Due to increasing concerns about global warming and energy crisis, intensive efforts have been made to explore renewable and clean energy sources. Single-atom metals and two-dimensional (2D) nanomaterials have attracted extensive attention in the fields of energy and environment because of their unique electronic structures and excellent properties.
- single-atom metal
- two-dimensional nanomaterials
- water splitting
- CO2 reduction
- catalysis
- DFT
1. Introduction
2. Synthesis Methods of Single-Atom Metal/2D MoS2 Hybrid Nanomaterials
The catalytic performances are determined by concrete improvements of synthetic methodologies [44]. The most widely employed approaches for SACs are pyrolysis, atomic layer deposition (ALD) method, physical vapor deposition (PVD), wet-chemistry strategy, and electronic deposition [45][46][47]. However, it is still hard to manipulate atoms in a highly accurate way for the control synthesis of theoretically designed SACs due to ultrahigh surface free energy. The synthesis methodologies for 2D MoS2 can be divided into two categories: top-down and bottom-up methods. The former mainly includes chemical vapor deposition (CVD) and solvothermal or hydrothermal methods [48][49][50][51][52]; the latter includes mechanical exfoliation, chemical or electrochemical exfoliation methods, and liquid-phase exfoliation [53]. However, the number of successful cases for single-atom metal/2D MoS2 hybrid nanomaterials is still limited compared to the synthesis methods for single-atom metal modified 3D supports, SACs preparation, and/or 2D materials. The methods for single-atom metal/2D MoS2 hybrid nanomaterials are derived from the approaches for SAC@3D supports, such as pyrolysis and coprecipitation. Considering that the common synthetic methods of SACs or 2D materials have been discussed in depth in previous reviews, this revisewarch focuses on some novel methods for single-atom metal/2D MoS2 hybrid nanomaterials, including the one-pot chemical method, the electrochemical process, and the polyoxometalate template-based synthetic strategy (Figure 1).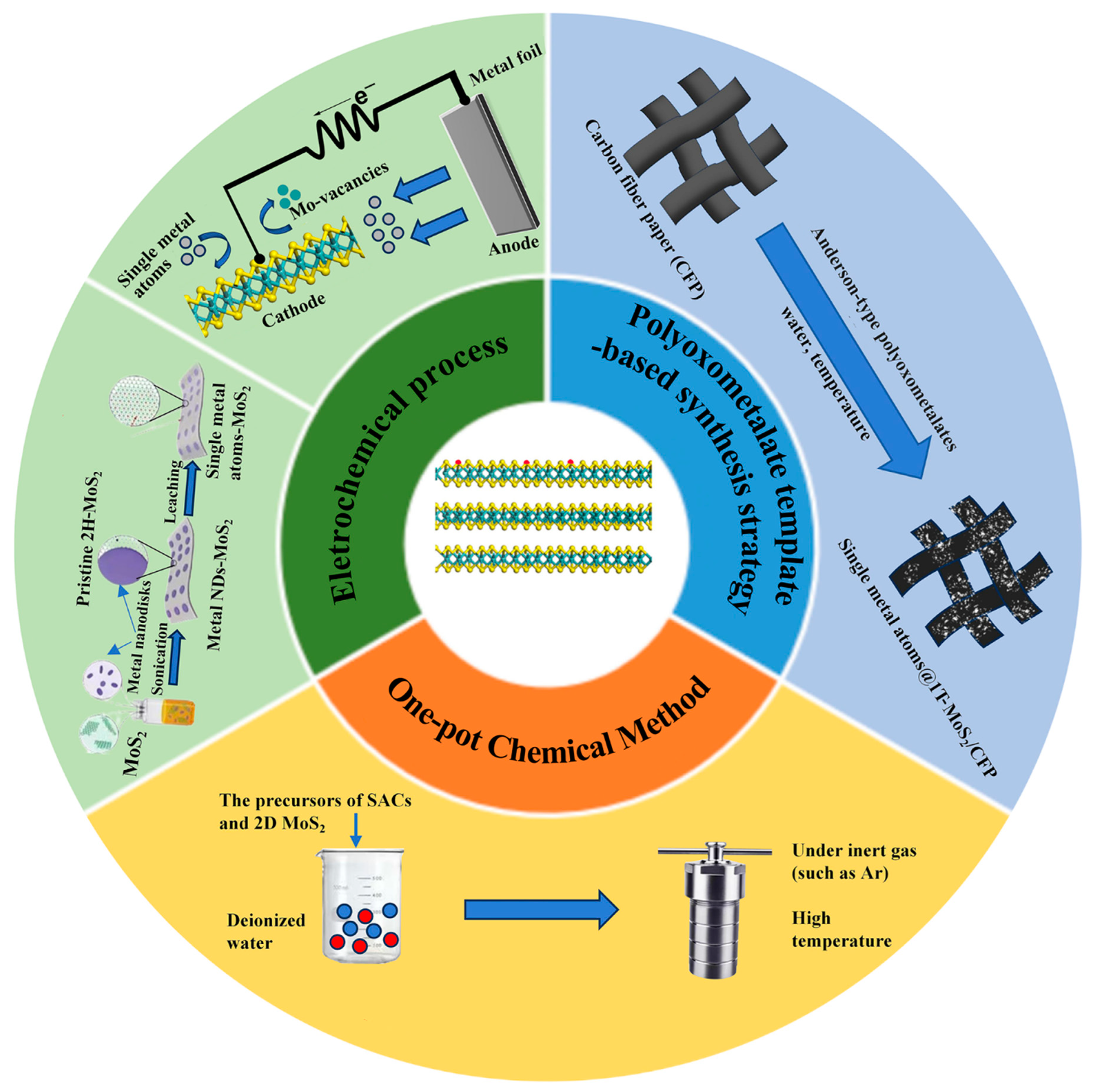
2.1. One-Pot Chemical Method
The principle of this method is directly to mix the precursors of SACs and 2D MoS2 for the following reactions under inert gas (e.g., Ar). The typical processes are described as follows: firstly, all precursors (e.g., (NH4)6Mo7O24·4H2O, H2PtCl6, and CS2) are dissolved in a certain amount of deionized water to form a homogeneous solution [20]; then, the resulting solution is transferred to a Teflon-lined