You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Doaa Alqaidy.
Thymomas are considered one of the most prevalent types of mediastinal epithelial tumors, which frequently develop in the anterior mediastinum.
- thymus
- mediastinum
- thymoma
- staging
- classification
1. Introduction
Thymic epithelial lesions are mostly thymomas. They are a common cause of anterior mediastinal mass in adults [1]. However, in general practice, thymomas account for approximately 0.2–1.5% of all malignant neoplasms. Among the anterior mediastinal tumors, thymoma is the most common. It accounts for 20% of all mediastinal neoplasm in adults [2,3][2][3]. Thymomas occur in 0.13–0.26 cases per 100,000 people per year [1,4,5][1][4][5]. They are extremely uncommon in young people and are more prevalent among individuals in their fifth and sixth decades of life. Only 11% of thymomas were diagnosed before the age of 35 [1,6][1][6]. It affects females slightly more commonly than males [3,4,7][3][4][7]. In a more detailed analysis of the epidemiology of thymoma, Engels stated in his study that Asian and Pacific Islanders had a greater prevalence of thymomas in the USA [8].
Patients are commonly presented as a single well-circumscribed mass, readily identified by the computed tomography (CT) technique. The majority of thymomas present as round or oval lobulated lesions on magnetic resonance imaging (MRI) scans and computed tomography (CT) [6,9,10][6][9][10]. Areas of calcification and hemorrhage (low densities on CT scan) are rarely seen, although irregularities are often indicators of local invasion and aggressive behavior [1,9,10][1][9][10].
2. Clinical Features
Thymoma can present in different ways, including asymptomatically as a mediastinal mass on chest radiography (about one-third of cases), local compressive symptoms as chest pain, a neck mass, or superior vena cava syndrome, or concurrently with myasthenia gravis in one-third of cases [1,7,8][1][7][8].
Although thymoma patients present with a variety of symptomatologies, it is important to emphasize the link between thymomas and paraneoplastic diseases [11,12][11][12]. The numerous associations between thymomas and other illnesses, such as autoimmune disorders, collagen vascular disorders, hematological disorders, neoplasia, and others, are well established [1,7,8][1][7][8]. The medical condition that seems to have the strongest association with thymoma is myasthenia gravis. It is widely believed that thymomas are present in roughly 10–15% of myasthenia gravis (MG) patients [13]. Additionally, it has been shown that 20–25% of individuals with MG and 40% of those with thymoma had one or more paraneoplastic autoimmune diseases [2,7,14,15][2][7][14][15]. In a study by Mao et al. [14[14][16],16], the authors stated that out of 2206 potentially relevant studies, the incidence of thymoma in MG patients was 21%. Additionally, thymoma appears to be much more common in male MG patients and those who were older than 40 at the time of MG’s diagnosis [14]. Furthermore, in a study by Okuma et al. [17], immunological abnormalities were found in 21.8% of the thymoma group in a clinicopathological investigation of 187 thymic malignancies, including thymomas, thymic carcinomas, and neuroendocrine carcinomas. In total, 13.3% of these individuals had secondary malignancy [17]. Weissferdt et al., in one of the largest series on thymomas ever reported [18], found that 807 patients out of 1470 patients with thymomas had pertinent clinical data available to them. The authors found the following connections in their examination of 807 patients:
-
Myasthenia gravis—17%;
-
Neoplasia—6.8%;
-
Other autoimmune diseases—3.8%.
In terms of therapy and prognosis, the preferred course of therapy for thymomas is surgical resection, which is typically successful [7,15][7][15]. The extent of the tumor’s invasiveness and its resectability, however, are significant factors that are considered when determining if surgical resection is appropriate [19]. The degree of invasion is directly related to the clinical prognosis for patients with thymomas. Complete surgical resection seems to be the preferred course of treatment for individuals whose illness has only spread to the mediastinum and the risk of recurrence is low [20]. On the other hand, invasive disease is more likely to be treated with additional medical techniques, such as chemotherapy and/or radiation therapy [10,16,19][10][16][19]. Even when a full resection is performed, thymoma often recurred, so it is important to plan for a long period of follow-up. Recurrences of thymoma affect between 10 and 29% of individuals. Distant metastasis commonly manifests in the lungs, liver, and bone [7]. The lung is widely recognized as the most frequent location for distant metastases.
