Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Xiomar Gómez.
The fermentation of syngas is an attractive technology that can be integrated with gasification of lignocellulosic biomass. The coupling of these two technologies allows for treating a great variety of raw materials. Lignin usually hinders microbial fermentations; thus, the thermal decomposition of the whole material into small molecules allows for the production of fuels and other types of molecules using syngas as substrate, a process performed at mild conditions.
- fermentation
- syngas
- biomass gasification
1. Introduction
Syngas fermentation is a technology that uses specific biomass to transform small gaseous molecules into other gaseous compounds such as methane, but also other compounds such as ethanol, butanol, and hexanol may be obtained. Alcohols are released into the liquid phase, thus requiring additional separation stages. The main advantages of the process are the mild conditions applied to the fermentation (low temperature and pressure) [1] to achieve the conversion of hydrogen and carbon monoxide (CO)/carbon dioxide (CO2), in an equivalent way as in the Fischer–Tropsch synthesis. Current interest in reducing the use of fossil sources for energy production and attaining a circular economy model in Europe to decouple economic growth and resource use [2] have led to the search for technologies capable of reusing materials and reducing the demand for virgin raw materials.

2. Gasification/Pyrolysis Technologies
The gasification process is a proven technology used to produce syngas from coal for electricity generation in gas turbines. Synthesis of syngas molecules is carried out by catalytic processes such as Fischer–Tropsch, producing liquid hydrocarbons, generating methanol from the Basf process, or methane from the Sabatier reaction [40,41,42][3][4][5]. The use of syngas dates back to 1812 when the Westminster and London Gas Light and Coke Co. was provided with a royal grant to light London streets in 1812 [43][6]. Although the gasification process has been widely studied, the technology deployment has suffered several drawbacks, still being at a demonstration scale or needing government subsidies to make plant installation feasible. The integrated gasification combined cycle (IGCC) concept counted with four demonstration plants. Two were located in the United States (Wabash River Coal Gasification Repowering Project and the Tampa Electric Integrated Gasification Combined-Cycle Project), and the other two were located in Europe (Willem Alexander IGCC Plant in Buggenum, near Roermond (Latitude 51.222980, Longitude 5.971580) The Netherlands and ELCOGAS IGCC Plant in Puertollano, Spain) [44][7]. These plants were either shut down due to air pollutant constraints or retired due to finalizing project activities [45,46][8][9]. These large-scale demonstration projects allowed the development of the technology to reach a status close to commercial scale, although high installation costs are still a significant burden. However, experience has been gained regarding syngas composition and direct valorization in turbines for energy production, which is a valuable feature for the deployment of the technology when using other fuels. The gasification technologies currently available are based on fixed-bed downdraft systems, up-draft, bubbling bed, circulating fluidized bed or dual configurations to increase process efficiency (see Figure 1). It is worth mentioning that any technology should comply with current environmental legislation. Therefore, gasification/pyrolysis is not an exception. The technology must meet restrictions regarding volatile compounds production, NOx, sulfur compounds, and particulate matter (PM2.5). Configurations exploring the feasibility of treating mixtures of coal and biomass capable of complying with emission standards were studied by Ward et al. [47][10] as part of the Alaska Syngas Project using a gasifier designed by Hamilton Maurer International, Inc. Large-scale configurations capable of producing a significant amount of energy also required high input rates, for this reason the combined use of coal and biomass allows adapting scale and materials availability in addition to reducing the costs of gas cleaning technologies.
Figure 1. Schematization of different gasification technologies.
3. Syngas Fermentation
3.1. Fundamentals of Syngas Fermentation
Alcohols and organic acids can be produced by acetogens using the acetyl-CoA reductive pathway, which is their main unifying feature given their wide genetic diversity. Ethanol and acetate production has been extensively studied, but C4 to C8 chemicals can also be produced from this pathway using different types of clostridium organisms [148]. This pathway is known as Wood–Ljungdahl and allows for reducing CO/CO
Schematization of different gasification technologies.
