [100,103][47][50].
The synthetic derivative of oleanolic acid, 2-Cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO) (Figure 53), is endowed with not only anticancer and anti-inflammatory activity but also partial agonistic PPARγ agonism. Intriguingly, its methyl ester, called CDDO-Me, is reported as a PPARγ antagonist [104][51]. PPARγ binding competition in the presence of rosiglitazone using scintillation proximity assay (SPA) showed that the ki values for binding to PPARγ are 12 nM and 130 nM for CDDO and CDDO-Me, respectively [104][51]. As evidence of selectivity, neither of them could interact with PPARα.
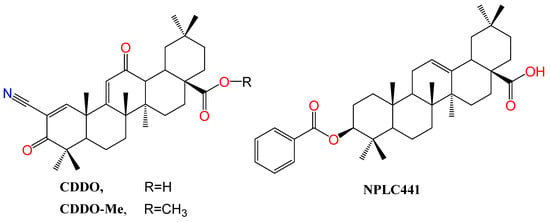
Figure 53. Structure of OA derivatives CDDO and CDDO-Me, PPAR modulators, and NPLC441, an RXR modulator. Atoms were colored by element.
3. Modulation of LXR
LXR (α,β) are naturally activated by oxysterols and implicated in glucose homeostasis, lipid homeostasis, cancer, atherosclerosis, and NASH [16,105][52][53]. LXR plays a crucial role in regulating hepatic de novo lipogenesis (DNL) and cholesterol homeostasis [106][54]. LXRα activation promotes the expression of hepatic lipogenic genes through the activation of SREBP-1c2. Hepatic expression of LXR is elevated in non-alcoholic fatty liver disease (NAFLD) patients; hence, antagonizing LXR may be of therapeutic benefit [107,108][55][56]. On the other side, synthetic LXR agonists such as compound T090 are anti-atherosclerotic agents, albeit with severe undesirable effects, including elevated de novo lipogenesis and consequent development of hepatic steatosis. Both LXR subtypes share over 70% amino acid identity and architecture with PPARs.
OA was identified as an LXR antagonist, which is similar to the effect of its congener ursolic acid [55,109][57][58]. In differentiated HepaRG, hepatocyte-like cells, OA reversed the T090-indued lipid accumulation and elevation of mRNA expression and protein level of the SREBP-1c promoter in a dose-dependent manner. In other words, OA downregulated the mRNA and protein levels of target genes involved in the LXRα-SREBP-1c signaling pathway. OA activity is observed in HepaRG but not in colon cells LS174T, confirming the cellular-context paradox. OA fitted snugly in LXRα-LBD, adopting a similar orientation of T090 as calculated by molecular modeling [109][58].
On the contrary, in rats, another cellular model, OA oral administration promoted mRNA expression and protein levels of liver detoxification enzymes, including hydroxylation, glucuronidation, sulfation, and glutathione conjugation enzymes. This helped protect the liver from bile acids-induced toxicity in BDL rats. OA hepatoprotective effect is basically attributed to increasing the mRNA expression of LXRα and other transcription factors. At the protein level, not only LXRα was elevated but also PXR, RAR, and VDR [43][19].
References
- Lamers, C.; Schubert-Zsilavecz, M.; Merk, D. Medicinal Chemistry and Pharmacological Effects of Farnesoid X Receptor (FXR) Antagonists. Curr. Top. Med. Chem. 2014, 14, 2188–2205.
- Sun, L.; Cai, J.; Gonzalez, F.J. The Role of Farnesoid X Receptor in Metabolic Diseases, and Gastrointestinal and Liver Cancer. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 335–347.
- Jiang, L.; Zhang, H.; Xiao, D.; Wei, H.; Chen, Y. Farnesoid X Receptor (FXR): Structures and Ligands. Comput. Struct. Biotechnol. J. 2021, 19, 2148–2159.
- Makishima, M.; Okamoto, A.Y.; Repa, J.J.; Tu, H.; Learned, R.M.; Luk, A.; Hull, M.V.; Lustig, K.D.; Mangelsdorf, D.J.; Shan, B. Identification of a Nuclear Receptor for Bile Acids. Science 1999, 284, 1362–1365.
- Parks, D.J.; Blanchard, S.G.; Bledsoe, R.K.; Chandra, G.; Consler, T.G.; Kliewer, S.A.; Stimmel, J.B.; Willson, T.M.; Zavacki, A.M.; Moore, D.D.; et al. Bile Acids: Natural Ligands for an Orphan Nuclear Receptor. Science 1999, 284, 1365–1368.