3. Thymoma Classification
Even though many publications have noted the difficulty in classifying thymomas, specifically due to their heterogeneity and the risk of predicting outcomes based on specific cell types, this is likely one of the most debatable issues in the history of thymomas [2,21][2][21]. For classifying thymomas, several histological schemes have been suggested and proposed over the years [2[2][11][21],11,21], Table 1.
Table 1.
Different histological classification systems.
| Bernatz | Muller-Hermelink | Moran–Suster | WHO, 6th Edition |
|---|---|---|---|
| Spindle cell thymomas Mixed thymomas Lymphocytes-rich thymomas |
Medullary thymoma Mixed thymoma Predominantly cortical Cortical thymoma |
Thymoma Thymoma Thymoma Thymoma |
Type A Type AB Type B1 Type B2 |
| Epithelial-rich thymomas with cytologic atypia | Well-differentiated thymic carcinoma | Atypical thymoma | Type B3 |
| Thymic carcinoma | Thymic carcinoma | Thymic carcinoma |
Prior to the original 1999 World Health Organization consensus publication on thymoma, the first attempt to classify this heterogenous group of tumors in 1961 was made by Bernatz [22] et al. based on the amount of lymphocytes present in each kind of thymoma into the following categories:
-
Lymphocyte-rich thymomas are those tumors where lymphocytes predominate over epithelial cells;
-
Epithelial-rich thymomas are those where lymphocytes are present in smaller amounts than the epithelial cells;
-
Mixed thymomas, also known as lymphoepithelial thymomas, are those tumors where lymphocytes and epithelial cells are present in roughly equal amounts.
The “histologic-based classification”, which Marino and Muller-Hermelink suggested in 1985 [23], is based on the medulla and cortex of the normal thymus compartment. The tumors are categorized into three categories in this proposal: Tumors that are intended to recapitulate the healthy thymic medulla are known as “medullary thymomas”. However, thymomas that mimic the normal thymic cortex are referred to as cortical thymomas. Finally, mixed thymomas are tumors with medullary and cortical components. Furthermore, a more histologic classification attempt was proposed, and in 1999, because of several differences in how thymomas are classified histologically, the World Health Organization (WHO) proposed an “official” classification system [4,6,21][4][6][21]. The classification depends on the concept that there are two primary histologic kinds of tumor cells in thymomas: round/epithelioid (named type B) and spindle/oval (designated type A) [21], (Figure 1). Type AB was given to tumors that included elements of these two categories. In the current 2021 WHO classification [4], type AB thymoma is also compassing cases of mixtures with a lymphocyte-depleted type A component and a type B-like lymphocyte-rich component. These elements may combine into distinct, independent lobules or may be intermingled. For thymoma type B, three subgroups designated as B1, B2, and B3 were further subclassified on the basis of the proportional increase (in relation to the lymphocytes) and the presence of atypia of the neoplastic epithelial cells [2,4,21][2][4][21]. The former WHO type C thymoma, which was designated to cases that revealed significant cytologic atypia, nuclear pleomorphism, and notable mitotic activity, all of which are signs of malignancy, was removed from the 2004 WHO classification, and since then, these cases are considered thymic carcinoma [2,4,21][2][4][21]. The diagnosis should describe all observable histological types, starting with the main component, and minor components should be estimated in 10% increments for thymomas that have several histological patterns [4].