3. Syngas Fermentation
3.1. Fundamentals of Syngas Fermentation
Alcohols and organic acids can be produced by acetogens using the acetyl-CoA reductive pathway, which is their main unifying feature given their wide genetic diversity. Ethanol and acetate production has been extensively studied, but C4 to C8 chemicals can also be produced from this pathway using different types of clostridium organisms [11]. This pathway is known as Wood–Ljungdahl and allows for reducing CO/CO
2
using H
2, obtaining energy, and assimilating carbon dioxide to produce cell carbon [149]. The pathway contains two branches, the methyl and carbonyl branch. In the methyl branch, the methyl group obtained from the reduction of CO/CO
, obtaining energy, and assimilating carbon dioxide to produce cell carbon [12]. The pathway contains two branches, the methyl and carbonyl branch. In the methyl branch, the methyl group obtained from the reduction of CO/CO
2 is transformed into formate by formate dehydrogenase and by a sequence of multiple steps forms methyl radicles that are further assimilated along with CO, forming acetyl-CoA [37]. CO can also be oxidized by biological water gas shift reaction into CO
is transformed into formate by formate dehydrogenase and by a sequence of multiple steps forms methyl radicles that are further assimilated along with CO, forming acetyl-CoA [13]. CO can also be oxidized by biological water gas shift reaction into CO
2, and the reducing equivalents derived from the reaction are conserved as reduced ferredoxin [150,151]. The production of acetyl-CoA is mediated by acetyl-CoA synthase (ACS) and carbon monoxide dehydrogenase complex (CODH) [1]. The bifunctional CO dehydrogenase enzyme reduces CO
2
into CO, thus serving as the carbonyl group. In contrast, the methyl group is derived by a multistep reduction of CO
2 where formyl, methenyl, methylene, and methyl intermediates are bound to a pterin cofactor [152]. The carbonyl branch is exclusive of anaerobic microorganisms.
Acetate is formed from acetyl-CoA to recover energy, and ethanol is produced from further reduction of acetate [152]. Hydrogenases are inhibited by the presence of CO, causing a decrease in hydrogen uptake, but as CO levels decrease, hydrogen uptake increases again [153]. Some acetyl-CoA is used to produce complex organic cell components, carbohydrates, proteins, and lipids [154].
Ethanol production from syngas is usually carried out by using
where formyl, methenyl, methylene, and methyl intermediates are bound to a pterin cofactor [16]. The carbonyl branch is exclusive of anaerobic microorganisms.
Acetate is formed from acetyl-CoA to recover energy, and ethanol is produced from further reduction of acetate [16]. Hydrogenases are inhibited by the presence of CO, causing a decrease in hydrogen uptake, but as CO levels decrease, hydrogen uptake increases again [17]. Some acetyl-CoA is used to produce complex organic cell components, carbohydrates, proteins, and lipids [18].
Ethanol production from syngas is usually carried out by using
Clostridium ljungdahlii strains. The fermentation requires a change in conditions to promote solventogenesis, transforming organic acids into alcohol. Thus, ethanol production should be initially favored by biomass growth along with organic acid production (acetate) and, by shifting conditions, ethanol is produced in a subsequent stage [159].
Keeping high ethanol productivity under continuous conditions is still a challenge due to the loss of culture viability and low levels of metabolic activity, as demonstrated by Mohammadi et al. [162], who reported that recovery stages and pH adjustments are needed to keep active microflora. These authors obtained an ethanol concentration of 6.5 g/L, but this value was just a peak concentration attained during a short period. The fermentation showed irregular performance for the 30-day operation period due to changes in biomass activity and pH, with ethanol evolution initially increasing until reaching peak values, suffering a decrease and posterior recovery afterward.
The number of scientific publications regarding syngas fermentation has increased exponentially in recent years [165]. Alcohols with longer chains than ethanol can also be obtained from syngas fermentation.
strains. The fermentation requires a change in conditions to promote solventogenesis, transforming organic acids into alcohol. Thus, ethanol production should be initially favored by biomass growth along with organic acid production (acetate) and, by shifting conditions, ethanol is produced in a subsequent stage [19].
