Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Mongkhon Sompornrattanaphan and Version 3 by Jessie Wu.
Food-dependent exercise-induced allergic reactions (FDEIA) represent a distinct clinical phenomenon where symptoms arise during exercise following the consumption of specific trigger foods, with the most severe manifestation being anaphylaxis—a condition distinct from typical exercise-induced or food-induced anaphylaxis.
- anaphylaxis
- challenge test
- food allergy
- food-dependent exercise-induced anaphylaxis
- gluten
- IgE
- oral food challenge
1. Clinical Diagnosis
Diagnosing food-dependent exercise-induced allergic reactions (FDEIA) is a complex task that relies on clinical evaluation, and it remains challenging on multiple fronts. Patients often experience recurrent episodes of reactions, leading to delayed and sometimes inaccurate diagnoses [1][2][3][1,4,19].
Traditionally, it has been understood that individuals with FDEIA can tolerate specific foods or augmenting factors independently, but reactions occur when exercise or other cofactors coincide within 4 h of food ingestion [4][5]. Interestingly, the reverse scenario, where food is consumed just after exercise, can also trigger reactions [5][12]. The clinical manifestations of FDEIA span a spectrum from mild cutaneous reactions (e.g., urticaria) to potentially life-threatening anaphylaxis [6][2]. Notably, anaphylactic responses in FDEIA tend to be more severe compared to typical IgE-mediated food-induced anaphylaxis. This heightened severity is often due to the frequent involvement of cardiovascular symptoms (e.g., hypotension, syncope, loss of consciousness), along with respiratory symptoms (e.g., chest tightness, wheezing, cough, desaturation) [5][7][8][9][10][11,12,13,22,23].
Furthermore, FDEIA is frequently misdiagnosed, as it shares clinical features with other conditions, including chronic or acute recurrent urticaria, exercise-induced anaphylaxis, idiopathic anaphylaxis, hereditary angioedema, and mastocytosis [11][12][13][3,24,25].
2. In Vivo and In Vitro Tests
In confirming the diagnosis of FDEIA, it is crucial to consider both the patient’s clinical history and the presence of positive specific IgE (sIgE) and/or a positive skin prick test (SPT) related to the suspected food [1][3][1,19]. While wheat has emerged as the most commonly reported trigger for FDEIA, a wide range of other foods have also been implicated, including seafood, red meat, eggs, buckwheat, peanuts, tree nuts, fruits, and vegetables [3][5][14][15][16][17][12,16,17,19,26,27].
In cases where allergen extracts or sIgE for the suspected foods are not commercially available, a prick-to-prick test (PTP) can be conducted. It is important to note that a positive outcome in either in vivo or in vitro tests does not always definitively confirm an allergy. Therefore, the selection of tests should be based on clinical history and limited to the relevant causative food. In cases where a suspected allergen cannot be identified or does not align with the clinical history, the consideration of an exercise–food challenge test becomes necessary to achieve an accurate diagnosis [4][5].
In the context of WDEIA, a significant allergen component known as omega-5-gliadin (ω5-gliadin) has been identified—initially observed in 18 Finnish adults with WDEIA, all exhibiting IgE antibodies to this novel ω5-gliadin [18][28]. Matsuo H, et al. [19][29] demonstrated an 80% sensitivity in 50 Japanese children and adults with WDEIA using sIgE to ω5-gliadin (with a cut-off value > 0.35 kUA/L) determined via ImmunoCAPTM (Phadia, Uppsala, Sweden). From the ROC analysis, an optimal cut-off value for ω5-gliadin sIgE was determined to be 0.89 kUA/L. This value, which yielded a sensitivity of 78% and a specificity of 96%, was recommended for use [19][29]. A study from Brockow K, et al. [20][18] produced similar findings among 16 adults with challenge-confirmed WDEIA. The sensitivities of sIgE to wheat, gluten, and ω5-gliadin were reported as 81%, 100%, and 100%, respectively, with corresponding specificities of 87%, 95%, and 97%, using a cut-off value > 0.35 kUA/L. Additionally, the PTP employing gluten demonstrated a sensitivity of 100% and a specificity of 96%.