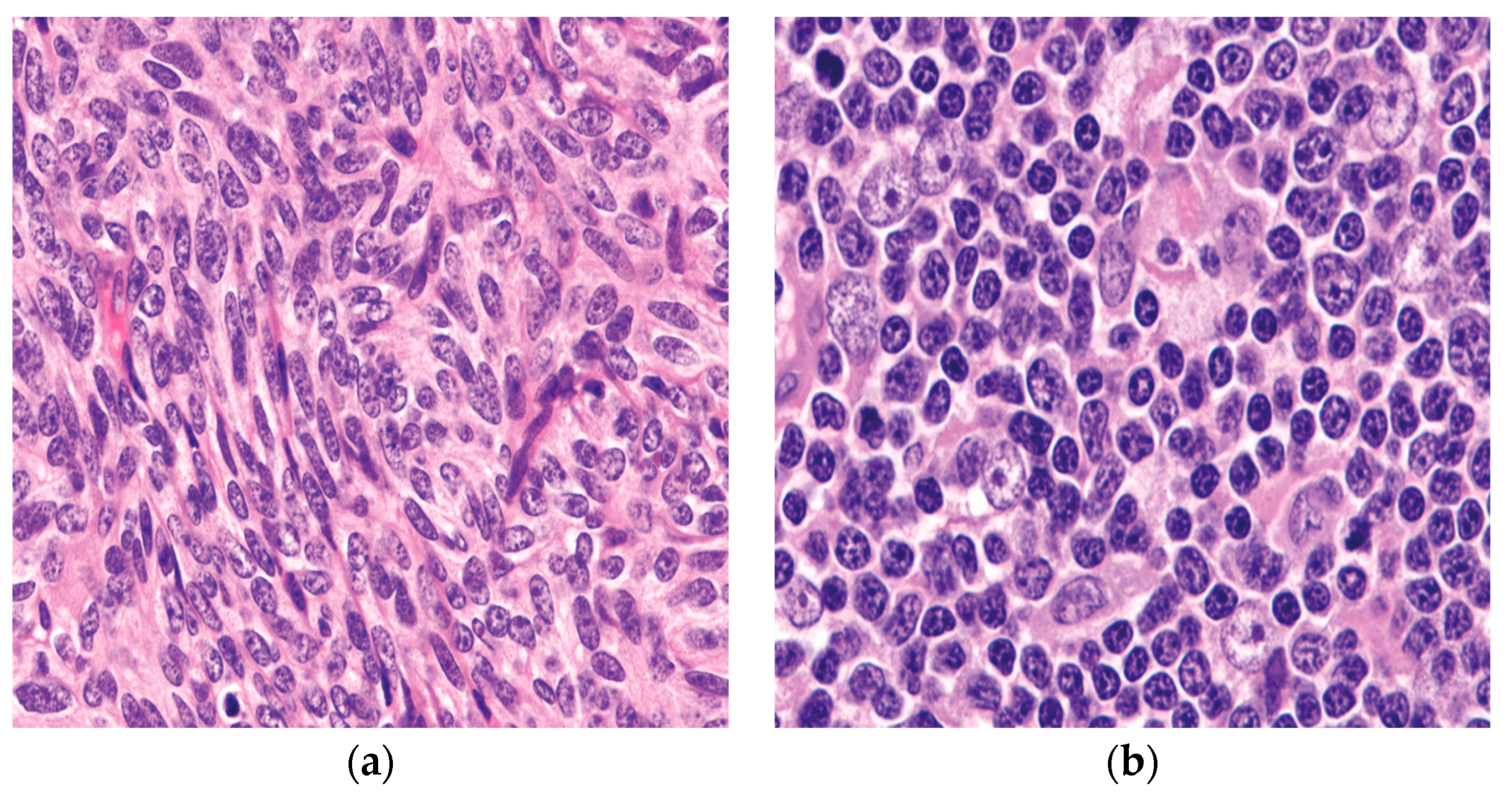
Figure 1. The two epithelial components characteristically seen in thymoma: (a) the spindle/oval cells commonly seen in type A and type AB thymoma; (b) round/epithelioid epithelial cells found in type B thymoma. (a,b) (H&E, 40×).
When discussing thymoma classification, it is crucial to highlight the Suster–Moran proposal which was first introduced in 1999 [24]. According to their proposal, primary thymic epithelial neoplasms are a spectrum of lesions that range from well to poorly differentiated tumors. This assumption served as the foundation for their histologic grading of the tumors [21,24][21][24]. Based on the presence or absence of the distinctive organotypical signs of differentiation in the normal thymus and the cytologic atypia, the degree of differentiation for every specific subtype was established as follows:
-
Thymoma: Well-differentiated thymic epithelial neoplasms are tumors that exhibit the majority or all the organotypical characteristics of thymic differentiation and lack of cytologic atypia.
-
Atypical thymoma: Tumors that exhibit mild to moderate cytologic atypia and only certain organotypical characteristics of thymic differentiation are categorized as moderately differentiated thymic epithelial neoplasms.
-
Thymic carcinoma: These tumors are poorly differentiated thymic epithelial neoplasms and are defined by the complete lack of the organotypical characteristics of the thymus and overt cytological signs of malignancy.
It is also vital to note that thymomas are tumors with a complex heterogeneity and many growth patterns, making it challenging to propose a single and simple classification scheme [25,26][25][26]. Because of this heterogeneity, any histological schema is rather unrealistic, and the staging of the tumor at the time of diagnosis is more important than ever for determining the best course of treatment [27,28][27][28].