Keeping high ethanol productivity under continuous conditions is still a challenge due to the loss of culture viability and low levels of metabolic activity, as demonstrated by Mohammadi et al. [20], who reported that recovery stages and pH adjustments are needed to keep active microflora. These authors obtained an ethanol concentration of 6.5 g/L, but this value was just a peak concentration attained during a short period. The fermentation showed irregular performance for the 30-day operation period due to changes in biomass activity and pH, with ethanol evolution initially increasing until reaching peak values, suffering a decrease and posterior recovery afterward.
The number of scientific publications regarding syngas fermentation has increased exponentially in recent years [21]. Alcohols with longer chains than ethanol can also be obtained from syngas fermentation.
Clostridium carboxidivorans
and
C. kluyveri have been proposed as organisms suitable for producing elongated alcohols and fatty acids in co-cultures or mixed microbial cultures [166,167,168]. Hexanol is produced along with butanol and ethanol, with the latter being the predominant product [169]. In this type of syngas fermentation, butanol and hexanol concentrations hardly surpass the threshold of 1 g/L, thus setting great difficulties in subsequent alcohol recovery stages.
have been proposed as organisms suitable for producing elongated alcohols and fatty acids in co-cultures or mixed microbial cultures [22][23][24]. Hexanol is produced along with butanol and ethanol, with the latter being the predominant product [25]. In this type of syngas fermentation, butanol and hexanol concentrations hardly surpass the threshold of 1 g/L, thus setting great difficulties in subsequent alcohol recovery stages.
3.2. Experimental Work and Integration with Biomass Gasification
The experimental work available in the scientific literature deals mainly with small-batch scale reactors using synthetic syngas free of inhibitory components. Data obtained from large-scale experiences are scarce and even more if the use of syngas derived from biomass gasifiers is considered. Kundiyana et al. [201] evaluated aThe experimental work available in the scientific literature deals mainly with small-batch scale reactors using synthetic syngas free of inhibitory components. Data obtained from large-scale experiences are scarce and even more if the use of syngas derived from biomass gasifiers is considered. Kundiyana et al. [26] evaluated a
Clostridium strain P11T in a semi-pilot scale system. These authors tested a biomass gasifier and a 100 L reactor to ferment the resulting syngas. The main fermentation products were ethanol, acetic acid, and 2-propanol. Ethanol concentration reached a value of 25.3 g/L. The fermentation was tested under batch conditions, and cell concentration in the reactor was kept low, at about 0.84 g/L. Ethanol showed an increasing trend during the whole operation period (about two months), indicating promising future performance on a larger scale. However, the presence of trace contaminants resulted in severe fermentation failure.
Gasification of low-quality biomass tends to produce more tars and inhibitors, thus demanding specific cleaning operations to remove contaminants, increasing cleaning-associated costs and adversely affecting process feasibility. Ramachandriya et al. [202] reviewed the effect of contaminants such as HCN, NOx, sulfide compounds, oxygen, and tars, among others, highlighting the relevance of producing clean syngas to integrate the two processes safely. Given the difficulties found in attaining stable performance when coupling small-scale biomass gasification and CHP energy production, it is expected that syngas fermentation can be linked to large-scale gasification units, which can stand a costly syngas upgrading process and afford additional equipment needed for controlling and removing contaminants.Gasification of low-quality biomass tends to produce more tars and inhibitors, thus demanding specific cleaning operations to remove contaminants, increasing cleaning-associated costs and adversely affecting process feasibility. Ramachandriya et al. [27] reviewed the effect of contaminants such as HCN, NOx, sulfide compounds, oxygen, and tars, among others, highlighting the relevance of producing clean syngas to integrate the two processes safely. Given the difficulties found in attaining stable performance when coupling small-scale biomass gasification and CHP energy production, it is expected that syngas fermentation can be linked to large-scale gasification units, which can stand a costly syngas upgrading process and afford additional equipment needed for controlling and removing contaminants.
Liakakou et al. [203] tested Clostridium ljungdahlii in a fermenting system coupled to a biomass gasifier. These authors studied the inhibitory effect of trace substances and tar components, evaluating the efficiency of the syngas cleaning processes developed to remove benzene, toluene, and xylene (BTX) mixtures using a specific oil absorber. The cleaning system also included a hydrodesulfurization and a bed adsorption unit for removing H
2S and COS. The high efficiency of the cleaning process allowed for running a fermentation free of inhibitory conditions. The final ethanol concentration attained when feeding the clean gas was 2.2 g/L, using a 1.5 L CSTR.
