Autoimmune thyroid disease (AITD) is the most common organ-specific autoimmune disorder clinically presented as Hashimoto thyroiditis (HT) and Graves’ disease (GD). The pathogenesis of AITD is caused by an inappropriate immune response related to genetic, non-genetic, and environmental factors. Pregnancy is one of the factors that have a great influence on the function of the thyroid gland because of the increased metabolic demand and the effects of hormones related to pregnancy. During pregnancy, an adaptation of the maternal immune system occurs, especially of the innate immune system engaged in maintaining adaptive immunity in the tolerant state, preventing the rejection of the fetus. Pregnancy-related hormonal changes (estrogen, progesterone, hCG) may modulate the activity of innate immune cells, potentially worsening the course of AITD during pregnancy. This especially applies to NK cells, which are associated with exacerbation of HD and GD. On the other hand, previous thyroid disorders can affect fertility and cause adverse outcomes of pregnancy, such as placental abruption, spontaneous abortion, and premature delivery. Additionally, it can cause fetal growth retardation and may contribute to impaired neuropsychological development of the fetus.
- autoimmune thyroid disease
- pregnancy
- neutrophils
- dendritic cells
1. Introduction
2. The Delicate Thyroid Equilibrium in Pregnancy
Thyroid dysfunction is a pathological condition commonly seen in pregnant women, and likewise, AITD can undesirably affect fertility. Its association with adverse outcomes such as placental abruption, spontaneous abortion, and premature delivery [21][18], gestational hypertension, and fetal growth retardation is confirmed by a plethora of studies [22][19]. Maternal biochemical hypothyroidism is usually a priori defined as TSH above 6.0 mIU/L, and exposure to TSH above 10 mIU/L is used as a marker of overt maternal hypothyroidism. It is known that subclinical thyroid dysfunction before conception is related to an increased risk of adverse pregnancy outcomes [26][20], which significantly occur in pregnant women having TSH above 10 mIU/L. Interestingly, no significant association between thyroid autoantibodies and pregnancy outcomes was found in recent research by Knøsgaard et al. [27][21]. Increasing evidence emphasizes an elevated risk of adverse gestational and neonatal outcomes induced by subclinical hypothyroidism in women during the early gestational period. Particularly, the risk of pregnancy-specific complications has been increased in TPOAb-positive women having serum TSH levels exceeding 2.5 mIU/L, especially in those with serum TSH levels greater than 4.0 mIU/L [28][22]. Concerns were raised about thyroid dysfunction in newborns whose mothers have (autoimmune) hypothyroidism, which led to the practice of thyroid hormone testing in the early neonatal period.3. Autoimmune Hyperthyroidism in Pregnancy
The most common cause of hyperthyroidism in pregnancy is GD, which occurs in about 0.2% of all pregnancies in iodine-rich areas [20[17][23],31], whereas in iodine-deficient countries, excessive thyroid hormone production is usually caused by autonomous thyroid nodules [32][24], GD often improves during the third trimester of pregnancy and may worsen during the first year after birth. It is characterized by the production of thyroid-stimulating immunoglobulin (TSI) autoantibodies that bind to the TSH receptor and stimulate the overproduction of thyroid hormones. TSI antibodies cross the placenta and cause fetal or neonatal hyperthyroidism (incidence between 1% and 5% in women with GD) [33][25]. However, if the mother is taking antithyroid drugs (ATDs) for GD, fetal hyperthyroidism is rare because ATDs also cross the placenta, preventing fetal hyperthyroidism [34][26]. Elevated TPOAb or TgAb were found in 2% to 17% of pregnant women [35][27]. It is very difficult to distinguish GD from