- Zhang, Y.; Lee, F.Y.; Barrera, G.; Lee, H.; Vales, C.; Gonzalez, F.J.; Willson, T.M.; Edwards, P.A. Activation of the Nuclear Receptor FXR Improves Hyperglycemia and Hyperlipidemia in Diabetic Mice. Proc. Natl. Acad. Sci. USA 2006, 103, 1006–1011.
- Fang, Y.; Hegazy, L.; Finck, B.N.; Elgendy, B. Recent Advances in the Medicinal Chemistry of Farnesoid X Receptor. J. Med. Chem. 2021, 64, 17545–17571.
- Pellicciari, R.; Costantino, G.; Fiorucci, S. Farnesoid X Receptor: From Structure to Potential Clinical Applications. J. Med. Chem. 2005, 48, 5383–5403.
- Xu, X.; Shi, X.; Chen, Y.; Zhou, T.; Wang, J.; Xu, X.; Chen, L.; Hu, L.; Shen, X. HS218 as an FXR Antagonist Suppresses Gluconeogenesis by Inhibiting FXR Binding to PGC-1α Promoter. Metabolism 2018, 85, 126–138.
- Festa, C.; Finamore, C.; Marchianò, S.; Di Leva, F.S.; Carino, A.; Monti, M.C.; del Gaudio, F.; Ceccacci, S.; Limongelli, V.; Zampella, A.; et al. Investigation around the Oxadiazole Core in the Discovery of a New Chemotype of Potent and Selective FXR Antagonists. ACS Med. Chem. Lett. 2019, 10, 504–510.
- Shishodia, S.; Azu, N.; Rosenzweig, J.A.; Jackson, D.A. Guggulsterone for Chemoprevention of Cancer. Curr. Pharm. Des. 2016, 22, 294–306.
- Mudaliar, S.; Henry, R.R.; Sanyal, A.J.; Morrow, L.; Marschall, H.-U.; Kipnes, M.; Adorini, L.; Sciacca, C.I.; Clopton, P.; Castelloe, E.; et al. Efficacy and Safety of the Farnesoid X Receptor Agonist Obeticholic Acid in Patients with Type 2 Diabetes and Non-alcoholic Fatty Liver Disease. Gastroenterology 2013, 145, 574–582.e1.
- Modica, S.; Murzilli, S.; Salvatore, L.; Schmidt, D.R.; Moschetta, A. Nuclear Bile Acid Receptor FXR Protects against Intestinal Tumorigenesis. Cancer Res. 2008, 68, 9589–9594.
- Kainuma, M.; Takada, I.; Makishima, M.; Sano, K. Farnesoid X Receptor Activation Enhances Transforming Growth Factor β-Induced Epithelial-Mesenchymal Transition in Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2018, 19, 1898.
- Liu, W.; Wong, C. Oleanolic Acid Is a Selective Farnesoid X Receptor Modulator. Phytother. Res. 2010, 24, 369–373.
- Kanno, Y.; Tanuma, N.; Takahashi, A.; Inouye, Y. TO901317, a Potent LXR Agonist, Is an Inverse Agonist of CAR. J. Toxicol. Sci. 2013, 38, 309–315.
- Chen, P.; Li, J.; Fan, X.; Zeng, H.; Deng, R.; Li, D.; Huang, M.; Bi, H. Oleanolic Acid Attenuates Obstructive Cholestasis in Bile Duct-Ligated Mice, Possibly via Activation of NRF2-MRPs and FXR Antagonism. Eur. J. Pharmacol. 2015, 765, 131–139.
- Chen, P.; Zeng, H.; Wang, Y.; Fan, X.; Xu, C.; Deng, R.; Zhou, X.; Bi, H.; Huang, M. Low Dose of Oleanolic Acid Protects against Lithocholic Acid–Induced Cholestasis in Mice: Potential Involvement of Nuclear Factor-E2-Related Factor 2-Mediated Upregulation of Multidrug Resistance-Associated Proteins. Drug Metab. Dispos. 2014, 42, 844–852.
- Chai, J.; Du, X.; Chen, S.; Feng, X.; Cheng, Y.; Zhang, L.; Gao, Y.; Li, S.; He, X.; Wang, R.; et al. Oral Administration of Oleanolic Acid, Isolated from Swertia Mussotii Franch, Attenuates Liver Injury, Inflammation, and Cholestasis in Bile Duct-Ligated Rats. Int. J. Clin. Exp. Med. 2015, 8, 1691–1702.