In cases where patients manifest multiple episodes of severe allergic reactions or anaphylaxis and are suspected of having FDEIA, it is advisable to measure basal serum tryptase levels. Elevated baseline values should then trigger an assessment for mastocytosis or other mast cell disorders [1].
3. Food–Exercise Cofactor Challenge Test
The exercise challenge test, conducted subsequent to the oral food challenge test (OFC), henceforth referred to as the exercise–food challenge test, is a valuable diagnostic tool when a definitive diagnosis cannot rely solely on clinical history, in combination with SPT and/or sIgE. Despite being considered the gold standard test, the exercise–food challenge test’s principal limitation is its positivity rate, ranging from 50 to 100% across protocols [3][7][8][16][20][21][22][23][24][6,7,8,11,13,18,19,26,30]. This variability arises from complex interactions between factors like exercise type/duration/intensity, ambient room conditions, temperature, and humidity, collectively influencing reaction reproducibility. Therefore, the negative challenge test may indicate challenges in selecting the causative foods, identifying contributing factors, or ensuring the appropriate intensity and duration of exercise during testing. Although several protocols have been established, there is currently a lack of standardization or consensus, encompassing factors such as food protein challenge quantity, exercise duration/intensity, cofactor type/dosage, and protocol duration. Typically, the exercise–food challenge test begins with substantial food protein consumption, optionally supplemented with ASA (10 mg/kg/dose in children, 300–1000 mg in adults) and/or alcohol. This is followed by aerobic and/or anaerobic exercise, lasting 4–6 min or extending up to 60 min, initiated 30–60 min after food ingestion. Some authors recommend maintaining the challenge room at 25–30 °C with 40–50% humidity [3][7][8][16][20][22][24][25][26][7,11,13,18,19,26,30,31,32]. Table 1 summarizes the studies conducting food–exercise cofactor challenge tests in the previous literature.
Table 1.
Summary of studies conducting food and cofactor challenge tests in individuals suspected of food-dependent exercise-induced anaphylaxis (FDEIA).
| Study, Country | Population | Protocol | Challenge Food | Exercise | Cofactors | Positive Challenge Rate |
|---|---|---|---|---|---|---|
| Studies in children and adolescents | ||||||
| Motomura et al. [23][8] 2015, Japan | 51 children | 3 days | Noodles (70 g of wheat), boiled shrimp (50 g) | 30 min of aerobic exercise followed by 6 min of anaerobic exercise | ASA 10 mg/kg/dose (Max 500 mg) |
51% |
| Asauimi et al. [16][26] 2016, Japan | 41 children and adolescents | 1 day | Udon noodles (200–400 g of wheat, 5.2–10.4 g of wheat protein), shrimp (40–100 g), or one whole apple | Ergometer ≥ 6 min | ASA 10 mg/kg/dose (Max 500 mg) |
49% |
| Studies in children, adolescents, and adults | ||||||
| Aihara et al. [21][6] 2002, Japan | 10 children, adolescents, and adults |
2–5 days | Wheat (100 g of wheat) | No detail | ASA 500 mg | 80% |
| Srisuwatchari et al., [7][11] 2021, Thailand | 14 children, adolescents, and adults |
3 days | Bread (60–75 g of wheat, 7.6–9.5 g of wheat protein) | Motor-driven treadmill Children and adolescents ≥ 4 min and adults ≥ 15 min |
ASA Children 10 mg/kg/dose (Max 300 mg) Adults 300–381 mg |
71.4% |
| Studies in adults | ||||||
| Brockow et al. [20][18] 2015, Germany | 16 adults | 6 days | Baked bread (10–80 g of pure gluten flour) | 45 min of aerobic exercise followed by 8 min of anaerobic exercise | ASA 500–1000 mg and 10–30 mL of 95% ethanol | 100% |
| Christensen et al. [22][7] 2018 Denmark | 71 adults | 2 days | Baked bread (80 g of pure gluten flour) | 4 treadmill aerobic phases of 15 min | None | 62% |
| Christensen et al. [27][15] 2019, Denmark | 25 adults | 5 days | Baked gluten rolls (80 g) | Treadmill aerobic exercise for 15 min | ASA 1000 mg, 37.5% alcohol |
100% |
| Thongngarm et al., [8][13] 2020, Thailand | 18 adults | 3 days | Bread (60–75 g of wheat, 7.6–9.5 g of wheat protein) | Motor-driven treadmill for 15 min | ASA 300 mg | 94.4% |
Abbreviation: ASA, acetyl salicylic acid; g, gram(s); kg, kilogram(s); mg, milligram(s); min, minute(s).