gestational transient thyrotoxicosis (GTT) in early pregnancy since both conditions have similar symptoms: palpitations, tremors, and heat intolerance. The likelihood of transient thyrotoxicosis during pregnancy is higher in pregnant women who have not had previous thyroid disease, goiter, or orbitopathy and have a mild vomiting disorder [37][28]. Hyperthyroidism in pregnancy can also be caused by toxic multinodular goiter and toxic adenoma [38][29]. Subacute painful or silent thyroiditis are very rare causes of thyrotoxicosis in pregnancy, while TSH-secreting pituitary adenoma, struma ovarii, functional thyroid cancer metastases, or a TSH receptor mutation leading to functional hypersensitivity to hCG [39][30] are even rarer.4. Autoimmune Hypothyroidism in Pregnancy
Hypothyroidism in pregnancy is commonly defined as the presence of elevated TSH and decreased serum T4 concentration during gestation, with both concentrations outside the trimester-specific reference ranges. For women with a TSH above the population and trimester-specific upper limit, or above 4.0 mIU/L when local reference ranges are not available, free T4 should be measured [41][31]. The most common cause of thyroid hypofunction during pregnancy is HT. It is estimated that 2–3% of healthy non-pregnant women of reproductive age have elevated TSH, and thyroid autoantibodies are present in around 30–60% of those cases. [42][32]. Continuing pregnancies with overt hypothyroidism have been associated with an increased risk of several complications such as preeclampsia, gestational hypertension, placental abruption, preterm delivery, low birth weight, increased rate of cesarean section, postpartum hemorrhage, perinatal morbidity and mortality, and neuropsychological and cognitive impairment in the child. De Leo reports the presence of TPOAbs and TgAbs in 5–14% and 3–18% of pregnant women, respectively [43][33]. In addition to this, TPOAbs were found in 9.5% of women with previous pregnancy loss or subfertility [44][34]. AITD is the most common autoimmune disease in women of reproductive age, affecting nearly 10% of that group. Keeping this in mind is of great interest to clinicians because of the potential negative impact on female fertility and pregnancy outcomes [45][35].5. The Immunological Background of AITD
The immunological background of AITD includes innate and adaptive mechanisms. In general, innate mechanisms are cell-mediated and ensure a prompt response to pathogens, whereas adaptive mechanisms are specific and involve the formation of antibodies targeted to specific antigens [46][36] but also seem to be directed by innate factors. HT is primarily characterized by cell-mediated autoimmunity in the form of lymphocytic infiltration, causing the destruction of thyroid follicles. It has recently been proposed that the presence of specific stromal cells in the thyroid of patients with HT stimulates inflammatory cell recruitment, even the formation of highly organized lymphocytic infiltrates termed tertiary lymphoid organs [47][37]. Tissue destruction and the consequent exposure of thyroid antigens cause autoantibody production. Contrary to adaptive immunity that has been extensively described in AITD, the cells of innate immunity involved in the pathophysiology of AITD have recently gained more attention. The main cells involved in the innate immunological processes are polymorphonuclear leukocytes (mainly neutrophils), innate lymphoid cells including natural killer (NK) cells, natural killer T (NKT) cells, monocytes, macrophages, and dendritic cells (DCs) [51,52,53,54][38][39][40][41].6. Innate Immunity during Normal Pregnancy