Other cleaning technologies developed include catalytic decomposition, BTX absorbers, removal of acid gases using adsorbents, and removal of nitrogen species [92,207]. A new cleaning process was tested recently by Frilund et al. [208] using low-cost adsorption systems and organic solvent-free removal concepts to reduce costs and complexity of operation. On the contrary, Calì et al. [209] tested a water scrubber along with a chemical and physical wastewater management unit containing an oil skimmer and an activated carbon adsorption filter, attaining a significant reduction in the water demanded by the cleaning stage. The use of an innovative cleaning system was tested by Hai et al. [210], proposing a mop fan with water spry, obtaining a tar removal efficiency of 89.6%.Other cleaning technologies developed include catalytic decomposition, BTX absorbers, removal of acid gases using adsorbents, and removal of nitrogen species [29][30]. A new cleaning process was tested recently by Frilund et al. [31] using low-cost adsorption systems and organic solvent-free removal concepts to reduce costs and complexity of operation. On the contrary, Calì et al. [32] tested a water scrubber along with a chemical and physical wastewater management unit containing an oil skimmer and an activated carbon adsorption filter, attaining a significant reduction in the water demanded by the cleaning stage. The use of an innovative cleaning system was tested by Hai et al. [33], proposing a mop fan with water spry, obtaining a tar removal efficiency of 89.6%.
It should be noted that CO can also exert a toxic effect on hydrogenase enzymes, thus making the CO route favorable for reducing power and decreasing ethanol production efficiency [202]. This effect explains the results obtained by Lanzillo et al. [137] and Kim et al. [195]. However, results reported by Hurstand and Lewis [158] indicated a better performance when increasing the gas reactor pressure from 0.35 to 2.0 atm, reporting almost nil ethanol production at the lowest pressure tested and reaching an ethanol concentration up to 2.5 g/L using It should be noted that CO can also exert a toxic effect on hydrogenase enzymes, thus making the CO route favorable for reducing power and decreasing ethanol production efficiency [27]. This effect explains the results obtained by Lanzillo et al. [34] and Kim et al. [35]. However, results reported by Hurstand and Lewis [36] indicated a better performance when increasing the gas reactor pressure from 0.35 to 2.0 atm, reporting almost nil ethanol production at the lowest pressure tested and reaching an ethanol concentration up to 2.5 g/L using
Clostridium carboxidivorans P7
T. Experiments performed at an even higher pressure, on the contrary, confirm the decrease experienced in product yield.
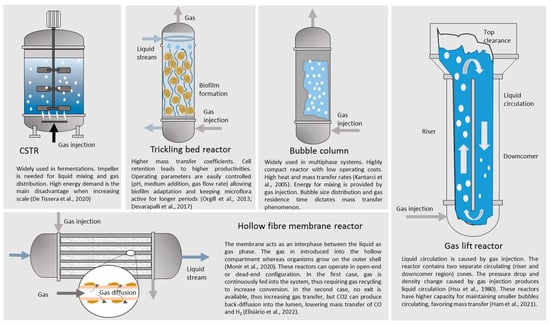
CSTR are the most widely used laboratory reactors tested due to the simplified operation of the system at a small scale. However, other configurations offer outstanding performance when attempting an increase in mass transfer. Factors that affect the mass transfer phenomenon of gaseous species into the liquid phase include impeller configuration, agitation speed, partial pressure, temperature, the surface area available for mass transfer, mean bubble size, bubble size distribution, and bubble residence time [220,221,222]. In the present case, the inhibitory effect caused by CO sets limits to the amount of substrate safely transferred from the gas phase.CSTR are the most widely used laboratory reactors tested due to the simplified operation of the system at a small scale. However, other configurations offer outstanding performance when attempting an increase in mass transfer. Factors that affect the mass transfer phenomenon of gaseous species into the liquid phase include impeller configuration, agitation speed, partial pressure, temperature, the surface area available for mass transfer, mean bubble size, bubble size distribution, and bubble residence time [37][38][39]. In the present case, the inhibitory effect caused by CO sets limits to the amount of substrate safely transferred from the gas phase.
