Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Wi Hyoung Lee.
Fiber-type electronics is a crucial field for realizing wearable electronic devices with a wide range of sensing applications. Fiber-type transistors have been applied in various fields, such as memory, energy storage, chemical and biosensors, light-emitting devices, and solar cells.
- fiber
- transistor
- conjugated polymer
- chemical sensor
1. Chemical Sensors
1.1. Glucose Sensors
Glucose sensors are vital medical devices that provide their users with important information about sugar levels in the blood, and are particularly significant for managing diseases such as diabetes mellitus [49][1]. Notably, glucose levels can be non-invasively monitored in bodily fluids like saliva, tears, and sweat [50][2]. Among these fluids, sweat emerges as a particularly accessible and promising medium for glucose sensing [51][3]. Therefore, there is substantial research interest in developing glucose sensors using fiber-type transistors with a focus on their applications in non-invasive and continuous glucose monitoring. These sensors can be seamlessly integrated into fabrics and clothing, efficiently collecting sweat from the skin for analysis. A research team from Wuhan University has successfully developed glucose sensors-based organic electrochemical transistors (OECTs) using a combination of polypyrrole nanowires and reduced graphene oxide (rGO) [52][4]. They achieved this by integrating polypyrrole into rGO sheets through a straightforward in situ polymerization process, resulting in the creation of a hybrid active layer for fiber-based organic thin-film transistors. This PPy/rGO composite was applied to a polyamide (PA) filament, and a glucose sensor was developed by immobilizing glucose oxidase on the filament with Nafion. The chemical reactions occurring near the gate electrode are outlined below:
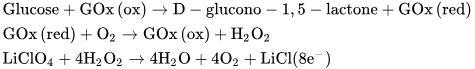
Glucose undergoes catalysis by glucose oxidase (GOx), which is attached to the surface of the gate electrode. This catalytic process generates hydrogen peroxide (H2O2) and enables electron transfer near the gate electrode’s surface [53][5]. Consequently, this alteration in the effective gate voltage applied to the transistor affects the channel current. Since the concentration of H2O2 is directly correlated with the concentration of glucose, H2O2 is commonly utilized for detecting and quantifying glucose levels [54,55,56][6][7][8].
In a PBS environment, the negatively charged CHIT layer can repel anionic interferences like UA and AA due to electrostatic forces. The authors then weaved their devices using cotton yarns on a conventional weaving machine and subsequently tested them with various glucose concentrations in a PBS solution. Notably, the devices exhibited a detectable response at a concentration as low as 30 × 10−9 M, similar to the detection limit of the devices before the weaving process. To illustrate the device’s practical application, they incorporated a fabric-based OECT into a diaper and used it to monitor glucose levels in artificial urine absorbed by the diaper. The device detected the glucose concentration as low as 3 × 10−3 M and showed good selectivity when 1 × 10−3 M uric acid was added. Also, note that the diaper underwent repeated bending and stretching during measurements. The device, however, exhibited minimal impact on its performance, suggesting the excellent stability of the device’s performance.