3.1. Pathological Features
Thymomas can have a wide range of macroscopic characteristics, such as solid, cystic, and areas of infarction/necrosis [27]. The majority of thymomas are well-defined tumors, however, a subset can be ill-defined [1,7,27][1][7][27] with ill-defined capsules. In thymoma cases with prominent cystic or hemorrhagic changes, special attention should be given to proper sampling of the solid areas which usually illustrate the classic thymoma features. Failure to do so will delay the diagnosis and the proper management [1]. Size wise, tumors can range from as tiny as 1 cm to ones with a maximum diameter of far over 10 cm [29].
Microscopically, as was already mentioned, there is a broad variety of histological growth patterns that can be observed in thymomas and they can be diagnostic pitfalls.
3.1.1. Conventional Thymoma with Lymphocytes
Included in this category are the tumors that have been classified as mixed thymoma—lymphoepithelial, cortical, and WHO type B2, as well as those that have been identified as lymphocyte-rich, cortical, and WHO type B1 in other nomenclatures [1,21][1][21]. Microscopically, this thymoma is lymphocytic rich with minimal epithelial components. It usually has the characteristic perivascular space [7,11][7][11] and three distinct growth patterns may be visible in the low-power image of these tumors:
-
A lobulated growth pattern with considerable hyalinization or bands of connective tissue between the lobules (Figure 2a).
-
The tumor lacks well-formed lobules, but there is the presence of collagenous material mixed with the biphasic populations of lymphocytes and epithelial cells (Figure 2b).
-
A diffuse growth pattern in which the tumor exhibits sheets of lymphocytes mixed with epithelial cells in various ratios (Figure 2c,d).
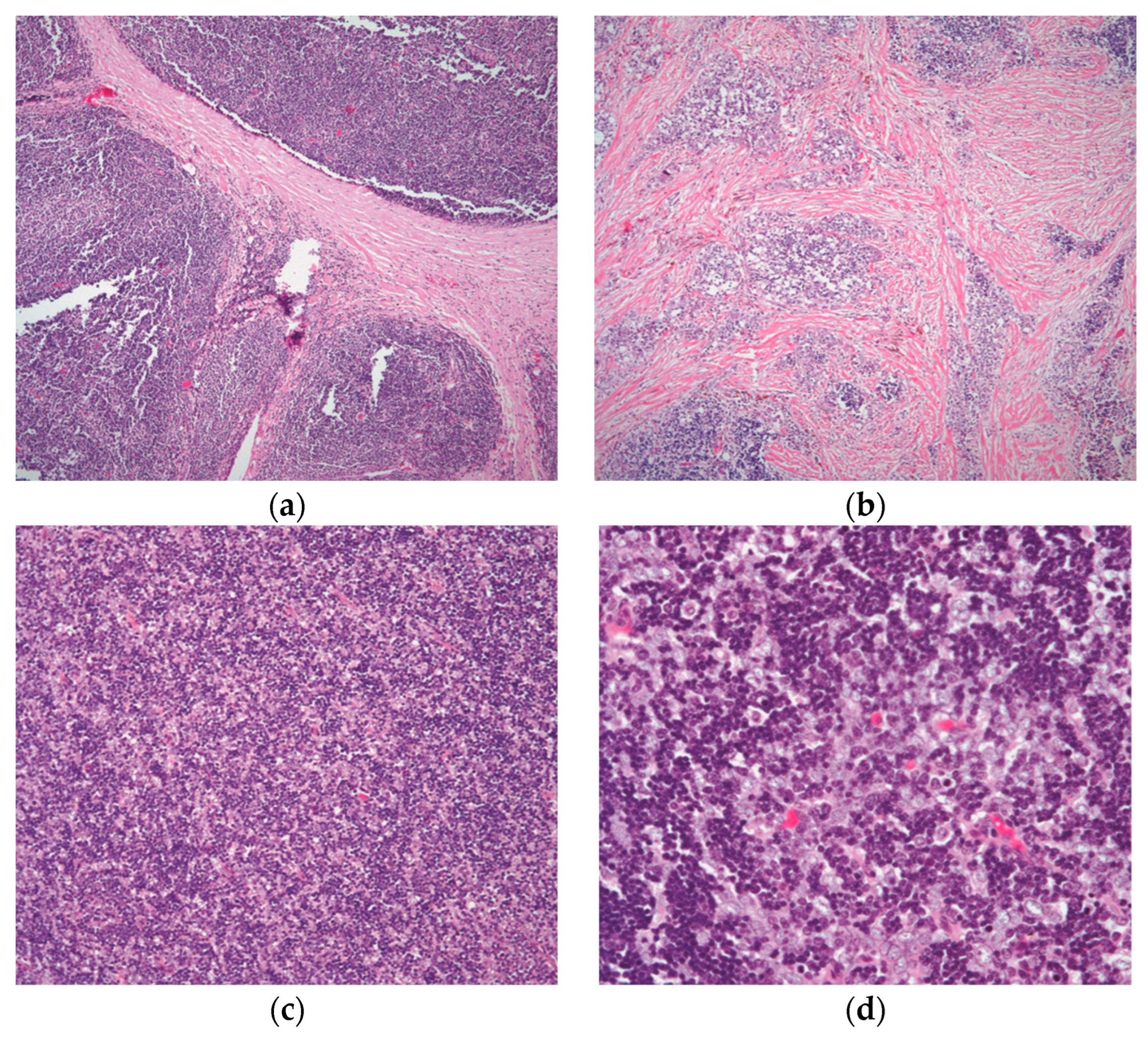
Figure 2. The different growth patterns of conventional thymoma: (a) type B1 thymoma showing lobulation by thick fibrous bands; (b) thymoma in areas of fibrocollagenous stroma with no well-defined lobulation; (c) thymoma with an even distribution of lymphocytes and epithelial cells and a diffuse development pattern; and (d) higher magnification displaying the mixed population of lymphocytes and polygonal epithelial cells. (a,b) (H&E, 4×); (c) (H&E, 10×); (d) (H&E, 20×).
