Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by SEYED SAEED MADANI and Version 2 by Sirius Huang.
The interaction between temperature regulation and lithium-ion batteries is pivotal due to the intrinsic heat generation within these energy storage systems. A profound understanding of the thermal behaviors exhibited by lithium-ion batteries, along with the implementation of advanced temperature control strategies for battery packs, remains a critical pursuit. Utilizing tailored models to dissect the thermal dynamics of lithium-ion batteries significantly enhances our comprehension of their thermal management across a wide range of operational scenarios.
- lithium-ion batteries
- safety
- thermal management
1. The Reasons for Choosing Lithium-Ion Batteries
Lithium-ion (Li-ion) batteries have gained widespread popularity in various applications due to their numerous beneficial features. Below are some compelling reasons for selecting lithium-ion batteries:
Impressive Energy Density: Li-ion batteries boast a remarkable energy density, enabling them to store substantial energy within a compact and lightweight package. This makes them particularly suitable for portable electronic devices, electric vehicles (EVs), and scenarios where size and weight are crucial factors.
Reusability: Li-ion batteries are rechargeable, allowing for multiple uses throughout their lifespan. This translates to cost-effectiveness compared to disposable single-use batteries.
Extended Cycle Life: Li-ion batteries typically endure a longer cycle life when compared to many other rechargeable battery types, capable of enduring hundreds to thousands of charge and discharge cycles before experiencing significant capacity degradation.
Low Self-Discharge Rate: Li-ion batteries exhibit a relatively low self-discharge rate, ensuring they retain their charge for extended periods when not in active use. This feature is valuable for devices that may remain idle for prolonged periods between uses.
Swift Charging: Li-ion batteries can be charged rapidly, especially when compared to certain other battery technologies. This rapid charging capability is essential for applications like smartphones and electric vehicles.
Versatility: Lithium-ion batteries come in various shapes and sizes, making them adaptable and suitable for a wide array of applications, ranging from small consumer electronics to large-scale energy storage systems.
Minimal Memory Effect: Unlike some other rechargeable battery types, Li-ion batteries exhibit a minimal memory effect. Consequently, they do not require complete discharge before recharging, making them more user-friendly.
Elevated Voltage Output: Li-ion batteries provide a relatively high nominal voltage. This elevated voltage output is advantageous for many applications, facilitating efficient device operation.
Reliability and Safety: While Li-ion batteries can present safety concerns if mishandled, they feature enhanced safety measures compared to older battery technologies. Manufacturers have integrated various safety mechanisms, including thermal protection and pressure relief valves, to mitigate the risk of thermal runaway and other safety issues.
Environmental Considerations: Li-ion batteries are considered more environmentally friendly than certain other battery chemistries, such as lead-acid batteries, due to their lower toxicity and potential for recycling. Recycling programs for Li-ion batteries are becoming increasingly widespread.
It is important to note that while Li-ion batteries offer numerous advantages, they are not without limitations, including environmental concerns, the potential for thermal runaway, and the finite lifespan of the cells. However, ongoing research and development endeavors are aimed at addressing these challenges and further enhancing Li-ion battery technology.
2. Impact of Temperature on Lithium-Ion Batteries
The impact of temperature on lithium-ion batteries’ performance degradation is depicted in Figure 1. This deterioration primarily results from the intricate interplay of battery materials and the chemical reactions occurring within. Thermal fluctuations have the potential to induce variations in the kinetics of electrochemical reactions taking place within the battery matrix. Moreover, temperature significantly influences fundamental parameters, including the ionic conductivities of both electrolytes and electrodes. The consequences of extreme temperature conditions become even more complex when compared to milder thermal environments. The ambient temperature, influenced by variables such as seasonal changes, meteorological factors, and broader climate influences, displays location-specific fluctuations based on vehicular geography. This modulation of environmental temperature emerges as a pivotal factor determining battery longevity. Calendar life, a crucial metric representing battery lifespan, is influenced by the dynamic interplay between state of charge and temperature. Locations with elevated ambient temperatures often experience noticeable declines in battery capacity during storage conditions. Additionally, it is noteworthy that temperature also significantly affects battery cycle life, exerting a substantial influence on this critical parameter [1][2][20,21].
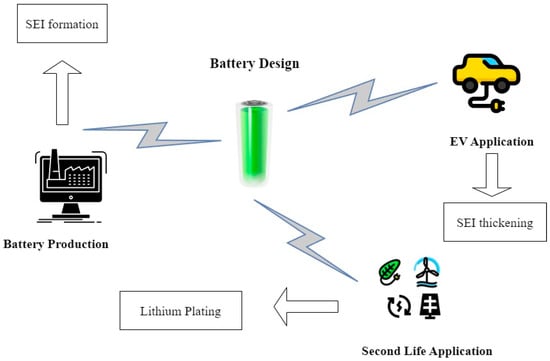
Presenting a comprehensive view of the battery’s journey, Figure 2 encapsulates the battery lifecycle. This encompassing trajectory begins with the production phase, navigates through intricate design considerations, and extends to second-life applications and the integration of electric vehicles (EVs). Thus, the profound impact of temperature fluctuations on lithium-ion batteries unravels a nuanced tapestry where diverse parameters and intricate interactions converge to shape the battery’s performance, longevity, and overall lifecycle.