stainless autoclave under Ar and maintained at a high temperature for a certain reaction time. The one-pot method has the advantages of simple operation and saving synthetic costs. In addition, the catalysts prepared by this method can have sulfur vacancies and the doping features of metal atoms at the same time [54]. Until now, Cu@1T-MoS2, Ni@1T-MoS2, Fe@1T-MoS2, and Co@1T-MoS2 have been easily prepared through this method [55].2.2. Electrochemical Process
The electrochemical process is used to synthesize SAC-modified 2D MoS2 via electrochemical etching of big-size metal precursor. Taking the single-atom cobalt (Co) array modified 2D MoS2 as an example, firstly 2D MoS2 and Co nanodisks (NDs) are synthesized using standard solvothermal procedures and standard air-free procedures, respectively. Then, the combination of Co NDs and 2D MoS2 is realized via an assembly process. Finally, single-atom Co array covalently bound onto distorted 1T-MoS2 nanosheets (denoted as SA Co-D 1T-MoS2) via Co-S bonds can be synthesized through electrochemical cyclic voltammetry (CV) leaching of Co nanodisks (NDs). In addition to electrochemical CV leaching, the electrochemical deposition can be used to synthesize the nanocomposites of SACs modified 2D MoS2, such as Pt, Cu, Sn, and Pd anchored on the 2D MoS2, because these single metal atoms (from metal ions in the electrolyte solution) can be introduced onto the MoS2 monolayer driven by applying the bias potential [56].2.3. Polyoxometalate Template-Based Synthetic Strategy
Highly purified and stable metallic 1T-MoS2 can be obtained via a hydrothermal method which introduces organic sulfur sources into (NH4)6Mo7O24·4H2O (denoted as Mo7). Here, Mo7 is a precursor that is a butterfly-shaped metal oxide cluster, and it belongs to the β-isomer of Anderson-type polyoxometalates (POMs). In addition, its unique structure makes it possible to tune the chemical environment of 1T-MoS2 with various metal atoms. Using Anderson-type polyoxometalates ([XH6Mo6O24]n−) as precursors, atomically designing metal doping sites onto metallic 1T-MoS2 can be achieved. [XH6Mo6O24]n− is denoted as XMo6 (X = FeIII, CoIII, n = 3; X = NiII, n = 4) [53].3. Applications of Single-Atom Metal/2D MoS2 Hybrid Nanomaterials
3.1. Electrochemical CO2 Reduction