Statistically, these tumors represented 31.1% of the total and 51.9% of the invasive thymoma in that category in a study of 1470 patients that was conducted by Weissferdt et al. [18]. The authors concluded that half of all cases of these types of thymomas may be invasive at the time of diagnosis from the total number of cases of these tumors.
3.1.2. Spindle Cell Thymoma
Spindle cell thymoma has a high degree of morphologic diversity. The tumor cells may be arranged in ways that may resemble other epithelial or mesenchymal neoplasms, but their fundamental morphology is that of a spindle cellular proliferation made up of fusiform cells with sparse cytoplasm, elongated nuclei, dispersed chromatin, and inconspicuous nucleoli [19,25][19][25] (Figure 3).
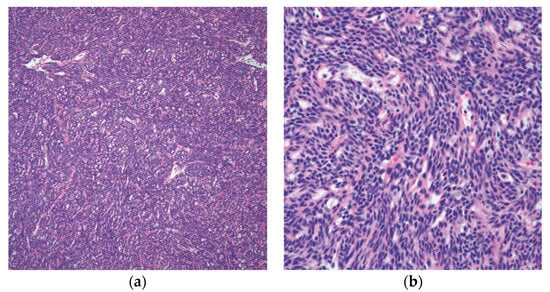
Figure 3. Spindle cell thymoma histologic feature: (a) low power of thymoma type A/spindle cell thymoma; (b) high power view showing the characteristic spindle cell cytology. (a) (H&E, 10×); (b) (H&E, 20×).
These tumors can exhibit three distinct growth patterns at low power magnification: lobulated, diffuse, and vascular or HPC growth pattern [30,31][30][31]. Additionally, regions of hyalinization, which can range from focal to widespread, may be seen in spindle cell thymomas. The lymphocyte component can vary in this thymoma from none or few lymphocytes to prominent lymphocytes intermixed with the spindle cells. In the WHO classification, this thymoma is classified as type A or type AB. From a statistical perspective, spindle cell thymomas make up around 13% of all thymomas [31,32][31][32].
3.1.3. Atypical Thymoma, WHO Type B3 Thymoma
This entity has histological characteristics and clinical behavior that are more similar to thymomas than to carcinomas, and as a result, the term “atypical thymoma” is best used to describe an entity with characteristics that fall somewhere between thymomas and thymic carcinomas [2,24,33][2][24][33]. Morphologically, the epithelial proliferation in this specific type of thymoma is characterized by round to polygonal cells with an abundance of eosinophilic cytoplasm, vesicular nuclei, several cells with conspicuous nucleoli, and occasionally mitotic figures [27,34][27][34]. More significantly, these tumors have a sparse population of T-immature lymphocytes, which is essential (Figure 4).