3. Electrochemical-Thermal Behavior of Lithium-Ion Batteries
The comprehensive spectrum of electrochemical and thermal models developed to elucidate the intricate behaviors of lithium-ion batteries is represented in Table 1. This compilation underscores the diversity inherent in these models, all crafted with the overarching goal of simulating and understanding the multifaceted thermal and electrochemical dynamics exhibited by lithium-ion batteries across various environmental temperatures and current profiles. The crux of lithium-ion battery modeling lies in the formulation of complex sets of equations meticulously designed to capture the battery’s dynamic response and performance. These efforts have primarily aimed to foster the development and deployment of electrochemical-thermal battery simulation models, often involving the integration of heat transfer experiments and simulations, further enhanced by techniques like computational fluid dynamics and a range of battery-centric experiments.
Table 1.
Compilation of Electrochemical and Thermal Models for Lithium-ion Batteries.
| Battery | Description | Reference |
|---|---|---|
| Lithium-ion battery | A dynamic electro-thermal model -The model was developed employing MATLAB (Simulink). |
I. Baghdadi et al. [3][22] |
| Lithium polymer batteries | Electrochemical-Thermal Model | Song et al. [4][23] |
| Lithium-ion battery | Electro-thermal model and core temperature estimation | L. Chen et al. [5][24] |
| LiCoO2 -A wound prismatic cell with a capacity of 900 mAh, -A LiCoO2 positive electrode (cathode) and a synthetic graphite negative electrode (anode). |
The results show no appreciable difference in capacity fade from changing only the discharge cutoff voltage. | Choi S.S. et al. [6][25] |
| Lithium-ion battery | -Investigation of the power fade when the battery is soaked at a higher SOC and temperature. -The results show that the power fades in the first 4-week period appeared to be a strong function of temperature. -Multiple cells were exposed to various static aging conditions, ranging from 25 °C and 60% SOC to 55 °C and 100% SOC. |
Thomas E.V. et al. [7][26] |
| MCMB/Li1−xNiyCo1−yO2 | Experimentally investigate the irreversible capacity loss on a fully charged mesocarbon microbead MCMB/Li1−xNiyCo1−yO2 cell to determine the impact on capacity fade from using different electrolytes. | Smart M.C. et al. [8][27] |
| Lithium-ion battery | A review of the exothermic reactions inside the battery. | Spotnitz R. et al. [9][28] |
| Lithium-ion batteries | Development of a general energy balance for insertion battery systems by using enthalpy potentials. | Rao L. et al. [10][29] |
| Li/LiPF6 LiCoO2, LiNi0.8Co0.2O2, and LiC6 |
-Study of the heat of mixing effect inside a battery containing a porous insertion electrode. -The study introduced measurements of the entropy of reaction as a function of state of charge for LiCoO2, LiNi0.8Co0.2O2, and LiC6. |
Thomas K.E. et al. [11][30] |
| Lithium-polymer battery | -Study of the effect of the electrode configuration on the thermal behavior of a lithium-polymer battery. -Scale-up modeling |
Kim U.S. et al. [12][31] |
| Lithium-polymer battery | Presenting an approach for modeling the large-scale lithium-ion polymer battery. | Kim U.S. et al. [13][32] |
| Lithium-ion batteries | A non-uniform degradation model has been presented for large-scale lithium-Ion. | Smith K.A. et al. [14][33] |
| Lithium-ion battery | -Long-term coulombic efficiency behaviors of LFP and NMC cells are investigated. -Aging mechanisms of LFP and NMC cells are analyzed by incremental capacity curves. -The relationship between coulombic efficiency and capacity fading is clarified. -Some applications of our research outcomes to battery management systems are discussed. |
Yang F. et al. [15][34] |
| LiAl/FeS | Investigation of battery heat using a thermodynamic energy balance on a complete cell. | Bernardi D. et al. [16][35] |
In a significant contribution, Baghdadi et al. [3][22] introduced a precisely tailored, dynamic electro-thermal model calibrated specifically for high-power utilization of lithium batteries. The model’s parameters were derived from empirical insights obtained through experimental investigations, with many parameters dependent on current magnitude, temperature, and battery state of charge (SOC). Validation across a variety of operational conditions, including static and dynamic current profiles, demonstrated the model’s accuracy. Impressively, simulation results showed close agreement with extensive test data spanning twenty hours. Another noteworthy effort by Song et al. [4][23] involved the development of a mathematical model for the comprehensive exploration of heat transfer and thermal management aspects inherent to lithium polymer batteries. Building upon an existing electrochemical model, this enhanced version incorporated temperature-dependent parameters such as the diffusion coefficient of lithium ions, ionic conductivity of lithium ions, and transference number of lithium ions to provide a deeper understanding of thermal intricacies within the lithium polymer framework. Experimental validation, coupled with analyses of discharge behaviors and heat generation rates within lithium polymer cells, enabled a thorough comparison of experimental observations with model-derived outcomes, accompanied by a detailed discussion of diverse thermal management strategies. Similarly, Choi S.S. et al. [6][25] conducted a comprehensive investigation into the factors influencing the cycle life of lithium-ion cells. The insights gained emphasized the substantial impact of charge conditions on cycle life, with discharge conditions exhibiting relatively lesser sensitivity. Charging cells at rates exceeding the 1C rate, prolonged float-charge periods above 4.2 V, and high charge cut-off voltages were identified as factors negatively affecting cycle life. Interestingly, unlike other battery types, the depth of discharge displayed a limited correlation with cycle life enhancement. The association between degradation rate and charge voltage, along with the duration of exposure to high charge voltage, implicated electrochemical oxidation as a fundamental degradation mechanism.