Electrocatalytic CO2RR includes three steps, namely, the chemisorption of CO2 on the surface of electrocatalysts, the transfer of high-energy electrons and protons between two elements to break C=O bonds, and the desorption of products from the surface of the electrocatalysts [57]. For the hybrid system composed of single-atom metals and 2D MoS2, CO2RR more frequently occurred on metal, and the reaction paths or products are strongly dependent on the components of metal. These reaction products or paths include three types: (1) the reduction of CO2 to CO (e.g., on Au or Ag), (2) the reduction of CO2 to formic acid (e.g., on Sn and Pb), and (3) the reduction of CO or carbon–oxygen compounds to hydrocarbons or alcohols (e.g., on Cu, Fe, and Mn) (Table 1).|
Catalyst |
Potential Determining Steps |
Limiting Potentials (V) |
Overpotential (V) |
Production for Catalysts |
Ref. |
|---|---|---|---|---|---|
|
Fe@MoS2 |
*HCOO → *HCOOH |
−0.39 |
0.56 |
CH4 |
[58] |
|
Co@MoS2 |
*HCOO → *HCOOH |
−0.24 |
0.41 |
CH4 |
[58] |
|
Ni@MoS2 |
CO2 → *HCOO |
−0.45 |
0.62 |
CH4 |
[58] |
|
Cu@MoS2 |
*OCH3 → CH4 + *O |
−1.05 |
1.22 |
CH4 |
[58] |
|
Ru@MoS2 |
*CO → *CHO |
−0.73 |
0.9 |
CH4 |
[59] |
|
Pd@MoS2 |
CO2 → *HCOO |
−0.96 |
1.13 |
CH4 |
[60] |
|
Pt@MoS2 |
CO2 → *HCOO |
−0.50 |
0.67 |
CH4 |
[60] |
3.2. Electrochemical Water Splitting
In a conventional water electrolyzer, HER reaction occurs at the cathode and H2 is separated out, while OER reaction occurs at the anode and O2 is separated out (Figure 2a) [61][62][63]. Under standard conditions, a thermodynamic potential of 1.23 V is required to drive electrochemical water splitting (Figure 2b) for HER and OER. However, in real conditions, the input potential of water splitting in practical electrolyzers is much larger than 1.23 V. In general, a high-performance electrocatalyst for water splitting is still focused on noble-metal-based catalysts (e.g., Pt for HER and IrO2 or RuO2 for OER) (Table 2); however, it is necessary to develop noble metal-free electrocatalysts or decrease the loading amount of noble metals electrocatalysts because of the prohibitive cost and scarce reserve. For the reaction mechanisms (e.g., the Volmer–Heyrovsky mechanism) or paths for HER used in calculation models, there are three elementary steps regarding interactions between the water dissociates and a reactive hydrogen intermediate (absorbed hydrogen on the catalyst surface, Had), including the Volmer step followed by either the Heyrovsky step (H2O + Had + e– ↔ H2 + OH−) or the Tafel recombination step (2Had ↔ H2). The detailed mechanism or reaction path is dependent on the components of metal.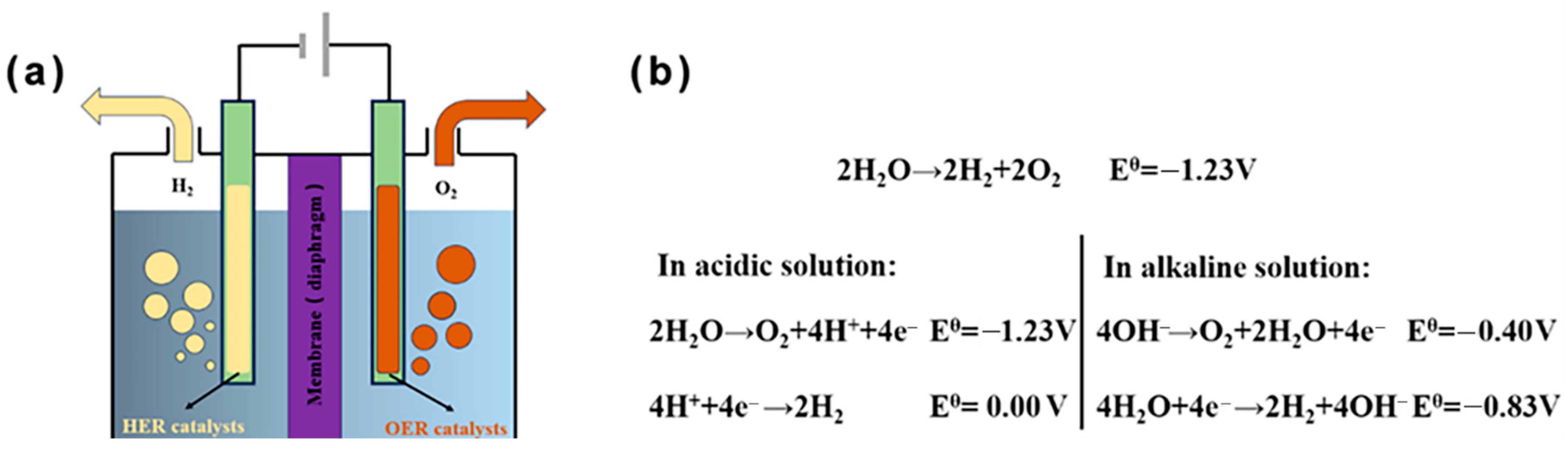
|
Catalyst |
Electrolyte |
η (mV)/Best Ratio (w.t.% or Concentration) |
Tafel Slope (mV dec −1) |
Stability Test |
Ref. |
|---|---|---|---|---|---|
|
Co/2D MoS2 |
0.5 M H2SO4 |