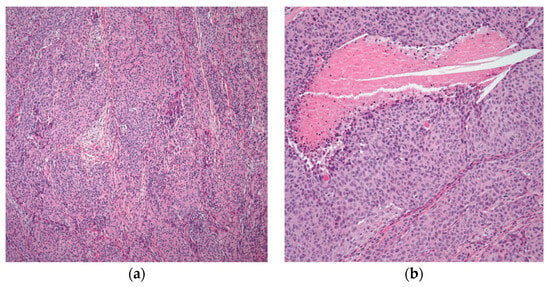
Figure 4. Atypical thymoma/WHO type B3: (a) atypical thymoma case showing nested pattern and rosette-like formation; (b) higher magnification showing atypia and focal comedo necrosis. (a) (H&E, 4×); (b) (H&E, 10×).
Atypical thymoma (WHO category B3) appears to be more frequently attributed to invasion, more rapid recurrence, and a higher risk of tumor-related fatalities than the better-differentiated forms of the disease [2,21,35][2][21][35]. In a large cohort study by Weissferdt et al. [18], they examined 1470 thymomas, of which 186 were atypical thymomas, accounting for around 12.7% of the total. In total, 139 of the 186 atypical thymomas in this cohort were invasive tumors at various clinical stages, roughly 75% of the cases. As a result, the distinction between thymomas and atypical thymomas became statistically significant when a survival curve was analyzed.
3.2. Unusual Histologic Subtypes
3.2.1. Micronodular Thymoma with B-Cell Lymphoid Hyperplasia
The first description of this specific type of thymoma was by Suster and Moran in 1999 [36], which is characterized by the presence of lymphoid nodules that have significant germinal centers and spindle cell nodules. The oval cells that make up the spindle cell nodules lack nuclear atypia and mitotic activity (Figure 5).
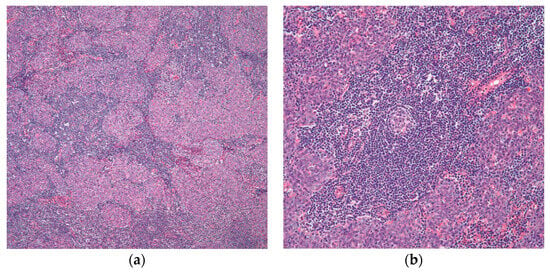
Figure 5. Micronodular thymoma with B-cell lymphoid hyperplasia: (a) islets of nodular epithelium embedded within a lymphocytic stroma; (b) many germinal centers are easily identifiable. (a) (H&E, 2×); (b) (H&E, 10×).
Cystic changes can be prominent in this type of thymoma. Oramas et al. reported the clinopathological features of 25 cases of micronodular thymomas with prominent cystic changes. In this case series, four cases were invasive tumors with invasion into the perithymic adipose tissue through the fibrous capsule. However, the majority of them (21 tumors) were encapsulated [37]. By immunohistochemistry, the epithelial spindle cells are positive for cytokeratin, while the lymphoid component was shown to be mainly B-lymphocytes [1,37][1][37]. In the clinical follow-up provided in this initial publication [36], as well as in the subsequent studies, the patients were free of recurrence in around half of the cases. This clinical behavior was like that seen in conventional thymomas.
3.2.2. Other Histologic Variants
Thymoma with significant plasma cells, also known as plasma cell-rich thymoma, is one of the rare histologic types that appears to frequently be associated with an underlying autoimmune illness, particularly myasthenia gravis, [27,38][27][38]. It shows a mixture of both epithelial and plasma cells. It is also important to note that plasma cells are one of the normal cellular components of the normal thymus, and they may be present in the normal thymus, although not in the same proportion as lymphocytes [3,7,39][3][7][39]. Metaplastic thymoma is another unusual histological type of thymoma. It is characterized by a spindle cell component mimicking sarcoma with minimal lymphoid component. This specific variant has been described in 1997 under the term of thymoma with pseudosarcomatous component [40]. Other rare variants of thymomas can have myoid cells, showing papillary/pseudopapillary, adenomatoid-like, alveolar, glandular, signet ring cell, and clear cell features. It is important to bring attention to the difficulty that these thymoma variations may present in tiny mediastinoscopic biopsies, potentially leading to an incorrect diagnosis. Those tumors, nonetheless, need to be treated appropriately under the term “thymoma”, and more crucially, they do belong to certain categories rather than a specific position in any classification scheme [27].
References
- Kalhor, N.; Moran, C. Mediastinal Pathology; Springer: Berlin/Heidelberg, Germany, 2019.
- Suster, S.; Moran, C.A. Thymoma classification: Current status and future trends. Am. J. Clin. Pathol. 2006, 125, 542–554.