Conversely, Thomas E.V. et al. [7][26] embarked on a meticulously designed accelerated aging study, thoroughly exploring the interplay between aging duration, temperature, and state-of-charge (SOC) in shaping lithium-ion cell performance. Through an extended monitoring process involving a hybrid pulse power characterization test at low current regimes over a 44-week period, a notable empirical model of power fade was established. This model comprehensively encapsulated two simultaneous degradation processes—one rapid and temperature-accelerated, and the other proceeding at a slower pace influenced by temperature and SOC. Addressing temperature-related challenges, Smart M.C. et al. [8][27] systematically explored the profound impact of electrolyte composition on low-temperature lithium-ion cell performance. By carefully selecting ester solvents for incorporation into multi-component electrolyte formulations, the study leveraged favorable physicochemical attributes such as low viscosity, high permittivity, and low melting points. The compatibility of these formulations with diverse electrode compositions, including LiCoO2 and LiNiCoAlO2, was demonstrated. The study also delved into lithium intercalation and deintercalation ease within Li-carbon cells at varying temperatures, employing conventional electrochemical techniques to uncover insights into surface film attributes.
Expanding into the realm of abuse testing, Spotnitz R. et al. [9][28] conducted a comprehensive synthesis of published studies investigating abuse testing involving lithium-ion cells and their components, complemented by the application of modeling techniques. These studies meticulously identified specific exothermic reactions and estimated heats of reaction for each, subsequently leading to model development. These models, enriched with estimated kinetic parameters and designed to address high-rate batteries, comprehensively captured cell behavior under diverse abuse conditions such as high temperatures, short-circuits, overcharging, nail penetration, and physical crushing. Notably, these models shed light on the role of fluorinated binders in thermal runaway, revealing the binder’s minimal contribution to this phenomenon. In summary, the orchestrated symphony of electrochemical and thermal models, exemplified in the table, aligns with a collective effort to decipher the intricate dynamics inherent to lithium-ion batteries. These models span a wide landscape, encompassing diverse perspectives and insights, thereby contributing to a deeper understanding of these pivotal energy storage systems.
In a distinct exploration, Rao L., Newman J. et al. [10][29] introduced an innovative energy balance framework for insertion battery systems, built upon enthalpy potentials as a foundational cornerstone. This pioneering approach facilitated the calculation of heat-generation rates through an inventive methodology. Additionally, an alternate model based on localized heat generation within an electrochemical cell was formulated, yielding consistent outcomes. The authors also introduced the concept of the effective open-circuit potential of an insertion battery, enhancing characterization during the open-circuit state of galvanostatic discharge. Specific simulation efforts focused on heat generation within a lithium cell during galvanostatic discharge, analyzing the interplay between the shape of the open-circuit potential and ohmic losses within the porous cathode. Remarkably, this study revealed that a single reaction could give the illusion of two reactions due to the presence of twin plateaus within the open-circuit potential. The cessation of current within electrochemical systems triggers heat generation, a consequence of the relaxation of concentration gradients. This phenomenon, referred to as the heat of mixing, results from this relaxation.
Thomas K.E. et al. [11][30] delved into this inquiry, providing two methodologies—a computational approach and an analytical approximation—to quantify the heat of mixing. While typically negligible within materials with robust transport properties ensuring satisfactory battery performance, exceptions arise, particularly with materials like lithium insertion electrodes engaged in insertion reactions. In such contexts, the entropy of reaction undergoes significant variations depending on the state of charge, introducing an entropy of reaction that manifests as a reversible heat effect comparable in magnitude to resistive heating. In a separate investigation, Kim U.S. et al. [12][31] conducted a comprehensive thermal analysis to examine the thermal performance of a lithium-polymer battery. This study delved into how electrode arrangement influences thermal behavior, considering factors such as electrode aspect ratio, positioning of current-collecting tabs, and discharge rates. Utilizing the finite element method, the study predicted potential and current density distribution across the battery’s electrodes during discharge, subsequently enabling calculations of temperature distribution within the lithium-polymer battery. Impressively, the temperature distributions derived from the model closely matched experimental measurements from batteries with different electrode types under various discharge rates. Expanding their horizons, Kim U.S. et al. [13][32] introduced a modeling approach to upscale a lithium-ion polymer battery (LIPB). Validation, confirmed by comparing experimental discharge curves with modeling results, demonstrated that parameters used for modeling small-scale LIPBs could be extended to larger scales, contingent on the consistency of electrode materials, composition, and manufacturing processes. Using the finite element method, the distribution of potential and current density across the LIPB electrodes during discharge was predicted and then utilized for calculations of temperature distributions within the LIPB. Notably, the temperature distributions derived from the model exhibited commendable agreement with corresponding experimental measurements.