- Wang, S.-R.; Xu, T.; Deng, K.; Wong, C.-W.; Liu, J.; Fang, W.-S. Discovery of Farnesoid X Receptor Antagonists Based on a Library of Oleanolic Acid 3-O-Esters through Diverse Substituent Design and Molecular Docking Methods. Molecules 2017, 22, 690.
- Pan, Y.; Zhou, F.; Song, Z.; Huang, H.; Chen, Y.; Shen, Y.; Jia, Y.; Chen, J. Oleanolic Acid Protects against Pathogenesis of Atherosclerosis, Possibly via FXR-Mediated Angiotensin (Ang)-(1–7) Upregulation. Biomed. Pharmacother. 2018, 97, 1694–1700.
- Żwawiak, J.; Pawełczyk, A.; Olender, D.; Zaprutko, L. Structure and Activity of Pentacyclic Triterpenes Codrugs. A Review. Mini-Rev. Med. Chem. 2021, 21, 1509–1526.
- Mariotti, V.; Strazzabosco, M.; Fabris, L.; Calvisi, D.F. Animal Models of Biliary Injury and Altered Bile Acid Metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864 Pt B, 1254–1261.
- Liu, J.; Liu, J.; Meng, C.; Huang, C.; Liu, F.; Xia, C. Oleanolic Acid Alleviates ANIT-Induced Cholestatic Liver Injury by Activating Fxr and Nrf2 Pathways to Ameliorate Disordered Bile Acids Homeostasis. Phytomedicine 2022, 102, 154173.
- Liu, J.; Liu, J.; Meng, C.; Gu, Q.; Huang, C.; Liu, F.; Xia, C. NRF2 and FXR Dual Signaling Pathways Cooperatively Regulate the Effects of Oleanolic Acid on Cholestatic Liver Injury. Phytomedicine 2023, 108, 154529.
- Fallon, C.M.; Smyth, J.S.; Quach, A.; Lajczak-McGinley, N.; O’Toole, A.; Barrett, K.E.; Sheridan, H.; Keely, S.J. Pentacyclic Triterpenes Modulate Farnesoid X Receptor Expression in Colonic Epithelial Cells: Implications for Colonic Secretory Function. J. Biol. Chem. 2022, 298, 102569.
- Wang, S.; Huan, Y.; Niu, S.; Cao, H.; Yang, M.; Zhou, X.; Gao, X.; Wang, X.; Shen, Z.; Fang, W.-S. Discovery of 12β-Oxygenated Oleanolic Acid Alkyl Esters as Potent and Selective FXR Modulators Exhibiting Hyperglycemia Amelioration in Vivo. Bioorganic Chem. 2022, 129, 106203.
- Jin, L.; Feng, X.; Rong, H.; Pan, Z.; Inaba, Y.; Qiu, L.; Zheng, W.; Lin, S.; Wang, R.; Wang, Z.; et al. The Antiparasitic Drug Ivermectin Is a Novel FXR Ligand That Regulates Metabolism. Nat. Commun. 2013, 4, 1937.
- Ma, H.; Bao, Y.; Niu, S.; Wang, S.; Li, Y.; He, H.; Zhang, N.; Fang, W. Structure Optimization of 12β-O-γ-Glutamyl Oleanolic Acid Derivatives Resulting in Potent FXR Antagonist/Modulator for NASH Therapy. Pharmaceuticals 2023, 16, 758.
- Zhao, T.; Wang, J.; He, A.; Wang, S.; Chen, Y.; Lu, J.; Lv, J.; Li, S.; Wang, J.; Qian, M.; et al. Mebhydrolin Ameliorates Glucose Homeostasis in Type 2 Diabetic Mice by Functioning as a Selective FXR Antagonist. Metabolism 2021, 119, 154771.
- Liu, J.; Lu, Y.-F.; Wu, Q.; Xu, S.-F.; Shi, F.-G.; Klaassen, C.D. Oleanolic Acid Reprograms the Liver to Protect against Hepatotoxicants, but Is Hepatotoxic at High Doses. Liver Int. 2019, 39, 427–439.
- Feng, H.; Hu, Y.; Zhou, S.; Lu, Y. Farnesoid X Receptor Contributes to Oleanolic Acid-Induced Cholestatic Liver Injury in Mice. J. Appl. Toxicol. 2022, 42, 1323–1336.