During normal pregnancy, a complex and extensive adaptation of the maternal immune system occurs to protect the mother and the fetus from infection and activation of detrimental immune response against the semi-allogeneic fetus. Throughout pregnancy, the innate immune system changes and plays a critical role in maintaining adaptive immunity in the tolerant state (Figure 1). This tolerogenic state during pregnancy is well established systemically and especially locally where maternal and fetal tissues are in direct contact with each other. It is considered that decidua and, as lately described, placenta play pivotal roles in the innate immune response [57][42].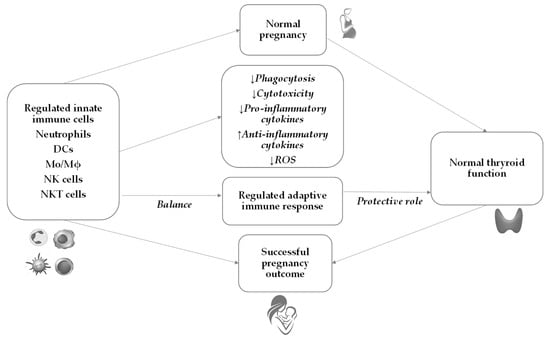
7. Innate Immunity in AITD and Pregnancy
The innate immune system plays a pivotal role in the early recognition and initiation of the immune response against the thyroid gland in AITD. The subsequent activation of the adaptive immune response further perpetuates the autoimmune process, leading to thyroid dysfunction (Figure 2).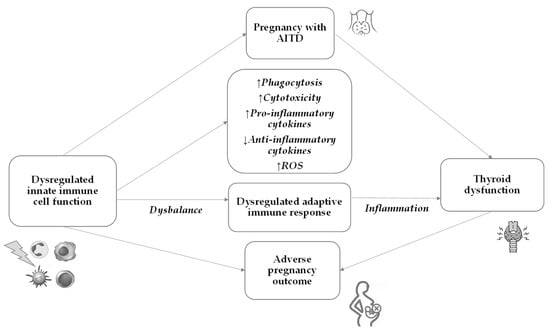
8. Emerging Causal Connection: Microbiota and the Thyroid (Dis)balance
9. Conclusions
The etiopathogenesis of AITD includes an immune reaction against own body antigens in thyroid cells of genetically susceptible individuals, in interaction with epigenetic and environmental factors. An activated cascade of immunological changes, which leads to the loss of immune tolerance, includes innate immunity, which further activates cells and mediators of adaptive immunity. Trigger antigen in this cascade as well as some other molecular events are still not known. For instance, a new area of investigation is the influence of gut dysbiosis on AITD. Until further studies resolve the background and help in new therapeutical approaches, it is of the utmost importance to identify and manage AITD in pregnant women to avoid adverse pregnancy outcomes and to ensure appropriate development of the newborns’ nervous system. Monitoring the thyroid function and proper thyroid hormone replacement medication are crucial to ensure that thyroid levels are within the appropriate range throughout the pregnancy. Regular follow-up visits and additional blood tests may be required to assess thyroid function and adjust medication dosage as needed. Proper diagnosis and management of autoimmune thyroiditis during pregnancy can significantly reduce the risk of complications for both the mother and the fetus. However, extensive studies are still needed to understand the complex immunopathogenesis of thyroid autoimmunity.References
- Franco, J.S.; Amaya-Amaya, J.; Anaya, J.M. Thyroid disease and autoimmune diseases. In Autoimmunity: From Bench to Bedside ; Anaya, J.M., Shoenfeld, Y., Rojas-Villarraga, A., Levy, R.A., Cervera, R., Eds.; El Rosario University Press: Bogota, Colombia, 2013; Chapter 30. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459466/ (accessed on 8 July 2023).
- Zhang, X.; Wang, X.; Hu, H.; Qu, H.; Xu, Y.; Li, O. Prevalence and Trends of Thyroid Disease among Adults, 1999–2018. Endocr. Pract. 2023, in press.
- Taylor, P.; Albrecht, D.; Scholz, A.; Gutierrez-Buey, G.; Lazarus, J.H.; Dayan, C.M.; Okosieme, O.E. Global epidemiology of hyperthyroidism and hypothyroidism. Nat. Rev. Endocrinol. 2018, 14, 301–316.
- Vargas-Uricoechea, H. Molecular Mechanisms in Autoimmune Thyroid Disease. Cells 2023, 12, 918.
- Kalarani, I.B.; Veerabathiran, R. Impact of iodine intake on the pathogenesis of autoimmune thyroid disease in children and adults. Ann. Pediatr. Endocrinol. Metab. 2022, 27, 256–264.
- Strikić Đula, I.; Pleić, N.; Babić Leko, M.; Gunjača, I.; Torlak, V.; Brdar, D.; Punda, A.; Polašek, O.; Hayward, C.; Zemunik, T. Epidemiology of Hypothyroidism, Hyperthyroidism and Positive Thyroid Antibodies in the Croatian Population. Biology 2022, 11, 394.
- Tomer, Y.; Huber, A. The etiology of autoimmune thyroid disease: A story of genes and environment. J. Autoimmun. 2009, 32, 231–239.
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506.
- Klubo-Gwiezdzinska, J.; Wartofsky, L. Hashimoto thyroiditis: An evidence-based guide to etiology, diagnosis and treatment. Pol. Arch. Intern. Med. 2022, 132, 16222.