Furthermore, Smith K.A. et al. [14][33] employed empirical correlations to examine the influence of temperature-dependent electrode film impedance growth (thermal stress) and cycling-dependent capacity fade (mechanical stress) on cell degradation and performance decline. This study aimed to quantify the non-uniform imbalance and performance deterioration that traverse the cell’s lifespan as degradation evolves. The results were compared with those from a 1D electrochemical/lumped thermal model. Simulations spanned varying temperatures, cycling intensities, and states-of-life, providing insight into diverse scenarios where the internal reaction field was influenced by temperature, potential, and degradation state. Shifting the focus, Yang F. et al. [15][34] centered their investigation on the interplay between long-term coulombic efficiency (CE) and battery degradation—an area still shrouded in mystery. This study, driven by cycle life tests on commercially available lithium-ion batteries, explored the behavior of long-term CE and its connection with capacity degradation. Through incremental capacity (IC) analysis, the study uncovered the underlying mechanisms of battery aging. The paper offered not only experimental observations but also profound discussions on battery degradation, aging mechanisms, and the evolution of CE. This inquiry unveiled two distinct degradation patterns, highlighting the link between active material loss and battery degradation and emphasizing the electrochemical interplay between the evolution of CE and capacity degradation.
To ensure accurate estimation of cell thermal characteristics, Bernardi D. et al. [16][35] devised a comprehensive energy balance equation for battery systems. Critical for battery system design and thermal management, this equation incorporates a range of factors driving temperature changes within cells, encompassing electrochemical reactions, phase transitions, mixing effects, and Joule heating. This versatile framework addresses multifaceted effects comprehensively while considering simplifications and practical scenarios. Demonstrating the equation’s practical utility, mathematical models of cell discharge with varying reaction mechanisms were analyzed. These examples illustrated how the energy equation facilitates the analysis of diverse term contributions, highlighting the intricate nature of heat generation processes within cells while emphasizing the benefits of adopting such a comprehensive energy equation for such analyses.
4. Thermal Characteristics of Lithium-Ion Batteries
Lithium-ion batteries, known for their nonhomogeneous composition, exhibit diverse heating patterns on the surface of battery cells. This intricate interplay poses significant challenges for effective thermal modeling and the design of efficient thermal management systems tailored to various lithium-ion battery applications. As illustrated in Table 2, researchers have employed a plethora of methods to scrutinize the thermal attributes of lithium-ion batteries. Furthermore, extensive laboratory-based experimental characterizations have been conducted on diverse lithium-ion batteries operating under varied conditions. This line of inquiry predominantly seeks to unravel the relationship between surface temperature gradients and the thermal dynamics of lithium-ion battery cells. Parameters including power, open-circuit voltage, capacity, entropic heat coefficient, heat capacity, internal resistance, temperature, and battery heat generation have been meticulously determined across diverse load currents and an expansive temperature range. The insights garnered from these experimental results are pivotal for refining thermal modeling approaches for these batteries.
Table 2.
Different methods for thermal analysis and characteristics of lithium-ion batteries.
| Battery | Description | Reference |
|---|---|---|
| A ternary material (NMC111) lithium battery. | Design and thermal analysis of a new topological cooling plate for prismatic lithium battery thermal management | [17][36] |
| Lithium-ion cell (LixC6/LiyNiO2) A 14Ah lithium-ion pouch cell, 220 mm × 130 mm × 7 mm, |
-Influence of different design variables on the thermal behavior. -The state of charge (SOC): 100–0% increments of 5%. Temperature: −20 °C to 55 °C, with increments of 5 °C. |
[18][37] |
| -Porous insertion electrodes -Including a mesocarbon microbead anode, LiCoO2 cathode, and a 1 M LiPF6 salt electrolyte mixture including ethylene carbonate, propylene carbonate, ethyl-methyl carbonate, and diethyl carbonate. |
Thermal modeling | [19][38] |
| Lithium/polymer (LilPEO15-LiCF3SO3ITiS2) LixC6/LiyNiO2 cell |
Thermal modeling and discharge behavior | [20][39] |
| Lithium-ion battery | Thermal model | [21][40] |
| Lithium-ion battery pack | Power and thermal characterization | [22][41] |
| Lithium-ion batteries Sony (US18650) cell |
Analysis of electrochemical and thermal Behavior |
[23][42] |
| Lithium/polymer-electrolyte | Heat transfer phenomena | [24][43] |
| Lithium-ion batteries | Thermal modeling and design considerations |
[25][44] |
| Lithium polymer lithium/polymer-electrolyte batteries |
Electrochemical–thermal model | [26][45] |
| Battery module | Three-dimensional temperature and current distribution |
[27][46] |
| Lithium-ion batteries | Modified air-cooled battery thermal management system | [28][47] |
| Cylindrical lithium-ion battery | Evaluating the heat generation characteristics of cylindrical lithium-ion battery considering the discharge rates and negative to positive electrode capacity ratio | [29][48] |
| Spirally wound lithium batteries | Thermal analysis | [30][49] |
| Lithium-ion polymer battery | Scale-up modeling | [31][50] |
| Lithium/polymer-electrolyte | Thermal analysis | [32][51] |
| Lithium-polymer battery | Effect of electrode configuration on the thermal behavior |
[33][52] |
| Lithium-polymer | Three-dimensional thermal modeling | [34][53] |
| cylindrical Li-ion battery |
Accelerated rate calorimeter for electrochemical-calorimetric studies. |
[35][54] |
| Lithium-ion batteries | Accelerated rate calorimeter for characterization using electrochemical–calorimetric measurements |
[36][55] |
| LixMn2O4 Spinel |
Temperature sensors and an aluminum heat sink for thermal characteristics. | [37][56] |
| LMO/carbon | Isothermal calorimeter for theoretical and experimental analysis of heat generation. | [38][57] |
| NMC/graphite | Adiabatic calorimeter for heat generation in a high-power battery. | [39][58] |
| NMC/carbon | Isoperibolic calorimeter for potentiometric and calorimetric measurement of entropy changes. | [40][59] |
| LCO/graphite | Adiabatic calorimeter for thermal modeling of big cells. | [41][60] |
| LFP/graphite | Adiabatic calorimeter for electro-thermal model. | [42][61] |
The intricacies embedded in the thermal modeling of lithium-ion batteries necessitate a nuanced approach, as the solution varies depending on pack topologies, battery cell designs, and specific application contexts. In essence, a tailored thermal modeling system is indispensable for each unique lithium-ion battery instance. Key aspects such as the entropic heat coefficient, internal resistance, battery heat generation, and thermal models serve as foundational elements enabling the simulation of diverse lithium-ion batteries, unlocking insights into their thermal dynamics.