42/3.5% Co/1T-MoS2 |
32 |
10,000 CVs |
[64] |
|
Pt/2D MoS2 |
0.1 M H2SO4 |
60/1.5% Pt/MoS2 |
96 |
5000 CVs |
[20] |
|
Pd/2D MoS2 |
0.5 M H2SO4 |
89/1% Pd/1T-MoS2 |
62 |
5000 CVs |
[65] |
|
Ni/2D MoS2 |
0.5 M H2SO4 |
98/Ni/MoS2 |
103 |
2000 CVs |
[66] |
|
Ru/2D MoS2 |
0.5 M H2SO4 |
114/46 μg cm−2 Ru/MoS2 |
- |
10 h |
[67] |
|
Cu/2D MoS2 |
0.5 M H2SO4 |
131/1% Cu/MoS2 |
51 |
7 h |
[54] |
|
Fe, Co, Ni, Pd, Pt/2D MoS2 |
0.5 M H2SO4 |
140/2.7% Pd/1T-MoS2 |
57 |
1000 CVs |
[68] |
|
Ni/2D MoS2 |
0.5 M H2SO4 |
174/1% Ni/MoS2 |
69 |
1000 CVs |
[69] |
|
Au, Pt, Pd/2D MoS2 |
0.5 M H2SO4 |
210/1.1% Pt/MoS2 |
104 |
5 h |
[70] |
|
Ni/2D MoS2 |
0.5 M H2SO4 |
263/2.7% Ni/MoS2 |
81 |
1000 CVs |
[71] |
|
Ru/2D MoS2 |
1.0 M PBS |
125/46 μg cm−2 Ru/MoS2 |
- |
10 h |
[67] |
|
Ru/2D MoS2 |
1.0 M KOH |
41/46 μg cm−2 Ru/MoS2 |
114 |
20 h |
[67] |
|
Ir/2D MoS2 |
1.0 M KOH |
44/Ir/1T-MoS2 |
32 |
9000 CVs |
[72] |
|
Ni/2D MoS2 |
1.0 M KOH |
110/Ni/MoS2 |
119 |
2000 CVs |
[66] |
Note: overpotential value (η) and stability test were performed at the current density of 10 mA cm−2; CVs, cycles.
References
- Chen, Y.; Ji, S.; Chen, C.; Peng, Q.; Wang, D.; Li, Y. Single-atom catalysts: Synthetic strategies and electrochemical applications. Joule 2018, 2, 1242–1264.
- Zhang, J.; Zhao, Y.; Guo, X.; Chen, C.; Dong, C.-L.; Liu, R.-S.; Han, C.-P.; Li, Y.; Gogotsi, Y.; Wang, G. Single platinum atoms immobilized on an MXene as an efficient catalyst for the hydrogen evolution reaction. Nat. Catal. 2018, 1, 985–992.
- Gu, J.; Hsu, C.-S.; Bai, L.; Chen, H.M.; Hu, X. Atomically dispersed Fe3+ sites catalyze efficient CO2 electroreduction to CO. Science 2019, 364, 1091–1094.
- Wang, A.; Li, J.; Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2018, 2, 65–81.
- Khan, I.; Yuan, A.; Khan, S.; Khan, A.; Khan, S.; Shah, S.A.; Luo, M.; Yaseen, W.; Shen, X.; Yaseen, M. Graphitic carbon nitride composites with gold and ZIF-67 nanoparticles as visible-light-promoted catalysts for CO2 conversion and bisphenol A degradation. ACS Appl. Nano Mater. 2022, 5, 13404–13416.
- Khan, I.; Kang, K.; Khan, A.; Jiyuan, G.; Khan, S.; Khan, S.; Basir, A.; Sadiq, S. Efficient CO2 conversion and organic pollutants degradation over Sm3+ doped and rutile TiO2 nanorods decorated-GdFeO3 nanorods. Int. J. Hydrogen Energy 2023, in press.
- Khan, I.; Luo, M.; Khan, S.; Asghar, H.; Saeed, M.; Khan, S.; Khan, A.; Humayun, M.; Guo, L.; Shi, B. Green synthesis of SrO bridged LaFeO3/g-C3N4 nanocomposites for CO2 conversion and bisphenol A degradation with new insights into mechanism. Environ. Res. 2022, 207, 112650.