1.2. Ionic Concentration Sensors in Human Sweat
A research team at the Gwangju Institute of Science and Technology has developed a wearable sweat sensor based on the conductive polymer PEDOT:PSS [28][9]. They have designed microfiber-based organic electrochemical transistors (OECTs) for use as channel dimension-independent wearable sensors to measure ion concentrations in human sweat . These OECT devices without a substrate were created by carefully considering the acidity of the coagulation medium’s impact on OECT characteristics. This impact was examined in terms of the device’s response to various concentrations of aqueous ionic solutions in contact with the channel layer and the Ag/AgCl gate electrode. A PEDOT:PSS microfiber was connected with copper wires using Ag epoxy for source and drain connections. In the electrical measurement, the source and drain electrodes of the microfiber OECT were connected to a source meter, and the current variation was recorded at VD = −0.1 V. Meanwhile, the gate bias was applied to the Ag/AgCl gate electrode immersed in the aqueous electrolyte reservoir (containing NaCl). Consequently, the PEDOT:PSS chains could be de-doped (or doped) by positive (or negative) gate bias and/or high (or low) ion concentration [61][10]. This modulation of the source-to-drain current through the PEDOT:PSS microfiber channel led to a reduction in drain current and an on/off current ratio of 102 when the gate bias was swept from −0.4 to 0.4 V at a fixed drain voltage of −0.6 V, particularly in a 100 mM NaCl solution. The transfer curve shifted more negatively with higher salt concentrations, indicating that higher small ion concentrations could more effectively interrupt the doped state. According to the authors, they achieved a sensitivity of 01.6/dec for their PEDOT:PSS OECT devices. The time-dependent drain and gate currents were monitored when the NaCl concentration in the reservoir was altered. Despite keeping the gate and drain voltages fixed at 0 V and −0.1 V, respectively, to maintain the OECT device in the linear region, the reservoir solution was replaced with fresh solutions of designated NaCl concentrations (ranging from 10−1 to 103 mM). As a result, the negative drain current immediately increased upon adding a more concentrated NaCl solution (from 10−1 to 103 mM) and decreased when a less concentrated NaCl solution was introduced (from 103 to 10−1 mM). Additionally, the migration of ions between the gate electrode and the PEDOT:PSS layer could be monitored by measuring IG when the reservoir solution was replaced. For practical applications, the authors attached their microfiber-based OECT device to an arm, suggesting that this fiber-type ion sensor can be embedded into clothes due to its small size and flexibility even with its small diameter of 10–100 µm. They also demonstrated the practicality of employing substrate-free OECTs made from single strands in wearable electronics for human use. These devices were employed to sense the total small ion concentration in PBS, artificial sweat, and human sweat samples. The cation concentrations measured with their OECT sensor for the PBS and artificial sweat samples matched well those obtained from ion chromatography inductively coupled plasma (IC-ICP) mass spectroscopy with only a 3% error. However, the human sweat showed a relatively large error of 10%. The reason is that their OECT-based sensors can only detect monovalent small cations (Na+, K+), while the IC-ICP can detect all cations, not only monovalent cations but also divalent cations (Ca2+, Zn2+, Cu2+, Fe2+, Ni2+). The authors additionally highlighted the exceptional stability of the device following repeated measurements of cation concentrations after washing it out with a 1 mM NaCl solution.
Another interesting example of using fiber-based OECT for the detection of the saline concentration in human sweat was demonstrated by Tarabella et al. [41][11]. The fiber-based OECT consists of a cotton-soaked PEDOT:PSS thread channel, an Ag wire gate parallel to the channel, and a drop of liquid electrolyte to connect the thread channel and the wire gate. They investigated the suitability of fiber-based OECT for the physiological range of human sweat (2 × 10−2 to 8 × 10−2 M). They detected the physiological range of chloride in the sweat (30–60 mM), suggesting that their fiber-based OECT sensors are suitable for healthcare and fitness applications. The same group also developed cotton-based OECT sensors to detect adrenaline concentrations in human sweat [62][12]. A cotton yarn was functionalized with PEDOT:PSS for the channel and a Pt wire was used as the gate electrode. The researchers found that by using different gate electrodes such as Ag wires they could independently detect saline concentration and adrenaline concentration in real human sweat. In a recent study, the researchers demonstrated a new textile-based PEDOT:PSS OECT with ion-selective membranes added to detect selectively electrolytes in human sweat [63][13]. Their devices showed excellent selectivity to sodium, potassium, and calcium ions. The electrolyte concentration of the electrolytes that the sensors can detect was 10−5 to 1 M, which is the concentration range found in human sweat. This confirms the ability of their OECT to be integrated into clothes for real-time monitoring of electrolytes in sweat.