In a parallel pursuit, Bazinski, S.J. et al. [18][37] meticulously explored the influence of reversible (entropic) heat sources on the thermal behavior of lithium-ion batteries, particularly during the initial charge and discharge stages. The entropic coefficient (EC) emerged as a pivotal factor shaping the magnitude and direction of this reversible heat. The researchers identified varying EC values for a lithium-iron phosphate battery, revealing the significant impact of cell temperature on EC, particularly at extreme state-of-charge (SOC) levels. Employing curve fitting of experimental data, a correlation emerged linking EC to temperature and SOC. To validate this, calorimetric data from test cells were integrated to demonstrate the impact of reversible heating on the overall heat generation rate within the cell. This synergistic fusion of calorimetric data and EC measurement facilitated the assessment of irreversible heat generation within the cell. This exploration not only offers insights into the relationship between temperature, SOC, and EC but also sheds light on the processes of reversible and irreversible heat generation inherent to lithium-ion batteries.
In a different vein, Kumaresan, K. et al. [19][38] developed a thermal model to predict the discharge performance of a lithium-ion cell across varying operating temperatures. The model’s predictions were validated with experimental data from lithium-ion pouch cells. This thermal model incorporated a parameter set tailored to the lithium-ion cell, accounting for concentration and temperature dependencies. These parameters were determined through a comparative analysis of model predictions and experimental discharge profiles encompassing diverse temperatures and discharge rates. The concentration and temperature dependencies of these parameters were subsequently correlated using empirical formulations. The study also examined the implications of incorporating the temperature dependencies of various parameters within the model into simulated discharge profiles. The integration of temperature-dependent parameters enhanced the model’s predictive accuracy for the discharge performance of lithium-ion cells operating across different temperatures.
Taking a different approach, Botte, G.G. et al. [20][39] used a mathematical model integrating an anode (carbon) decomposition reaction to predict the temperature trajectory of a lithium-ion cell under medium- and high-rate discharge conditions. The investigation explored the influence of distinct design parameters and the activation energy associated with the anode (carbon) decomposition reaction on the projected temperature within a LixC6/LiyNiO2 cell. Model predictions highlighted the critical role of particle size in the negative electrode as a crucial parameter for accurately predicting the cell’s temperature. On a similar trajectory, Srinivasan, V. et al. [21][40] aimed to enhance the understanding of Li-ion cell thermal behavior through a two-dimensional, first principles-based thermal-electrochemical modeling approach. The model encompassed reversible, irreversible, and ohmic heats within matrix and solution phases, incorporating temperature-dependent transport, kinetic, and mass-transfer parameters based on Arrhenius expressions. Experimental data on the entropic contribution of manganese oxide spinal and carbon electrodes were integrated to assess the significance of this term in overall heat generation. Through simulations, the study estimated thermal and electrical energy, along with active material utilization, at distinct rates, providing a comprehensive exploration of the interplay between temperature and electrochemistry. Moreover, the paper explored the prospect of using experimental data instead of an electrochemical model to deduce heat generation rates. Discrepancies between local and lumped thermal models were analyzed, and the feasibility of using a heat generation rate established under specific thermal conditions for other scenarios was evaluated. Model simulations offered valuable insights into the appropriateness of various approximations when developing comprehensive thermal models for Li-ion cells.
In a distinct study, Al Hallaj, S. et al. [23][42] utilized a simplified one-dimensional thermal mathematical model with lumped parameters to simulate temperature profiles within lithium-ion cells. The model seamlessly integrated experimentally derived heat-generation parameters specific to the Sony (US18650) cell. Simulation outcomes were impeccably aligned with temperature measurements for discharge rates spanning C/2, C/3, and C/6, although slight deviations were noted for the C/1 discharge rate. The model’s capabilities extended to simulating temperature profiles under diverse operational scenarios and cooling rates for scaled-up cylindrical lithium-ion cells with capacities of 10 and 100 Ah. Profound insights emerged—cooling rate had a substantial impact on cell temperature across all discharge rates. Notably, a noticeable temperature gradient within the cell occurred only at higher cooling rates, where the Biot number exceeded 0.1. Conversely, at lower cooling rates, the cell’s behavior resembled that of a lumped system with a uniform temperature distribution. Pioneering the establishment of temperature thresholds for scale-up using the simplified model, commercial lithium-ion cells with various open circuit potentials (OCV) were tested in an accelerated rate calorimeter (ARC) to determine onset-of-thermal-runaway (OTR) temperatures. Specifically, Sony (US18650) cells with OCVs of 4.06, 3.0, and 2.8 V were examined, yielding measured OTR temperatures of 104 °C, 109 °C, and 144 °C, respectively. A significant finding emerged—a sharp OCV decrease, indicative of an internal short circuit, occurred at temperatures near the separator material’s melting point across all OCV values.