- Lu, Y.-F.; Wan, X.-L.; Xu, Y.; Liu, J. Repeated Oral Administration of Oleanolic Acid Produces Cholestatic Liver Injury in Mice. Molecules 2013, 18, 3060–3071.
- Liu, J. Oleanolic Acid and Ursolic Acid: Research Perspectives. J. Ethnopharmacol. 2005, 100, 92–94.
- Berger, J.; Moller, D.E. The Mechanisms of Action of PPARs. Annu. Rev. Med. 2002, 53, 409–435.
- Zhang, F.; Lavan, B.E.; Gregoire, F.M. Selective Modulators of PPAR-Gamma Activity: Molecular Aspects Related to Obesity and Side-Effects. PPAR Res. 2007, 2007, 32696.
- Allen, T.; Zhang, F.; Moodie, S.A.; Clemens, L.E.; Smith, A.; Gregoire, F.; Bell, A.; Muscat, G.E.; Gustafson, T.A. Halofenate Is a Selective Peroxisome Proliferator-Activated Receptor Gamma Modulator with Antidiabetic Activity. Diabetes 2006, 55, 2523–2533.
- Zheng, W.; Feng, X.; Qiu, L.; Pan, Z.; Wang, R.; Lin, S.; Hou, D.; Jin, L.; Li, Y. Identification of the Antibiotic Ionomycin as an Unexpected Peroxisome Proliferator-Activated Receptor γ (PPARγ) Ligand with a Unique Binding Mode and Effective Glucose-Lowering Activity in a Mouse Model of Diabetes. Diabetologia 2013, 56, 401–411.
- Sanderson, L.M.; Degenhardt, T.; Koppen, A.; Kalkhoven, E.; Desvergne, B.; Müller, M.; Kersten, S. Peroxisome Proliferator-Activated Receptor Beta/Delta (PPARbeta/Delta) but Not PPARalpha Serves as a Plasma Free Fatty Acid Sensor in Liver. Mol. Cell Biol. 2009, 29, 6257–6267.
- Fajas, L.; Auboeuf, D.; Raspé, E.; Schoonjans, K.; Lefebvre, A.-M.; Saladin, R.; Najib, J.; Laville, M.; Fruchart, J.-C.; Deeb, S.; et al. The Organization, Promoter Analysis, and Expression of the Human PPARγ Gene*. J. Biol. Chem. 1997, 272, 18779–18789.
- Yang, C.; Li, Q.; Li, Y. Targeting Nuclear Receptors with Marine Natural Products. Mar. Drugs 2014, 12, 601–635.
- Orasanu, G.; Ziouzenkova, O.; Devchand, P.R.; Nehra, V.; Hamdy, O.; Horton, E.S.; Plutzky, J. The PPARγ Agonist Pioglitazone Represses Inflammation In A PPARα-Dependent Manner In Vitro and In Vivo In Mice. J. Am. Coll. Cardiol. 2008, 52, 869–881.
- Huang, T.H.-W.; Peng, G.; Kota, B.P.; Li, G.Q.; Yamahara, J.; Roufogalis, B.D.; Li, Y. Pomegranate Flower Improves Cardiac Lipid Metabolism in a Diabetic Rat Model: Role of Lowering Circulating Lipids. Br. J. Pharmacol. 2005, 145, 767–774.
- Christensen, K.B.; Jørgensen, M.; Kotowska, D.; Petersen, R.K.; Kristiansen, K.; Christensen, L.P. Activation of the Nuclear Receptor PPARγ by Metabolites Isolated from Sage (Salvia officinalis L.). J. Ethnopharmacol. 2010, 132, 127–133.
- Rau, O.; Wurglics, M.; Paulke, A.; Zitzkowski, J.; Meindl, N.; Bock, A.; Dingermann, T.; Abdel-Tawab, M.; Schubert-Zsilavecz, M. Carnosic Acid and Carnosol, Phenolic Diterpene Compounds of the Labiate Herbs Rosemary and Sage, Are Activators of the Human Peroxisome Proliferator-Activated Receptor Gamma. Planta Med. 2006, 72, 881–887.
- Petersen, R.K.; Christensen, K.B.; Assimopoulou, A.N.; Fretté, X.; Papageorgiou, V.P.; Kristiansen, K.; Kouskoumvekaki, I. Pharmacophore-Driven Identification of PPARγ Agonists from Natural Sources. J. Comput. Aided Mol. Des. 2011, 25, 107–116.