OFCs, combined with exercise cofactors, serve as the established standard for confirming FDEIA diagnosis. However, when employing the combined wheat–exercise challenge, negative outcomes have been documented. As effective alternatives or supplements, ASA and alcohol can elicit symptoms, with ASA not only facilitating reactions but also lowering the challenge threshold. This suggests potential enhancement of diagnostic yield, particularly for cases where patients are unable to attain the target exercise intensity.
Protocols employing multiple cofactors have led to an increase in test positivity; in contrast, they are sometimes associated with more severe reactions. In such multi-cofactor protocols, it remains essential to confirm that patients do not exhibit allergic reactivity to these factors. Figure 1 exemplifies a sequential multi-day challenge test protocol, employing ASA and exercise in tandem after wheat ingestion, for diagnosing wheat-dependent exercise-induced anaphylaxis (WDEIA) or omega-5 gliadin syndrome.
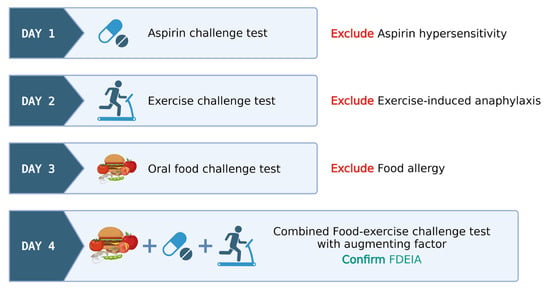
Figure 1. Illustrative model for food–exercise cofactor challenge test protocol in food-dependent exercise-induced anaphylaxis (FDEIA). Created via BioRender.com (accessed on 2 October 2023).
-
Day 1: ASA provocation test, cumulative 421.5 mg, observed for 6 h to rule out ASA hypersensitivity.
-
Day 2: Exercise challenge at 27–30 °C ambient temperature. Treadmill exercise adjusted for target heart rate (>80% max HR) for 15 min. Test ceased at any positive reaction or gradually tapered if negative.
-
Day 3: Wheat challenge using common wheat source (Farmhouse® bread, Thailand)—incremental dosing up to five slices observed for 6 h.
-
Day 4: Combined wheat–exercise–ASA challenge. ASA (300 mg) followed by four slices of bread, exercise as per Day 1.
The food–exercise challenge test protocols exhibit notable variability in their approaches. Nonetheless, these protocols consistently span several days and they are meticulously structured to encompass a broad range of scenarios, thereby precluding the influence of singular factors like exercise-induced anaphylaxis, food allergies, and hypersensitivity reactions to cofactors like aspirin. A favorable outcome in a combined food–exercise challenge test serves to conclusively establish the diagnosis of food-dependent exercise-induced anaphylaxis (FDEIA).
In cases where the exercise challenge yields a negative result on day 2, the patient proceeds to an open wheat challenge on day 3. Similarly, if a negative result occurs during the open wheat challenge on day 3, the patient advances to the combined wheat cofactor challenge on day 4. This protocol facilitates the diagnosis of EIA, classical IgE-mediated food allergy, and FDEIA on days 2, 3, and 4, respectively. If the physician chooses to exclusively employ exercise as the cofactor, the protocol may skip day 1, beginning directly with the exercise-only challenge.
Following the test, patients should undergo stringent monitoring for a minimum of 4 h, possibly extending to 24 h due to the possibility of late reactions [7][11]. It is important to emphasize that this test should be exclusively conducted under the supervision of a well-trained physician with adequate equipment and a trained personnel team, due to the risk of severe reactions [4][5].