In a parallel endeavor, Smith, K. et al. [24][43] employed a thermal model to dissect the limitations of pulse power and thermal dynamics in a Li-ion hybrid-electric vehicle (HEV) battery pack. The pack, housing 72 cells with a nominal voltage of 276 V and a capacity of 6 Ah, underwent scrutiny. High-rate pulse discharges, operational at approximately 25 °C, consistently reached their minimum voltage threshold of 2.7 V per cell due to active material Li depletion or saturation on electrode surfaces, highlighting solid-state diffusion as the limiting factor. In contrast, the maximum voltage threshold of 3.9 V per cell, designed to prevent lithium deposition on the negative electrode during charging, was considered overly conservative for high-rate pulses initiated from states-of-charge (SOCs) below 100%. The investigation revealed an intriguing insight—the maximum pulse charge rate, originating from a 50% SOC, could increase by up to 50% without risking lithium deposition, challenging the necessity for an excessively cautious maximum voltage threshold. While adhering to minimum and maximum voltage limits, the battery pack aligned with the power assist mode pulse power requirements of the Partnership for a New Generation of Vehicles (PNGV) at temperatures exceeding 16 °C. However, it fell short of achieving the desired energy output target.
In another venture, Verbrugge, M.W. et al. [25][44] proposed a technique to address current and temperature distributions in large-scale battery modules with three-dimensional configurations. Simulations focused on a specific module comprising cells with a lithium metal anode, polymer electrolyte, and vanadium oxide cathode. The findings highlighted the nonlinear correlation between power output and system temperature, primarily influenced by temperature’s impact on electrochemical reaction rates and ionic conductivity. The study also explored the estimation of physicochemical parameters, some of which were not readily available in existing literature but played a pivotal role as model inputs.
In a parallel investigation, Chen, Y. M.W. et al. [26][45] embarked on the mathematical modeling of heat generation and transport within lithium/polymer-electrolyte batteries, with a focus on their deployment in electric vehicles. Findings revealed that thermal management remains inconsequential for batteries operating at low discharge rates. However, at high discharge rates, battery temperature can rise significantly, particularly if the cell stack’s thickness exceeds a specific threshold. Interestingly, it was found that enhancing cooling conditions does not have a significant impact on increasing heat dissipation within large-scale battery systems due to the limited thermal conductivity of the polymer material. Model predictions can guide the design of appropriate battery structures and the selection of suitable cooling strategies to achieve the desired operational temperature range for a given discharge rate.
In another comprehensive overview, Gomadam, P.M. et al. [27][46] reviewed the mathematical models developed at the University of South Carolina for lithium and nickel battery systems. This encompassing survey covered models tailored for Li/Li-ion batteries, including simulations of single electrode particles, individual electrodes, full cells, and battery sets operating across diverse scenarios such as constant current discharge, pulse discharge, impedance, and cyclic voltammetry. Additionally, the review included models designed for nickel battery systems, elucidating complete cell performance and the behavior of nickel hydroxide as an active material. The robustness of these models, substantiated through recurrent comparisons with experimental data, showcased their accuracy in predicting real-world outcomes.
Shen et.al [28][47] introduced a modified air cooling system featuring a non-vertical, Z-shaped structure. They investigated how this innovative design affected the thermal properties of lithium iron phosphate power batteries. This system departs from the traditional Z-shaped cooling arrangement by tilting battery packs at different angles, resulting in a non-vertical airflow channel structure. When compared to the conventional Z-shaped air cooling system, the highest temperature within the battery pack decreased from an initial 38.15 °C to 34.14 °C, representing a 10.5% reduction. Moreover, the temperature variation decreased from an initial 2.59 °C to 1.97 °C, marking a 23.9% decrease. This modified air-cooled battery thermal management system improves the heat exchange rate between the battery pack and the surrounding air, leading to enhanced cooling performance and temperature uniformity. The outcomes of this study serve as a foundation for the development of a modified Z-shaped air cooling system, contributing to the safety improvements in electric vehicles and providing valuable insights for the further advancement of Battery Thermal Management Systems (BTMS).
Wu et.al [29][48] introduced an electrochemical-thermal model (ETM) designed to evaluate the heat generation characteristics of cylindrical Lithium-ion Batteries (LIBs). This model considers various discharge rates and the ratio of negative to positive electrode capacity (N/P ratio). To provide a comprehensive assessment of LIB thermal properties, the proposed ETM was validated using experimental data acquired at ambient temperatures of 25 °C and 35 °C. Subsequently, the study examined the distribution patterns of heat generation characteristics in LIBs under various conditions through numerical analysis. A notable aspect of this investigation was the thorough exploration of how different discharge rates and N/P ratios affect the heat generation in batteries. The results highlighted the significant role of heat generation in the negative electrode and emphasized the importance of considering the impact of the reversible term on the overall heat generation in LIB cells, particularly at lower discharge rates. Additionally, the research suggested that selecting the appropriate N/P ratio can improve the total heat generation of LIBs, offering advantages for optimizing performance in the early stages of battery design and thermal management.