- Liu, H.; Grasseschi, D.; Dodda, A.; Fujisawa, K.; Olson, D.; Kahn, E.; Zhang, F.; Zhang, T.; Lei, Y.; Branco, R.B.N. Spontaneous chemical functionalization via coordination of Au single atoms on monolayer MoS2. Sci. Adv. 2020, 6, eabc9308.
- Shan, J.; Ye, C.; Jiang, Y.; Jaroniec, M.; Zheng, Y.; Qiao, S.-Z. Metal-metal interactions in correlated single-atom catalysts. Sci. Adv. 2022, 8, eabo0762.
- Xu, J.; Lai, S.; Qi, D.; Hu, M.; Peng, X.; Liu, Y.; Liu, W.; Hu, G.; Xu, H.; Li, F. Atomic Fe-Zn dual-metal sites for high-efficiency pH-universal oxygen reduction catalysis. Nano Res. 2021, 14, 1374–1381.
- Zhang, N.; Zhang, X.; Tao, L.; Jiang, P.; Ye, C.; Lin, R.; Huang, Z.; Li, A.; Pang, D.; Yan, H. Silver single-atom catalyst for efficient electrochemical CO2 reduction synthesized from thermal transformation and surface reconstruction. Angew. Chem. Int. Ed. 2021, 60, 6170–6176.
- Gan, X.; Lei, D.; Ye, R.; Zhao, H.; Wong, K.-Y. Transition metal dichalcogenide-based mixed-dimensional heterostructures for visible-light-driven photocatalysis: Dimensionality and interface engineering. Nano Res. 2021, 14, 2003–2022.
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641.
- Yang, X.-F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-atom catalysts: A new frontier in heterogeneous catalysis. Acc. Chem. Res. 2013, 46, 1740–1748.
- Jones, J.; Xiong, H.; DeLaRiva, A.T.; Peterson, E.J.; Pham, H.; Challa, S.R.; Qi, G.; Oh, S.; Wiebenga, M.H.; Pereira Hernández, X.I. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 2016, 353, 150–154.
- Sun, J.-F.; Wu, J.-T.; Xu, Q.-Q.; Zhou, D.; Yin, J.-Z. CO2 electrochemical reduction using single-atom catalysts. Preparation, characterization and anchoring strategies: A review. Environ. Chem. Lett. 2020, 18, 1593–1623.
- Kwon, K.C.; Suh, J.M.; Varma, R.S.; Shokouhimehr, M.; Jang, H.W. Electrocatalytic water splitting and CO2 reduction: Sustainable solutions via single-atom catalysts supported on 2D materials. Small Methods 2019, 3, 1800492.
- Lee, W.H.; Ko, Y.-J.; Kim, J.-Y.; Min, B.K.; Hwang, Y.J.; Oh, H.-S. Single-atom catalysts for the oxygen evolution reaction: Recent developments and future perspectives. Chem. Commun. 2020, 56, 12687–12697.
- Li, X.; Cui, P.; Zhong, W.; Li, J.; Wang, X.; Wang, Z.; Jiang, J. Graphitic carbon nitride supported single-atom catalysts for efficient oxygen evolution reaction. Chem. Commun. 2016, 52, 13233–13236.
- Deng, J.; Li, H.; Xiao, J.; Tu, Y.; Deng, D.; Yang, H.; Tian, H.; Li, J.; Ren, P.; Bao, X. Triggering the electrocatalytic hydrogen evolution activity of the inert two-dimensional MoS2 surface via single-atom metal doping. Energy Environ. Sci. 2015, 8, 1594–1601.
- He, T.; Zhang, C.; Du, A. Single-atom supported on graphene grain boundary as an efficient electrocatalyst for hydrogen evolution reaction. Chem. Eng. Sci. 2019, 194, 58–63.
- Vancsó, P.; Popov, Z.I.; Pető, J.n.; Ollár, T.; Dobrik, G.; Pap, J.z.S.; Hwang, C.; Sorokin, P.B.; Tapasztó, L. Transition metal chalcogenide single layers as an active platform for single-atom catalysis. ACS Energy Lett. 2019, 4, 1947–1953.
- Zhou, X.; Li, K.; Lin, Y.; Song, L.; Liu, J.; Liu, Y.; Zhang, L.; Wu, Z.; Song, S.; Li, J. A single-atom manipulation approach for synthesis of atomically mixed nanoalloys as efficient catalysts. Angew. Chem. 2020, 132, 13670–13676.
- Su, H.; Zhou, W.; Zhang, H.; Zhou, W.; Zhao, X.; Li, Y.; Liu, M.; Cheng, W.; Liu, Q. Dynamic evolution of solid–liquid electrochemical interfaces over single-atom active sites. J. Am. Chem. Soc. 2020, 142, 12306–12313.