1.3. Ascorbic Acid Sensors
Ascorbic acid (AA) is widely known as vitamin C with primary functions as an antioxidant, protecting cells from the harmful effects of reactive oxygen species [65][14]. AA is also a neuromodulator in the central nervous system that is linked to neurological diseases such as epilepsy [64,66][15][16]. A research team from Fudan University developed ascorbic acid sensors based on all polymer fiber organic electrochemical transistors (PF-OECT [64][15]. To address the sensitivity reduction caused by rigid metal and carbon fiber-based electrodes and protein adhesion to the electrode surface in conventional electrochemical transistors, they used PEDOT:PSS fibers to create PF-OECT. These PF-OECTs had a soft, brain-tissue-like Young modulus and minimized protein adhesion due to the high hydrophilicity of PEDOT:PSS. There is a significant current change in the OECT sensors when exposed to ascorbic acid, even in the presence of interferences at similar physiological concentrations. The research team also evaluated PF-OECT, focusing on its flexibility, stability, and resistance to biofouling. When the polymer fiber was subjected to simulated in vivo conditions, it showed an exceptional level of flexibility. Remarkably, the PF-OECT maintained its performance consistently under dynamic deformations in a physiological environment, even after being immersed in artificial cerebrospinal fluid at 37 °C for 14 days. Regarding its anti-biofouling characteristics, the PF-OECT demonstrated stability, with only minor fluctuations observed before and after exposure to BSA solutions for 14 days. Finally, the researchers implanted PF-OECTs into the brains of mice for in vivo testing. These devices effectively detected the presence of ascorbic acid, displaying high selectivity over other chemicals. Furthermore, they remained stable throughout the 14-day testing period, suggesting their potential as durable implantable sensors.
Another example of fiber-based OECTs for ascorbic acid sensors has been reported by Fang et al. [67][17]. Nylon fiber was covered with PEDOT:PSS to serve as a channel, and a CNT fiber gate was placed next to the channel to form a transistor structure. The CNT fiber without modification has catalytic activity for ascorbic acid. The coaxial fiber OECT showed a linear response with the concentration of ascorbic acid in the range from 0.1 to 1 mM, which is close to the concentration of ascorbic acid in human tissue. The sensitivity was 12.78 mA/decade, and the limit of detection was 1 µM. For real applications, the researchers implanted the coaxial fiber OECT with an unmodified CNT fiber gate into the deep brain of mice. The device impressively exhibited strong selectivity, accurately detecting ascorbic acid in the presence of interfering substances like dopamine and glucose. For the biocompatibility of the coaxial fiber OECT, they found minimal inflammation and excellent compatibility of their devices with the biological environment. An interesting point here is that they can use a modified CNT gate with an electron transfer agent, tetrathiafulvalene (TTF), and glucose oxidase (GOx) to easily detect glucose.
1.4. Dopamine Sensors
Dopamine (DA) is a vital neurotransmitter that holds a central role in various functions within the central nervous system [34,68][18][19]. Dysregulation of dopamine is notably associated with two prominent diseases: Parkinson’s syndrome, which is caused by dopamine deficiency, and Alzheimer’s disease, which is linked to dementia [34,69][18][20]. Organic thin-film transistors (OTFTs) have gained significant attention for their utility in dopamine detection, primarily owing to their advantages of being cost-effective, easily manufacturable, and highly portable [70,71,72,73][21][22][23][24]. Organic electrochemical transistors (OECTs), especially fiber-based OECTs have recently emerged as promising dopamine sensors due to their exceptional attributes, including high sensitivity, rapid response times, low detection limits, and remarkable flexibility [34,48,52,56,74,75][4][8][18][25][26][27]. These properties are largely attributed to their larger surface-to-volume ratio. Qing et al. [34][18] developed dopamine sensors based on fiber-based wearable organic transistors. They developed fiber-based organic transistors with superior performance compared to conventional transistors. The developed fiber transistors were used as dopamine sensors, utilizing the twisted structure of PVA-co-PE nanofibers (NFs), which provided fast response times, long-term stability, and high productivity. Additionally, the 3D polypyrrole structure induced by NFs enabled the production of high-performance dopamine sensors. Compared to metal gate electrode-based organic fiber transistors, fiber gate electrodes exhibited high initial channel current values, enabling the sensing of dopamine at low concentrations (1 nM) and providing high selectivity. PA6 filaments were adjusted in size in NFs slurry, followed by surface conductivity treatment with PPy to produce PPy/NFs/PA6 fiber transistors. Since these components were manufactured using a commercial weaving machine, they can be designed with various textile textures for diverse circuit configurations and efficient mass production.