Chen, S.C. et al. [30][49] developed a comprehensive three-dimensional thermal model to understand the thermal behavior of a lithium-ion battery. The model ingeniously incorporated the layered structure of cell stacks, battery pack casing, and the space between these elements to provide a detailed analysis of heat dissipation. It included location-dependent convection and radiation at boundaries to accurately represent distinct heat dissipation characteristics across all surfaces. The study also proposed a simplified thermal model that achieved comparable calculation speed to a one-dimensional model, with a maximum error of less than 0.54 K. Both models effectively captured the asymmetric temperature distribution within the battery and even predicted temperature anomalies on the surface when a metal case was used. Insights gained emphasized the importance of factors such as the metal battery case, contact layer, and heat-spreader effects in battery system design.
In the study conducted by Zhu and their team [31][50], a series of experiments were carried out using a cone calorimeter to investigate Lithium-ion Battery (LIB) packs of varying sizes (1 × 1, 1 × 2, 2 × 2, 2 × 3, 3 × 3) and at different states of charge (SOC) levels (100%, 50%, and 0%). The research examined several fire-related parameters, such as the heat release rate (HRR), mass loss, and concentrations of CO, CO2, and O2. Interestingly, the study observed similar combustion patterns characterized by intermittent jets for LIB packs at both 50% SOC and 100% SOC. The findings revealed a consistent positive correlation between the total mass loss (TML) and the peak value of HRR (pHRR), described by a power function, in relation to the surface area of the exposed heat source. Notably, for battery packs with a 100% SOC, the pHRR of the 3 × 3 cell module increased significantly, approximately by a factor of 8, reaching 12 kW. The study also assessed the presence of the toxic gas carbon monoxide (CO) by determining the fractional effective dose (FED). It was found that for battery packs with sizes smaller than 2 × 3, the FED remained below 1 for packs at both 50% SOC and 100% SOC. This research offers valuable insights into predicting the progression and fire risk associated with larger-scale battery fires and provides potential strategies for mitigating thermal runaway (TR) hazards in accident scenarios.
Chen, S.-C. et al. [32][51] developed a two-dimensional thermal model specifically tailored for spirally wound cells, aiming to establish a standardized simulation methodology for these battery configurations. The model carefully considered the geometric attributes and boundary conditions of the spiral architecture to avoid distorted results caused by improper approximations of the spiral geometry. While this versatile model architecture offered precision, it came at the expense of computational time. Simulations performed on lithium batteries exposed to natural convection revealed that peak temperatures clustered in a circular region near the liquid-filled hollow core rather than at its exact center. Additionally, radiation emerged as a significant contributor to heat dissipation, accounting for up to 53.6% of the total when surface emissivity approached unity. Introducing airflow parallel to the cylinder axis proved effective in maintaining surface temperatures, although internal temperatures remained elevated for batteries with a larger radius. Airflow perpendicular to the cylinder axis, while slightly less effective than parallel flow, still contributed to reduced heat dissipation. Ensuring temperature uniformity required a battery case with high thermal conductivity. Chen, Y. et al. [33][52] developed a three-dimensional model to simulate and compare heat generation and transport in a lithium polymer electrolyte battery during galvanostatic discharges and under a dynamic power profile, such as the Simplified Federal Urban Driving Schedule (SFUDS). The study aimed to achieve and maintain operational temperature and temperature uniformity within the battery through well-designed thermal management. The findings highlighted the crucial role of anisotropic thermal conductivity and emphasized its importance in battery design. The study offered insights into designing laminated cell stacks to achieve uniform operational temperatures, especially when cooling channels or electric heaters were applied to the stack’s extremities. Under the SFUDS power profile, the time-averaged heat generation rate was low, necessitating high-performance insulation materials to maintain desired operational temperatures. The thermal model served as a toolkit for evaluating different configurations of cooling channels and electric heaters, optimizing heating intensities, and selecting insulating materials. Chen, Y. et al. [34][53] conducted a thermal analysis of lithium polymer electrolyte batteries, aiming to understand the relationship between battery thermal behavior and various design parameters. The study aimed to guide the preservation of operational temperature by designing appropriate cell stack structures and selecting suitable cooling and insulating systems. The analysis explored the effects of stack size and different cooling/insulating conditions on battery temperature across a range of discharge rates. These investigations provided valuable insights for maintaining desired operational temperatures. The study also calculated temperature distributions within cell stacks for different cell designs, including variations in component thicknesses and current collector materials. This analysis not only identified optimal cell structures from a heat transfer perspective but also discussed the thermal properties of lithium polymer electrolyte batteries with different positive electrode materials, such as V6O13, TiS2, and redox polymers. The study shed light on the thermal conductivity of batteries influenced by varying electrode compositions. Lastly, Du, S. et al. [35][54] conducted a study focusing on irreversible heat generation in lithium-ion batteries and its implications for electronic device development. The primary factors contributing to internal irreversible heat generation in Li-ion batteries are polarization and ohmic heat generation. The study developed a thermo-electrochemical coupling model that integrated dynamic parameters and the electric double layer to uncover the mechanisms behind this phenomenon. Results revealed a key insight—irreversible heat production increases significantly with discharge rate, with polarization heat production being the dominant factor. Ohmic heat production primarily contributes to electrolyte heating, while heating at the negative active material is much smaller compared to the positive active material. Calculations demonstrated that the ratio of ohmic heat production to total irreversible heat production rises as the discharge rate increases, helping to balance the influence of polarization heating. The study further investigated the role of particle size in irreversible heat production and polarization heat production at the positive and negative electrodes. The findings underscored the greater impact of particle size at the negative electrode on these factors within the battery. In summary, this study highlighted the crucial role of irreversible heat generation in li-ion batteries, revealing polarization heat production’s dominance and the relatively smaller contribution of ohmic heat production from negative active materials. It also emphasized the influence of electrode particle size on irreversible heat production and polarization heat production, shedding light on an often overlooked but essential aspect of li-ion battery dynamics.