- Zhang, X.; Guo, J.; Guan, P.; Liu, C.; Huang, H.; Xue, F.; Dong, X.; Pennycook, S.J.; Chisholm, M.F. Catalytically active single-atom niobium in graphitic layers. Nat. Commun. 2013, 4, 1924.
- Gan, X.; Zhang, J.; Liu, J.; Bai, Y.; Su, X.; Wang, W.; Cao, Z.; Zhao, H.; Ao, Y.; Wang, P. Polyaniline Functionalization of Defective 1T-MoS2 Nanosheets for Improved Electron and Mass Transfer: Implications for Electrochemical Sensors. ACS Appl. Nano Mater. 2023, 6, 11725–11736.
- Hou, C.C.; Zou, L.; Sun, L.; Zhang, K.; Liu, Z.; Li, Y.; Li, C.; Zou, R.; Yu, J.; Xu, Q. Single-atom iron catalysts on overhang-eave carbon cages for high-performance oxygen reduction reaction. Angew. Chem. 2020, 132, 7454–7459.
- Zhu, J.; Cai, L.; Yin, X.; Wang, Z.; Zhang, L.; Ma, H.; Ke, Y.; Du, Y.; Xi, S.; Wee, A.T. Enhanced electrocatalytic hydrogen evolution activity in single-atom Pt-decorated VS2 nanosheets. ACS Nano 2020, 14, 5600–5608.
- Rao, R.G.; Blume, R.; Hansen, T.W.; Fuentes, E.; Dreyer, K.; Moldovan, S.; Ersen, O.; Hibbitts, D.D.; Chabal, Y.J.; Schlögl, R. Interfacial charge distributions in carbon-supported palladium catalysts. Nat. Commun. 2017, 8, 340.
- Gan, X.; Zhao, H.; Quan, X. Two-dimensional MoS2: A promising building block for biosensors. Biosens. Bioelectron. 2017, 89, 56–71.
- Liu, T.; Zhao, X.; Liu, X.; Xiao, W.; Luo, Z.; Wang, W.; Zhang, Y.; Liu, J.-C. Understanding the hydrogen evolution reaction activity of doped single-atom catalysts on two-dimensional GaPS4 by DFT and machine learning. J. Energy Chem. 2023, 81, 93–100.
- Xiao, Z.; Gan, X.; Zhu, T.; Lei, D.; Zhao, H.; Wang, P. Activating the Basal Planes in 2H-MoTe2 Monolayers by Incorporating Single-Atom Dispersed N or P for Enhanced Electrocatalytic Overall Water Splitting. Adv. Sustain. Syst. 2022, 6, 2100515.
- Gan, X.; Lei, D.; Wong, K.-Y. Two-dimensional layered nanomaterials for visible-light-driven photocatalytic water splitting. Mater. Today Energy 2018, 10, 352–367.
- Pan, U.N.; Paudel, D.R.; Das, A.K.; Singh, T.I.; Kim, N.H.; Lee, J.H. Ni-nanoclusters hybridized 1T–Mn–VTe2 mesoporous nanosheets for ultra-low potential water splitting. Appl. Catal. B Environ. 2022, 301, 120780.
- Ertl, G.; Knözinger, H.; Weitkamp, J. Handbook of Heterogeneous Catalysis; VCH: Weinheim, Germany, 1997; Volume 2.
- Yang, J.; Mohmad, A.R.; Wang, Y.; Fullon, R.; Song, X.; Zhao, F.; Bozkurt, I.; Augustin, M.; Santos, E.J.; Shin, H.S. Ultrahigh-current-density niobium disulfide catalysts for hydrogen evolution. Nat. Mater. 2019, 18, 1309–1314.
- Gusmão, R.; Veselý, M.; Sofer, Z.k. Recent developments on the single atom supported at 2D materials beyond graphene as catalysts. ACS Catal. 2020, 10, 9634–9648.
- Shi, Y.; Zhou, Y.; Yang, D.-R.; Xu, W.-X.; Wang, C.; Wang, F.-B.; Xu, J.-J.; Xia, X.-H.; Chen, H.-Y. Energy level engineering of MoS2 by transition-metal doping for accelerating hydrogen evolution reaction. J. Am. Chem. Soc. 2017, 139, 15479–15485.
- Asadi, M.; Kim, K.; Liu, C.; Addepalli, A.V.; Abbasi, P.; Yasaei, P.; Phillips, P.; Behranginia, A.; Cerrato, J.M.; Haasch, R. Nanostructured transition metal dichalcogenide electrocatalysts for CO2 reduction in ionic liquid. Science 2016, 353, 467–470.