- Anastasiadis, K.; Ratnatunga, C. The Thymus Gland; Springer: Berlin/Heidelberg, Germany, 2007.
- Tsao, M.-S.; Nicholson, A.G.; Maleszewski, J.J.; Marx, A.; Travis, W.D. Reprint of “Introduction to 2021 WHO Classification of Thoracic Tumors”. J. Thorac. Oncol. 2022, 17, 337–340.
- Rich, A.L. Epidemiology of thymoma. J. Thorac. Dis. 2020, 12, 7531.
- Detterbeck, F.C.; Stratton, K.; Giroux, D.; Asamura, H.; Crowley, J.; Falkson, C.; Filosso, P.L.; Frazier, A.A.; Giaccone, G.; Huang, J. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: Proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J. Thorac. Oncol. 2014, 9, S65–S72.
- Lavini, C.; Moran, C.A.; Morandi, U.; Schoenhuber, R. Thymus Gland Pathology: Clinical, Diagnostic and Therapeutic Features; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009.
- Engels, E.A. Epidemiology of thymoma and associated malignancies. J. Thorac. Oncol. 2010, 5, S260–S265.
- Harris, K.; Elsayegh, D.; Azab, B.; Alkaied, H.; Chalhoub, M. Thymoma calcification: Is it clinically meaningful? World J. Surg. Oncol. 2011, 9, 95.
- Benveniste, M.F.; Rosado-de-Christenson, M.L.; Sabloff, B.S.; Moran, C.A.; Swisher, S.G.; Marom, E.M. Role of imaging in the diagnosis, staging, and treatment of thymoma. Radiographics 2011, 31, 1847–1861.
- Kalhor, N.; Moran, C.A. Thymoma: Current concepts. Oncology 2012, 26, 975.
- Tian, D.; Shiiya, H.; Sato, M.; Sun, C.B.; Anraku, M.; Nakajima, J. Tumor location may affect the clinicopathological features and prognosis of thymomas. Thorac. Cancer 2019, 10, 2096–2105.
- Bernard, C.; Frih, H.; Pasquet, F.; Kerever, S.; Jamilloux, Y.; Tronc, F.; Guibert, B.; Isaac, S.; Devouassoux, M.; Chalabreysse, L. Thymoma associated with autoimmune diseases: 85 cases and literature review. Autoimmun. Rev. 2016, 15, 82–92.
- Mao, Z.-F.; Mo, X.-A.; Qin, C.; Lai, Y.-R.; Hackett, M.L. Incidence of thymoma in myasthenia gravis: A systematic review. J. Clin. Neurol. 2012, 8, 161–169.
- López-Cano, M.; Ponseti-Bosch, J.M.; Espin-Basany, E.; Sánchez-García, J.L.; Armengol-Carrasco, M. Clinical and pathologic predictors of outcome in thymoma-associated myasthenia gravis. Ann. Thorac. Surg. 2003, 76, 1643–1649.
- Gupta, R.; Marchevsky, A.M.; McKenna, R.J.; Wick, M.; Moran, C.; Zakowski, M.F.; Suster, S. Evidence-based pathology and the pathologic evaluation of thymomas: Transcapsular invasion is not a significant prognostic feature. Arch. Pathol. Lab. Med. 2008, 132, 926–930.
- Okuma, Y.; Hosomi, Y.; Watanabe, K.; Yamada, Y.; Horio, H.; Maeda, Y.; Okamura, T.; Hishima, T. Clinicopathological analysis of thymic malignancies with a consistent retrospective database in a single institution: From Tokyo Metropolitan Cancer Center. BMC Cancer 2014, 14, 349.
- Weissferdt, A.; Kalhor, N.; Bishop, J.A.; Jang, S.J.; Ro, J.; Petersson, F.; Wu, B.; Langman, G.; Bancroft, H.; Bi, Y. Thymoma: A clinicopathological correlation of 1470 cases. Hum. Pathol. 2018, 73, 7–15.
- Weis, C.-A.; Yao, X.; Deng, Y.; Detterbeck, F.C.; Marino, M.; Nicholson, A.G.; Huang, J.; Ströbel, P.; Antonicelli, A.; Marx, A. The impact of thymoma histotype on prognosis in a worldwide database. J. Thorac. Oncol. 2015, 10, 367–372.
- Luo, T.; Zhao, H.; Zhou, X. The clinical features, diagnosis and management of recurrent thymoma. J. Cardiothorac. Surg. 2016, 11, 140.