1.5. Uric Acid Sensors
Uric acid is the primary antioxidant and the ultimate byproduct of purine nucleosides, adenosine, and guanosine catabolism [76,77][28][29]. The high level of uric acid in body fluids can lead to diseases such as gout, cardiovascular disease, kidney stones, and some metabolic syndromes [65][14]. Hence, the measurement of uric acid in human urine is crucial for early detection and monitoring of potential disease symptoms. Organic electrochemical transistors (OECTs), especially fiber-based OECTs have gained attention for sensing applications due to their adaptable design, low-operational voltage, and inherent signal amplification capabilities [48,76,78,79][25][28][30][31]. They offer a relatively large specific surface area, easy integration into fabrics, and seamless incorporation into textiles, providing exceptional sensitivity for various sensing applications. Recently, Tao et al. [76][28] from Wuhan Textile University conducted a study on synthesizing fiber-based transistors with poly(3,4-ethylenedioxythiophene) (PEDOT) nano-cluster structures via a reverse microfluidic method and utilized them as uric acid sensors [76][28]. First, cotton fiber was reduced with hydrazine hydrate in a gaseous environment to create graphene oxide-coated cotton fiber. Subsequently, rGO/cotton fiber was synthesized through a forward microfluidic method and used as the channel and gate electrode. To create the gate electrode for uric acid sensing, PEDOT-doped carbon fiber was prepared using a similar process.
Hao et al. [80][32] also reported molecularly imprinted polymer-based OECT sensors for uric acid and adrenaline. Here, they used a viscose yarn modified with a multi-walled carbon nanotube (MWCNT) and then coated with PEDOT:PSS for the channel. A carbon fiber yarn modified by a molecularly imprinted polymer (MIP) was used as a gate. These two yarns were placed in parallel and were connected by a gel electrolyte to complete a viscose yarn-based OECT. Their devices exhibited a high transconductance of 6.7 mS, a fast response time of 2 s, and excellent bending stability with the drain current remaining > 80% even after 500 bending. The detection limits of uric acid were in the range from 1 pM to mM. The MIP film was introduced to enhance the selectivity toward uric acid or adrenaline detection.
2. Physical Sensors
A research team from the Research Institute for Intelligent Wearable Systems in Hong Kong [81][33] has presented exciting tactile sensors based on ultrathin all-solid organic electrochemical transistors (OECT). First, they introduced an innovative method for producing large-scale ultrafine polyaniline (PAni) fibers (UFPFs) using a modified wet-spinning technique. In contrast to conventional wet-spinning processes, they replaced less effective solvents with more suitable ones in the coagulation bath. This alteration reduced the viscosity of the gel fibers, enabling them to be stretched to an ultrafine morphology through an extremely high drawing ratio. Then, the team designed an ultrathin all-solid organic electrochemical transistor (OECT) leveraging the structural and electrochemical advantages of UFPFs. The OECT device operates with less than 1 V of driving voltage, significantly amplifies drain−source electrical signals while consuming minimal power, and effectively responds to both vertical pressure and horizontal friction forces at varying intensities.
All the sensors described are summarized in Table 1.
Table 1.
A summary of fiber-type transistor-based sensors is discussed.
| Transistor Type | Sensing Type | Configuration | Materials | Fiber Preparation Method | References |
|---|---|---|---|---|---|
| OECT | Glucose | Cross geometry | Polypyrrole/rGO/polyamide (PA) filament | In situ polymerization | [52][4] |
| OECT | Glucose | Cross geometry | PEDOT:PSS/Nylon fibers | Coating | [48][25] |
| OECT | Ions in human sweat | A single strand fiber | PEDOT:PSS fibers | Wet spinning | [28][9] |
| OECT | Saline concentration in human sweat | Two parallel fibers | PEDOT:PSS/cotton thread | Soaking | [41][11] |
| OECT | Ascorbic acid | Two parallel fibers | PEDOT:PSS fibers | Extrusion | [64][15] |
| OECT | Dopamine | Cross geometry | PPy/NFs/PA6 fiber | In situ polymerization | [34][18] |
| OECT | Uric acid | Two parallel fibers | PEDOT/rGO/cotton fiber | Reversed microemulsion polymerization | [76][28] |
| OECT | Tactile | Two parallel fibers | Polyaniline (PANi) fibers | Wet spinning | [81][33] |
References
- Zhang, M.; Liao, C.; Mak, C.H.; You, P.; Mak, C.L.; Yan, F. Highly sensitive glucose sensors based on enzyme-modified whole-graphene solution-gated transistors. Sci. Rep. 2015, 5, 8311.