- Gleicher, N.; Barad, D.H. Gender as risk factor for autoimmune diseases. J. Autoimmun. 2007, 28, 1–6.
- Biondi, B.; Cappola, A.R.; Cooper, D.S. Subclinical hypothyroidism: A review. JAMA 2019, 322, 153–160.
- Rodondi, N.; Den Elzen, W.P.; Bauer, D.C.; Cappola, A.R.; Razvi, S.; Walsh, J.P.; Asvold, B.O.; Iervasi, G.; Imaizumi, M.; Collet, T.H.; et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 2010, 304, 1365–1374.
- Chaker, L.; Baumgartner, C.; Den Elzen, W.P.; Ikram, M.A.; Blum, M.R.; Collet, T.H.; Bakker, S.J.; Dehghan, A.; Drechsler, C.; Luben, R.N.; et al. Subclinical hypothyroid¬ism and the risk of stroke events and fatal stroke: An individual participant data analysis. J. Clin. Endocrinol. Metab. 2015, 100, 2181–2191.
- Khosrotehrani, K.; Johnson, K.L.; Cha, D.H.; Salomon, R.N.; Bianchi, D.W. Transfer of fetal cells with multilineage potential to maternal tissue. J. Am. Med. Assoc. 2004, 292, 75–80.
- Yap, Y.W.; Onyekwelu, E.; Alam, U. Thyroid disease in pregnancy. Clin. Med. 2023, 23, 125–128.
- Ain, K.B.; Mori, Y.; Refetoff, S. Reduced clearance rate of thyroxine-binding globulin (TBG) with increased sialylation: A mechanism for estrogen-induced elevation of serum TBG concentration. J. Clin. Endocrinol. Metab. 1987, 65, 689–696.
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J.; et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 2017, 27, 315–389.
- Thangaratinam, S.; Tan, A.; Knox, E.; Kilby, M.D.; Franklyn, J.; Coomarasamy, A. Association between thyroid autoantibodies and miscarriage and preterm birth: Meta-analysis of evidence. BMJ 2011, 342, d2616.
- Tańska, K.; Gietka-Czernel, M.; Glinicki, P.; Kozakowski, J. Thyroid autoimmunity and its negative impact on female fertility and maternal pregnancy outcomes. Front. Endocrinol. 2023, 13, 1049665.
- Li, M.; He, Y.; Mao, Y.; Yang, L.; Chen, L.; Du, J.; Chen, Q.; Zhu, Q.; Liu, J.; Zhou, W. Preconception thyroid-stimulating hormone levels and adverse pregnancy outcomes. Clin. Endocrinol. 2022, 97, 339–346.
- Knøsgaard, L.; Andersen, S.; Hansen, A.B.; Vestergaard, P.; Andersen, S.L. Maternal hypothyroidism and adverse outcomes of pregnancy. Clin. Endocrinol. 2023, 98, 719–729.
- Moleti, M.; Alibrandi, A.; Di Mauro, M.; Paola, G.; Perdichizzi, L.G.; Granese, R.; Giacobbe, A.; Scilipoti, A.; Ragonese, M.; Ercoli, A.; et al. Preconception Thyrotropin Levels and Thyroid Function at Early Gestation in Women with Hashimoto Thyroiditis. J. Clin. Endocrinol. Metab. 2023, 108, e464–e473.
- Maganha, C.A.; Mattar, R.; Mesa Júnior, C.O.; Marui, S.; Solha, S.T.G.; Teixeira, P.F.D.S.; Zaconeta, A.C.M.; Souza, R.T. Screening, diagnosis and management of hyperthyroidism in pregnancy. Rev. Bras. Ginecol. Obstet. 2022, 44, 806–818.
- Cooper, D.S.; Laurberg, P. Hyperthyroidism in pregnancy. Lancet Diabetes Endocrinol. 2013, 1, 238–249.
- Samuels, S.L.; Namoc, S.M.; Bauer, A.J. Neonatal Thyrotoxicosis. Clin. Perinatol. 2018, 45, 31–40.