- Suster, S.; Moran, C.A. Histologic classification of thymoma: The World Health Organization and beyond. Hematol./Oncol. Clin. N. Am. 2008, 22, 381–392.
- Pe, B. Thymoma: A clinicopathologic study. J. Thorac. Cardiovasc. Surg. 1961, 42, 424–444.
- Marino, M.; Müller-Hermelink, H.K. Thymoma and thymic carcinoma: Relation of thymoma epithelial cells to the cortical and medullary differentiation of thymus. Virchows Arch. A 1985, 407, 119–149.
- Suster, S.; Moran, C.A. Thymoma, atypical thymoma, and thymic carcinoma: A novel conceptual approach to the classification of thymic epithelial neoplasms. Am. J. Clin. Pathol. 1999, 111, 826–833.
- Ruangchira-urai, R.; Treetipsatit, J. Update in Thymoma for Surgical Pathologists. Asian Arch. Pathol. 2015, 11, 114–132.
- Johnson, S.B.; Eng, T.Y.; Giaccone, G.; Thomas, C.R., Jr. Thymoma: Update for the new millenium. Oncologist 2001, 6, 239–246.
- Oramas, D.M.; Moran, C.A. Thymoma: Histologically a heterogenous group of tumors. Semin. Diagn. Pathol. 2022, 39, 99–104.
- Tian, W.; Sun, Y.; Wu, Q.; Jiao, P.; Ma, C.; Yu, H.; Huang, C.; Tong, H. Surgical outcomes of 215 patients with thymic epithelial tumors: A single-center experience. Thorac. Cancer 2020, 11, 1840–1847.
- Okumura, M.; Yoshino, I.; Yano, M.; Watanabe, S.-I.; Tsuboi, M.; Yoshida, K.; Date, H.; Yokoi, K.; Nakajima, J.; Toyooka, S.-I. Tumour size determines both recurrence-free survival and disease-specific survival after surgical treatment for thymoma. Eur. J. Cardio-Thorac. Surg. 2019, 56, 174–181.
- Weissferdt, A.; Hernandez, J.C.; Kalhor, N.; Moran, C.A. Spindle cell thymomas: An immunohistochemical study of 30 cases. Appl. Immunohistochem. Mol. Morphol. 2011, 19, 329–335.
- Weissferdt, A.; Moran, C.A. The histomorphologic spectrum of spindle cell thymoma. Hum. Pathol. 2014, 45, 437–445.
- Moran, C.A.; Kalhor, N.; Suster, S. Invasive spindle cell thymomas (WHO type a) a Clinicopathologic correlation of 41 cases. Am. J. Clin. Pathol. 2010, 134, 793–798.
- Kelly, R.J. Thymoma versus thymic carcinoma: Differences in biology impacting treatment. J. Natl. Compr. Cancer Netw. 2013, 11, 577–583.
- Moran, C.A.; Walsh, G.; Suster, S.; Kaiser, L. Thymomas II: A clinicopathologic correlation of 250 cases with a proposed staging system with emphasis on pathologic assessment. Am. J. Clin. Pathol. 2012, 137, 451–461.
- Frank, C.D.; Ahmad, Z. Thymoma: Current diagnosis and treatment. Chin. Med. J. 2013, 126, 2186–2191.
- Suster, S.; Moran, C.A. Micronodular thymoma with lymphoid B-cell hyperplasia: Clinicopathologic and immunohistochemical study of eighteen cases of a distinctive morphologic variant of thymic epithelial neoplasm. Am. J. Surg. Pathol. 1999, 23, 955.
- Oramas, D.M.; Moran, C.A. Micronodular thymomas with prominent cystic changes: A clinicopathological and immunohistochemical study of 25 cases. Int. J. Surg. Pathol. 2021, 29, 352–357.
- Moran, C.A.; Suster, S.; Koss, M.N. Plasma cell–rich thymoma. Am. J. Clin. Pathol. 1994, 102, 199–201.
- Perez, Y.E.; Moran, C.A. The thymus: General concepts on embryology, anatomy, histology and immunohistochemistry. Semin. Diagn. Pathol. 2022, 39, 86–91.
- Suster, S.; Moran, C.A.; Chan, J.K. Thymoma with pseudosarcomatous stroma: Report of an unusual histologic variant of thymic epithelial neoplasm that may simulate carcinosarcoma. Am. J. Surg. Pathol. 1997, 21, 1316–1323.
More