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825.
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171.
- Wang, Y.; Qing, X.; Zhou, Q.; Zhang, Y.; Liu, Q.; Liu, K.; Wang, W.; Li, M.; Lu, Z.; Chen, Y.; et al. The woven fiber organic electrochemical transistors based on polypyrrole nanowires/reduced graphene oxide composites for glucose sensing. Biosens. Bioelectron. 2017, 95, 138–145.
- Shim, N.Y.; Bernards, D.A.; Macaya, D.J.; DeFranco, J.A.; Nikolou, M.; Owens, R.M.; Malliaras, G.G. All-plastic electrochemical transistor for glucose sensing using a ferrocene mediator. Sensors 2009, 9, 9896–9902.
- Liao, C.; Zhang, M.; Niu, L.; Zheng, Z.; Yan, F. Highly selective and sensitive glucose sensors based on organic electrochemical transistors with graphene-modified gate electrodes. J. Mater. Chem. B 2013, 1, 3820–3829.
- Bernards, D.A.; Macaya, D.J.; Nikolou, M.; DeFranco, J.A.; Takamatsu, S.; Malliaras, G.G. Enzymatic sensing with organic electrochemical transistors. J. Mater. Chem. 2008, 18, 116–120.
- Tang, H.; Lin, P.; Chan, H.L.; Yan, F. Highly sensitive dopamine biosensors based on organic electrochemical transistors. Biosens. Bioelectron. 2011, 26, 4559–4563.
- Kim, Y.; Lim, T.; Kim, C.-H.; Yeo, C.S.; Seo, K.; Kim, S.-M.; Kim, J.; Park, S.Y.; Ju, S.; Yoon, M.-H. Organic electrochemical transistor-based channel dimension-independent single-strand wearable sweat sensors. NPG Asia Mater. 2018, 10, 1086–1095.
- Khodagholy, D.; Doublet, T.; Quilichini, P.; Gurfinkel, M.; Leleux, P.; Ghestem, A.; Ismailova, E.; Herve, T.; Sanaur, S.; Bernard, C.; et al. In vivo recordings of brain activity using organic transistors. Nat. Commun. 2013, 4, 1575.
- Tarabella, G.; Villani, M.; Calestani, D.; Mosca, R.; Iannotta, S.; Zappettini, A.; Coppedè, N. A single cotton fiber organic electrochemical transistor for liquid electrolyte saline sensing. J. Mater. Chem. 2012, 22, 23830–23834.
- Coppedè, N.; Tarabella, G.; Villani, M.; Calestani, D.; Iannotta, S.; Zappettini, A. Human stress monitoring through an organic cotton-fiber biosensor. J. Mater. Chem. B 2014, 2, 5620–5626.
- Coppedè, N.; Giannetto, M.; Villani, M.; Lucchini, V.; Battista, E.; Careri, M.; Zappettini, A. Ion selective textile organic electrochemical transistor for wearable sweat monitoring. Org. Electron. 2020, 78, 105579.
- Yang, Y.; Jo, A.; Lee, Y.; Lee, C. Electrodeposited nanoporous ruthenium oxide for simultaneous quantification of ascorbic acid and uric acid using chronoamperometry at two different potentials. Sens. Actuators B Chem. 2018, 255, 316–324.
- Feng, J.; Fang, Y.; Wang, C.; Chen, C.; Tang, C.; Guo, Y.; Wang, L.; Yang, Y.; Zhang, K.; Wang, J.; et al. All-Polymer Fiber Organic Electrochemical Transistor for Chronic Chemical Detection in the Brain. Adv. Funct. Mater. 2023, 33, 2214945.
- Cheng, H.; Li, L.; Zhang, M.; Jiang, Y.; Yu, P.; Ma, F.; Mao, L. Recent advances on in vivo analysis of ascorbic acid in brain functions. TrAC Trends Anal. Chem. 2018, 109, 247–259.
- Fang, Y.; Feng, J.; Shi, X.; Yang, Y.; Wang, J.; Sun, X.; Li, W.; Sun, X.; Peng, H. Coaxial fiber organic electrochemical transistor with high transconductance. Nano Res. 2023, 16, 11885–11892.