Drake, S.J. et al. [36][55] introduced an innovative method for measuring the heat generation rate of Li-ion cells at high discharge rates, reaching up to 9.6C. This approach involves simultaneous measurements of cell temperature and surface heat flux, providing insights into heat stored and lost from the cell. Unlike calorimetry-based methods, this in-situ approach allows measurements in laboratory or field settings. Prior to heat generation measurements, a preliminary test measures the temperature gradient within the cell under identical ambient conditions. This data is used to correct temperature discrepancies within the cell during subsequent heat generation measurements. The paper also introduces a method to measure the internal cell temperature, providing more precise temperature data for heat generation analysis. By comparing heat generation measurements with established theoretical models, the study demonstrates the agreement between experimental data and theoretical projections. This validation confirms the effectiveness of the proposed measurement method and reinforces trust in the theoretical models used to understand heat generation in Li-ion cells. The paper also briefly discusses the potential benefits of actively cooling the cell, highlighting the advantages of cooling in managing heat generation and improving cell performance. Active cooling strategies are identified as valuable tools for mitigating excessive heat generation and enhancing the comprehensive thermal management of Li-ion cells. Gümüşsu, E. et al. [37][56] introduced a three-dimensional computational fluid dynamics (CFD) model to investigate the thermal performance of lithium-ion batteries under natural convection. This model encompasses the entire flow field surrounding the battery and internal conduction, to predict the battery’s temperature during discharge. The model relies solely on electrical performance parameters, granting it predictive power in thermal analysis. By comparing macro-scale thermophysical properties such as specific heat and thermal conductivity, the study reveals the significant role of specific heat in moderating the battery’s temperature, while the influence of thermal conductivity remains comparatively limited. Interestingly, the study finds that experimental data can be closely predicted even without considering the entropic term in heat generation calculations. The discrepancy between experimental and predicted battery surface temperatures remains within 3 °C across all discharge rates, regardless of the battery’s operational history. This developed CFD model serves as a versatile platform for exploring the thermal behavior of lithium-ion batteries across various packaging configurations, encompassing both natural and forced convection conditions. It facilitates a nuanced exploration of battery thermal management and optimization strategies, ultimately resulting in improved performance and safety for lithium-ion batteries. Xiao, M. et al. [38][57] conducted an experimental investigation using a calorimeter to enhance an electrochemical thermal model with additional terms. Calorimetric measurements were compared with model predictions to assess the model’s accuracy and reliability. The inclusion of these supplementary heat source terms, validated through experimental measurements, enhances the electrochemical thermal model’s ability to provide a comprehensive understanding of heat generation in batteries. This augmentation enhances battery thermal management and elevates safety considerations in practical applications. Abdul-Quadir, Y. et al. [39][58] introduced a method for discerning heat generation in individual battery cells during charge and discharge, a crucial element in effective battery thermal management. This method accounts for overpotential resistances through four distinct measurement techniques, incorporating the contribution of entropic heat generation within the cell. The authors conducted calorimeter tests to directly quantify heat generation within the battery cell, and the accuracy of the proposed method was validated through a comparison of calculated and measured heat generation values. The study highlights a strong agreement between overpotential resistances obtained from various techniques, except for direct current resistance measured using impedance spectroscopy. These findings instill confidence in the proposed method’s capability to accurately estimate heat generation, making it an essential tool for precise heat generation estimation. Eddahech, A. et al. [40][59] conducted a series of tests using an accelerating rate calorimeter to explore the thermal behavior of high-power lithium-ion cells during charge and discharge cycles at various current rates. The study focused on characterizing cell heat capacity, quantifying cell entropy, and understanding the impact of state-of-charge fluctuations and charge-discharge current rates on battery heat generation. These insights provide a deeper understanding of the cells’ heat generation and thermal characteristics, contributing to a more comprehensive grasp of their thermal behavior. Nieto, N. et al. [41][60] developed a thermal model tailored for a large Li-ion pouch cell with a capacity of 10.5 Ah. This model was based on experimental measurements of internal resistance and the entropic heat coefficient. Adiabatic calorimetry data were used to validate the thermal model’s accuracy. The study covered higher discharge rates and broader temperature operation ranges compared to previous research. The results demonstrated the thermal model’s reasonable prediction error for discharge processes conducted at moderate and elevated rates. The paper also discussed the strengths and limitations of the thermal model, offering insights into its practical applicability and key considerations for designing thermal management systems. Overall, the thermal model proved to be an effective predictor of heat generation behavior in large-format Li-ion pouch cells. Vertiz et al. [42][61] conducted a combined approach of calculated and experimental methods to explore the fundamental thermal characteristics of a commercially available high-capacity (14 Ah) pouch cell using LiFePO4/graphite chemistry. This investigation involved dual comparative analyses. Firstly, it compared heat generation predictions from Newman’s model with experimental heat measurements. Secondly, it established a correlation between empirical thermal behavior and the response of a 1D electro-thermal model. This research methodology allowed for a comprehensive assessment of the cell’s thermal behavior and validated the accuracy of theoretical predictions against experimental data.