- Gheorghiu, M.L.; Bors, R.G.; Gheorghisan-Galateanu, A.A.; Pop, A.L.; Cretoiu, D.; Varlas, V.N. Hyperthyroidism in Pregnancy: The Delicate Balance between Too Much or Too Little Antithyroid Drug. J. Clin. Med. 2021, 10, 3742.
- Li, C.; Zhou, J.; Huang, Z.; Pan, X.; Leung, W.; Chen, L.; Zhang, Y.; Wang, L.; Sima, Y.; Gober, H.J.; et al. The Clinical Value and Variation of Antithyroid Antibodies during Pregnancy. Dis. Markers 2020, 2020, 8871951.
- Thyroid Disease in Pregnancy. ACOG Practice Bulletin, Number 223. Obstet. Gynecol. 2020, 135, e261–e274.
- Ross, D.S.; Burch, H.B.; Cooper, D.S.; Greenlee, M.C.; Laurberg, P.; Maia, A.L.; Rivkees, S.A.; Samuels, M.; Sosa, J.A.; Stan, M.N.; et al. 2016 American Thyroid Association Guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 2016, 26, 1343–1421.
- Caron, P. Management of thyrotoxicosis and pregnancy: Review of the current literature and an update of the care pathway. Ann. Endocrinol. 2022, 83, 226–231.
- Sullivan, S. Hypothyroidism in Pregnancy. Clin. Obstet. Gynecol. 2019, 62, 308–319.
- Cigrovski Berković, M.; Herman Mahečić, D.; Marinković Radošević, J.; Strinović Morić, M.; Bilić-Ćurčić, I. Hypothyroidism and pregnancy: Still a controversial issue. Gynecol. Endocrinol. 2020, 36, 776–780.
- De Leo, S.; Pearce, E.N. Autoimmune thyroid disease during pregnancy. Lancet Diabetes Endocrinol. 2018, 6, 575–586.
- Dhillon-Smith, R.K.; Tobias, A.; Smith, P.P.; Middleton, L.J.; Sunner, K.K.; Baker, K.; Farrell-Carver, S.; Bender-Atik, R.; Agrawal, R.; Bhatia, K.; et al. The prevalence of thyroid dysfunction and autoimmunity in women with history of miscarriage or subfertility. J. Clin. Endocrinol. Metab. 2020, 105, 2667–2677.
- Vissenberg, R.; Manders, V.D.; Mastenbroek, S.; Fliers, E.; Afink, G.B.; Ris-Stalpers, C.; Goddijn, M.; Bisschop, P.H. Pathophysiological aspects of thyroid hormone disorders/thyroid peroxidase autoantibodies and reproduction. Hum. Reprod. Update 2015, 21, 378–387.
- Yatim, K.M.; Lakkis, F.G. A brief journey through the immune system. Clin. J. Am. Soc. Nephrol. 2015, 10, 1274–1281.
- Zhang, Q.Y.; Ye, X.P.; Zhou, Z.; Zhu, C.F.; Li, R.; Fang, Y.; Zhang, R.J.; Li, L.; Liu, W.; Wang, Z.; et al. Lymphocyte infiltration and thyrocyte destruction are driven by stromal and immune cell components in Hashimoto’s thyroiditis. Nat. Commun. 2022, 13, 775.
- Diefenbach, A.; Colonna, M.; Koyasu, S. Development, differentiation, and diversity of innate lymphoid cells. Immunity 2014, 41, 354–365.
- Woo, S.R.; Corrales, L.; Gajewski, T.F. Innate immune recognition of cancer. Annu. Rev. Immunol. 2015, 33, 445–474.
- Dadi, S.; Chhangawala, S.; Whitlock, B.M.; Franklin, R.A.; Luo, C.T.; Oh, S.A.; Toure, A.; Pritykin, Y.; Huse, M.; Leslie, C.S.; et al. Cancer immunosurveillance by tissue-resident innate lymphoid cells and innate-like T cells. Cell 2016, 164, 365–377.