- Qing, X.; Wang, Y.; Zhang, Y.; Ding, X.; Zhong, W.; Wang, D.; Wang, W.; Liu, Q.; Liu, K.; Li, M.; et al. Wearable Fiber-Based Organic Electrochemical Transistors as a Platform for Highly Sensitive Dopamine Monitoring. ACS Appl. Mater. Interfaces 2019, 11, 13105–13113.
- Yang, W.; Yu, Y.; Tang, Y.; Li, K.; Zhao, Z.; Li, M.; Yin, G.; Li, H.; Sun, S. Enhancing electrochemical detection of dopamine via dumbbell-like FePt–Fe3O4 nanoparticles. Nanoscale 2017, 9, 1022–1027.
- Li, B.-R.; Hsieh, Y.-J.; Chen, Y.-X.; Chung, Y.-T.; Pan, C.-Y.; Chen, Y.-T. An Ultrasensitive Nanowire-Transistor Biosensor for Detecting Dopamine Release from Living PC12 Cells under Hypoxic Stimulation. J. Am. Chem. Soc. 2013, 135, 16034–16037.
- Sun, C.; Wang, X.; Auwalu, M.A.; Cheng, S.; Hu, W. Organic thin film transistors-based biosensors. EcoMat 2021, 3, e12094.
- Wang, N.; Yang, A.; Fu, Y.; Li, Y.; Yan, F. Functionalized Organic Thin Film Transistors for Biosensing. Acc. Chem. Res. 2019, 52, 277–287.
- Song, J.; Zheng, J.; Yang, A.; Liu, H.; Zhao, Z.; Wang, N.; Yan, F. Metal–organic framework transistors for dopamine sensing. Mater. Chem. Front. 2021, 5, 3422–3427.
- Casalini, S.; Leonardi, F.; Cramer, T.; Biscarini, F. Organic field-effect transistor for label-free dopamine sensing. Org. Electron. 2013, 14, 156–163.
- Yang, A.; Li, Y.; Yang, C.; Fu, Y.; Wang, N.; Li, L.; Yan, F. Fabric Organic Electrochemical Transistors for Biosensors. Adv. Mater. 2018, 30, e1800051.
- Wang, N.; Liu, Y.; Fu, Y.; Yan, F. AC Measurements Using Organic Electrochemical Transistors for Accurate Sensing. ACS Appl. Mater. Interfaces 2018, 10, 25834–25840.
- Gualandi, I.; Tonelli, D.; Mariani, F.; Scavetta, E.; Marzocchi, M.; Fraboni, B. Selective detection of dopamine with an all PEDOT:PSS Organic Electrochemical Transistor. Sci. Rep. 2016, 6, 35419.
- Tao, Y.; Wang, Y.; Zhu, R.; Chen, Y.; Liu, X.; Li, M.; Yang, L.; Wang, Y.; Wang, D. Fiber based organic electrochemical transistor integrated with molecularly imprinted membrane for uric acid detection. Talanta 2022, 238, 123055.
- El Ridi, R.; Tallima, H. Physiological functions and pathogenic potential of uric acid: A review. J. Adv. Res. 2017, 8, 487–493.
- Lin, P.; Yan, F. Organic Thin-Film Transistors for Chemical and Biological Sensing. Adv. Mater. 2012, 24, 34–51.
- Liao, C.; Mak, C.; Zhang, M.; Chan, H.L.; Yan, F. Flexible organic electrochemical transistors for highly selective enzyme biosensors and used for saliva testing. Adv. Mater. 2015, 27, 676–681.
- Hao, P.; Zhu, R.; Tao, Y.; Jiang, W.; Liu, X.; Tan, Y.; Wang, Y.; Wang, D. Dual-Analyte Sensing with a Molecularly Imprinted Polymer Based on Enhancement-Mode Organic Electrochemical Transistors. ACS Appl. Mater. Interfaces 2023, 15, 30567–30579.
- Fang, B.; Yan, J.; Chang, D.; Piao, J.; Ma, K.M.; Gu, Q.; Gao, P.; Chai, Y.; Tao, X. Scalable production of ultrafine polyaniline fibres for tactile organic electrochemical transistors. Nat. Commun. 2022, 13, 2101.
More