- Georgiadis, I.; Karatzas, T.; Korou, L.-M.; Katsilambros, N.; Perrea, D. Beneficial Health Effects of Chios Gum Mastic and Peroxisome Proliferator-Activated Receptors: Indications of Common Mechanisms. J. Med. Food 2015, 18, 1–10.
- Fukazawa, T.; Smyrnioudis, I.; Konishi, M.; Takahashi, M.; Kim, H.K.; Nishimaki, M.; Xiang, M.; Sakamoto, S. Effects of Chios Mastic Gum and Exercise on Physical Characteristics, Blood Lipid Markers, Insulin Resistance, and Hepatic Function in Healthy Japanese Men. Food Sci. Biotechnol. 2018, 27, 773–780.
- Tzani, A.; Konstantopoulos, P.; Doulamis, I.; Liakea, A.; Minia, A.; Antoranz, A.; Korou, L.-M.; Kavantzas, N.; Alexopoulos, L.; Stamatelopoulos, K.; et al. Chios Mastic Gum Inhibits Diet-Induced Non-Alcoholic Steatohepatitis in Mice via Activation of PPAR-α. Atherosclerosis 2020, 315, e47–e48.
- Soulaidopoulos, S.; Tsiogka, A.; Chrysohoou, C.; Lazarou, E.; Aznaouridis, K.; Doundoulakis, I.; Tyrovola, D.; Tousoulis, D.; Tsioufis, K.; Vlachopoulos, C.; et al. Overview of Chios Mastic Gum (Pistacia lentiscus) Effects on Human Health. Nutrients 2022, 14, 590.
- Wang, Y.; Porter, W.W.; Suh, N.; Honda, T.; Gribble, G.W.; Leesnitzer, L.M.; Plunket, K.D.; Mangelsdorf, D.J.; Blanchard, S.G.; Willson, T.M.; et al. A Synthetic Triterpenoid, 2-Cyano-3,12-Dioxooleana-1,9-Dien-28-Oic Acid (CDDO), Is a Ligand for the Peroxisome Proliferator-Activated Receptor Gamma. Mol. Endocrinol. 2000, 14, 1550–1556.
- Lin, Z.; Zhang, Y.; Zhang, Y.; Shen, H.; Hu, L.; Jiang, H.; Shen, X. Oleanolic Acid Derivative NPLC441 Potently Stimulates Glucose Transport in 3T3-L1 Adipocytes via a Multi-Target Mechanism. Biochem. Pharmacol. 2008, 76, 1251–1262.
- Edwards, P.A.; Kennedy, M.A.; Mak, P.A. LXRs; Oxysterol-Activated Nuclear Receptors That Regulate Genes Controlling Lipid Homeostasis. Vascul. Pharmacol. 2002, 38, 249–256.
- Dixon, E.D.; Nardo, A.D.; Claudel, T.; Trauner, M. The Role of Lipid Sensing Nuclear Receptors (PPARs and LXR) and Metabolic Lipases in Obesity, Diabetes and NAFLD. Genes 2021, 12, 645.
- Higuchi, N.; Kato, M.; Shundo, Y.; Tajiri, H.; Tanaka, M.; Yamashita, N.; Kohjima, M.; Kotoh, K.; Nakamuta, M.; Takayanagi, R.; et al. Liver X Receptor in Cooperation with SREBP-1c Is a Major Lipid Synthesis Regulator in Non-alcoholic Fatty Liver Disease. Hepatol. Res. 2008, 38, 1122–1129.
- Griffett, K.; Burris, T.P. Development of LXR Inverse Agonists to Treat MAFLD, NASH, and Other Metabolic Diseases. Front. Med. 2023, 10, 1102469.
- Lin, Y.-N.; Wang, C.C.N.; Chang, H.-Y.; Chu, F.-Y.; Hsu, Y.-A.; Cheng, W.-K.; Ma, W.-C.; Chen, C.-J.; Wan, L.; Lim, Y.-P. Ursolic Acid, a Novel Liver X Receptor α (LXRα) Antagonist Inhibiting Ligand-Induced Non-alcoholic Fatty Liver and Drug-Induced Lipogenesis. J. Agric. Food Chem. 2018, 66, 11647–11662.
- Lin, Y.-N.; Chang, H.-Y.; Wang, C.C.N.; Chu, F.-Y.; Shen, H.-Y.; Chen, C.-J.; Lim, Y.-P. Oleanolic Acid Inhibits Liver X Receptor Alpha and Pregnane X Receptor to Attenuate Ligand-Induced Lipogenesis. J. Agric. Food Chem. 2018, 66, 10964–10976.
More