- Ebbo, M.; Crinier, A.; Vely, F.; Vivier, E. Innate lymphoid cells: Major players in inflammatory diseases. Nat. Rev. Immunol. 2017, 17, 665–678.
- Olmos-Ortiz, A.; Flores-Espinosa, P.; Mancilla-Herrera, I.; Vega-Sánchez, R.; Díaz, L.; Zaga-Clavellina, V. Innate Immune Cells and Toll-like Receptor-Dependent Responses at the Maternal-Fetal Interface. Int. J. Mol. Sci. 2019, 20, 3654.
- Cristiani, C.M.; Palella, E.; Sottile, R.; Tallerico, R.; Garofalo, C.; Carbone, E. Human NK Cell Subsets in Pregnancy and Disease: Toward a New Biological Complexity. Front. Immunol. 2016, 7, 656.
- Lee, S.K.; Kim, C.J.; Kim, D.J.; Kang, J.H. Immune cells in the female reproductive tract. Immune Netw. 2015, 15, 16–26.
- Lurie, S.; Rahamim, E.; Piper, I.; Golan, A.; Sadan, O. Total and differential leukocyte counts percentiles in normal pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 136, 16–19.
- Silvestre-Roig, C.; Fridlender, Z.G.; Glogauer, M.; Scapini, P. Neutrophil Diversity in Health and Disease. Trends Immunol. 2019, 40, 565–583.
- Hahn, S.; Hasler, P.; Vokalova, L.; van Breda, S.V.; Lapaire, O.; Than, N.G.; Hoesli, I.; Rossi, S.W. The role of neutrophil activation in determining the outcome of pregnancy and modulation by hormones and/or cytokines. Clin. Exp. Immunol. 2019, 198, 24–36.
- True, H.; Blanton, M.; Sureshchandra, S.; Messaoudi, I. Monocytes and macrophages in pregnancy: The good, the bad, and the ugly. Immunol. Rev. 2022, 308, 77–92.
- Uematsu, S.; Akira, S. Toll-like receptors and innate immunity. J. Mol. Med. 2006, 84, 712–725.
- Lampé, R.; Kövér, Á.; Szűcs, S.; Pál, L.; Árnyas, E.; Ádány, R.; Póka, R. Phagocytic index of neutrophil granulocytes and monocytes in healthy and preeclamptic pregnancy. J. Reprod. Immunol. 2015, 107, 26–30.
- Faas, M.M.; Spaans, F.; De Vos, P. Monocytes and macrophages in pregnancy and pre-eclampsia. Front. Immunol. 2014, 5, 298.
- Ramhorst, R.; Grasso, E.; Paparini, D.; Hauk, V.; Gallino, L.; Calo, G.; Vota, D.; Perez Leiros, C. Decoding the chemokine network that links leukocytes with decidual cells and the trophoblast during early implantation. Cell Adhes. Migr. 2016, 10, 197–207.
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964.
- Faas, M.M.; de Vos, P. Maternal monocytes in pregnancy and preeclampsia in humans and in rats. J. Reprod. Immunol. 2017, 119, 91–97.
- Xu, X.; Zhou, Y.; Fu, B.; Wei, H. Uterine NK cell functions at maternal-fetal interface. Biol. Reprod. 2022, 107, 327–338.
- Weng, J.; Couture, C.; Girard, S. Innate and Adaptive Immune Systems in Physiological and Pathological Pregnancy. Biology 2023, 12, 402.
- Stramazzo, I.; Capriello, S.; Filardo, S.; Centanni, M.; Virili, C. Microbiota and Thyroid Disease: An Updated Systematic Review. Adv. Exp. Med. Biol. 2023, 1370, 125–144.
- Knezevic, J.; Starchl, C.; Tmava Berisha, A.; Amrein, K. Thyroid-Gut-Axis: How Does the Microbiota Influence Thyroid Function? Nutrients 2020, 12, 1769.
- Deng, Y.; Wang, J.; Xie, G.; Zou, G.; Li, S.; Zhang, J.; Cai, W.; Xu, J. Correlation between gut microbiota and the development of Graves’ disease: A prospective study. iScience 2023, 26, 107188.