- Gan, X.; Lee, L.Y.S.; Wong, K.-y.; Lo, T.W.; Ho, K.H.; Lei, D.Y.; Zhao, H. 2H/1T phase transition of multilayer MoS2 by electrochemical incorporation of S vacancies. ACS Appl. Energy Mater. 2018, 1, 4754–4765.
- Gan, X.; Zhao, H.; Lei, D.; Wang, P. Improving electrocatalytic activity of 2H-MoS2 nanosheets obtained by liquid phase exfoliation: Covalent surface modification versus interlayer interaction. J. Catal. 2020, 391, 424–434.
- Zhang, J.; Wang, T.; Pohl, D.; Rellinghaus, B.; Dong, R.; Liu, S.; Zhuang, X.; Feng, X. Interface engineering of MoS2/Ni3S2 heterostructures for highly enhanced electrochemical overall-water-splitting activity. Angew. Chem. 2016, 128, 6814–6819.
- Linghu, Y.; Tong, T.; Li, C.; Wu, C. The catalytic mechanism of CO2 electrochemical reduction over transition metal-modified 1T’-MoS2 monolayers. Appl. Surf. Sci. 2022, 590, 153001.
- Ji, S.; Chen, Y.; Wang, X.; Zhang, Z.; Wang, D.; Li, Y. Chemical synthesis of single atomic site catalysts. Chem. Rev. 2020, 120, 11900–11955.
- Cheng, N.; Stambula, S.; Wang, D.; Banis, M.N.; Liu, J.; Riese, A.; Xiao, B.; Li, R.; Sham, T.-K.; Liu, L.-M. Platinum single-atom and cluster catalysis of the hydrogen evolution reaction. Nat. Commun. 2016, 7, 13638.
- Lu, Y.; Kuo, C.-T.; Kovarik, L.; Hoffman, A.S.; Boubnov, A.; Driscoll, D.M.; Morris, J.R.; Bare, S.R.; Karim, A.M. A versatile approach for quantification of surface site fractions using reaction kinetics: The case of CO oxidation on supported Ir single atoms and nanoparticles. J. Catal. 2019, 378, 121–130.
- Repp, J.; Moresco, F.; Meyer, G.; Rieder, K.-H.; Hyldgaard, P.; Persson, M. Substrate mediated long-range oscillatory interaction between adatoms: Cu/Cu(111). Phys. Rev. Lett. 2000, 85, 2981.
- Ji, Q.; Zhang, Y.; Zhang, Y.; Liu, Z. Chemical vapour deposition of group-VIB metal dichalcogenide monolayers: Engineered substrates from amorphous to single crystalline. Chem. Soc. Rev. 2015, 44, 2587–2602.
- Xiong, Q.; Zhang, X.; Wang, H.; Liu, G.; Wang, G.; Zhang, H.; Zhao, H. One-step synthesis of cobalt-doped MoS2 nanosheets as bifunctional electrocatalysts for overall water splitting under both acidic and alkaline conditions. Chem. Commun. 2018, 54, 3859–3862.
- Zhang, F.; Momeni, K.; AlSaud, M.A.; Azizi, A.; Hainey, M.F.; Redwing, J.M.; Chen, L.-Q.; Alem, N. Controlled synthesis of 2D transition metal dichalcogenides: From vertical to planar MoS2. 2D Mater. 2017, 4, 025029.
- Zhang, H. Ultrathin two-dimensional nanomaterials. ACS Nano 2015, 9, 9451–9469.
- Li, J.; Chen, S.; Quan, F.; Zhan, G.; Jia, F.; Ai, Z.; Zhang, L. Accelerated dinitrogen electroreduction to ammonia via interfacial polarization triggered by single-atom protrusions. Chem 2020, 6, 885–901.
- Zeng, Z.; Yin, Z.; Huang, X.; Li, H.; He, Q.; Lu, G.; Boey, F.; Zhang, H. Single-layer semiconducting nanosheets: High-yield preparation and device fabrication. Angew. Chem. 2011, 123, 11289–11293.
- Ji, L.; Yan, P.; Zhu, C.; Ma, C.; Wu, W.; Wei, C.; Shen, Y.; Chu, S.; Wang, J.; Du, Y. One-pot synthesis of porous 1T-phase MoS2 integrated with single-atom Cu doping for enhancing electrocatalytic hydrogen evolution reaction. Appl. Catal. B Environ. 2019, 251, 87–93.
- Wang, G.; Zhang, G.; Ke, X.; Chen, X.; Chen, X.; Wang, Y.; Huang, G.; Dong, J.; Chu, S.; Sui, M. Direct Synthesis of Stable 1T-MoS2 Doped with Ni Single Atoms for Water Splitting in Alkaline Media. Small 2022, 18, 2107238.
- Wang, X.; Zhang, Y.; Wu, J.; Zhang, Z.; Liao, Q.; Kang, Z.; Zhang, Y. Single-atom engineering to ignite 2D transition metal dichalcogenide based catalysis: Fundamentals, progress, and beyond. Chem. Rev. 2021, 122, 1273–1348.
- Wang, Y.; Han, P.; Lv, X.; Zhang, L.; Zheng, G. Defect and interface engineering for aqueous electrocatalytic CO2 reduction. Joule 2018, 2, 2551–2582.
- Yu, Q. Theoretical studies of non-noble metal single-atom catalyst Ni1/MoS2: Electronic structure and electrocatalytic CO2 reduction. Sci. China Mater. 2022, 66, 1079–1088.
- Ilyas, T.; Raziq, F.; Ali, S.; Zada, A.; Ilyas, N.; Shaha, R.; Wang, Y.; Qiao, L. Facile synthesis of MoS2/Cu as trifunctional catalyst for electrochemical overall water splitting and photocatalytic CO2 conversion. Mater. Des. 2021, 204, 109674.
- Ren, Y.; Sun, X.; Qi, K.; Zhao, Z. Single atom supported on MoS2 as efficient electrocatalysts for the CO2 reduction reaction: A DFT study. Appl. Surf. Sci. 2022, 602, 154211.
- Wang, X.; Zheng, Y.; Sheng, W.; Xu, Z.J.; Jaroniec, M.; Qiao, S.-Z. Strategies for design of electrocatalysts for hydrogen evolution under alkaline conditions. Mater. Today 2020, 36, 125–138.
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.-Y. Recent advances in electrocatalytic hydrogen evolution using nanoparticles. Chem. Rev. 2019, 120, 851–918.
- Pham, V.P.; Yeom, G.Y. Recent advances in doping of molybdenum disulfide: Industrial applications and future prospects. Adv. Mater. 2016, 28, 9024–9059.
- Qi, K.; Cui, X.; Gu, L.; Yu, S.; Fan, X.; Luo, M.; Xu, S.; Li, N.; Zheng, L.; Zhang, Q. Single-atom cobalt array bound to distorted 1T MoS2 with ensemble effect for hydrogen evolution catalysis. Nat. Commun. 2019, 10, 5231.
- Luo, Z.; Ouyang, Y.; Zhang, H.; Xiao, M.; Ge, J.; Jiang, Z.; Wang, J.; Tang, D.; Cao, X.; Liu, C. Chemically activating MoS2 via spontaneous atomic palladium interfacial doping towards efficient hydrogen evolution. Nat. Commun. 2018, 9, 2120.
- Wang, Q.; Zhao, Z.L.; Dong, S.; He, D.; Lawrence, M.J.; Han, S.; Cai, C.; Xiang, S.; Rodriguez, P.; Xiang, B. Design of active nickel single-atom decorated MoS2 as a pH-universal catalyst for hydrogen evolution reaction. Nano Energy 2018, 53, 458–467.
- Wang, D.; Li, Q.; Han, C.; Xing, Z.; Yang, X. Single-atom ruthenium based catalyst for enhanced hydrogen evolution. Appl. Catal. B Environ. 2019, 249, 91–97.
- Lau, T.H.; Wu, S.; Kato, R.; Wu, T.-S.; Kulhavy, J.; Mo, J.; Zheng, J.; Foord, J.S.; Soo, Y.-L.; Suenaga, K. Engineering monolayer 1T-MoS2 into a bifunctional electrocatalyst via sonochemical doping of isolated transition metal atoms. ACS Catal. 2019, 9, 7527–7534.
- Luo, R.; Luo, M.; Wang, Z.; Liu, P.; Song, S.; Wang, X.; Chen, M. The atomic origin of nickel-doping-induced catalytic enhancement in MoS2 for electrochemical hydrogen production. Nanoscale 2019, 11, 7123–7128.
- Xuan, N.; Chen, J.; Shi, J.; Yue, Y.; Zhuang, P.; Ba, K.; Sun, Y.; Shen, J.; Liu, Y.; Ge, B. Single-atom electroplating on two dimensional materials. Chem. Mater. 2018, 31, 429–435.
- Zhang, H.; Yu, L.; Chen, T.; Zhou, W.; Lou, X.W. Surface modulation of hierarchical MoS2 nanosheets by Ni single atoms for enhanced electrocatalytic hydrogen evolution. Adv. Funct. Mater. 2018, 28, 1807086.
- Wei, S.; Cui, X.; Xu, Y.; Shang, B.; Zhang, Q.; Gu, L.; Fan, X.; Zheng, L.; Hou, C.; Huang, H. Iridium-triggered phase transition of MoS2 nanosheets boosts overall water splitting in alkaline media. ACS Energy Lett. 2018, 4, 368–374.