Munasinghe and Khanal [223] tested different reactors and diffuser types, indicating that gas lift reactors with bulb diffusers showed better performance regarding mass transfer, surpassing the performance of hollow fiber membrane modules. Impeller configuration also has a great effect on mass transfer. The propensity to gas flooding is greatly reduced if impellers are changed from standard Rushton turbines to concave-shaped turbines. However, impellers are not convenient when large-volume reactors are considered due to the high increase in the energy demand experimented when scale increases [224]. Munasinghe and Khanal [40] tested different reactors and diffuser types, indicating that gas lift reactors with bulb diffusers showed better performance regarding mass transfer, surpassing the performance of hollow fiber membrane modules. Impeller configuration also has a great effect on mass transfer. The propensity to gas flooding is greatly reduced if impellers are changed from standard Rushton turbines to concave-shaped turbines. However, impellers are not convenient when large-volume reactors are considered due to the high increase in the energy demand experimented when scale increases [41].
Figure 3 shows different reactor configurations suitable for fermenting gaseous substrates.2 shows different reactor configurations suitable for fermenting gaseous substrates.

Figure 32. Schematization of different reactor configurations suitable for the fermentation of gaseous substrates. References: Monir et al. (2020) [216], De Tissera et al. (2020) [225], Orgill et al. (2013) [226], Devarapalli et al. (2017) [227], Kantarci et al. (2005) [228], Hsu et al. (1980) [229], Ham et al. (2021) [230], Elisiário et al. (2022) [231].
Schematization of different reactor configurations suitable for the fermentation of gaseous substrates. References: Monir et al. (2020) [42], De Tissera et al. (2020) [43], Orgill et al. (2013) [44], Devarapalli et al. (2017) [45], Kantarci et al. (2005) [46], Hsu et al. (1980) [47], Ham et al. (2021) [48], Elisiário et al. (2022) [49].
It must be kept in mind that the fermentation is not a final stage of a global process. Separation units are necessary to obtain the desired product, and energy demand is highly dependent on the fermentation yield attained. In addition, sterilization requirements to keep a pure culture free of external microbial contamination increase the process energy demand. Syngas fermentation has similarities with acetone-butanol-ethanol (ABE) fermentation, a process that, after experiencing an industrial boom, suffered from high energy prices, bacteriophage infection, and more efficient process competitors [232,233,234], being relegated to experimental studies and waiting for a new opportunity. Configurations capable of overcoming inhibitory conditions and achieving higher titer and separation efficiency may make this fermentation a viable alternative to petroleum-based processes.
It must be kept in mind that the fermentation is not a final stage of a global process. Separation units are necessary to obtain the desired product, and energy demand is highly dependent on the fermentation yield attained. In addition, sterilization requirements to keep a pure culture free of external microbial contamination increase the process energy demand. Syngas fermentation has similarities with acetone-butanol-ethanol (ABE) fermentation, a process that, after experiencing an industrial boom, suffered from high energy prices, bacteriophage infection, and more efficient process competitors [50][51][52], being relegated to experimental studies and waiting for a new opportunity. Configurations capable of overcoming inhibitory conditions and achieving higher titer and separation efficiency may make this fermentation a viable alternative to petroleum-based processes.
The capabilities of clostridium species to be integrated into a biorefinery context have been reviewed by Liberato et al. [235], considering these species the ideal candidates due to their versatility in using different types of substrates and operating under anaerobic conditions. Although the variety of species and products generated by different strains include alcohols and organic acids, the process of utilizing the conversion of simple sugars, lignocellulosic biomass, or gaseous streams remains to become feasible at an industrial scale. Efforts to reduce fermentation costs have been attempted by using low-cost fermenting media and supplements [194,196,236].
The capabilities of clostridium species to be integrated into a biorefinery context have been reviewed by Liberato et al. [53], considering these species the ideal candidates due to their versatility in using different types of substrates and operating under anaerobic conditions. Although the variety of species and products generated by different strains include alcohols and organic acids, the process of utilizing the conversion of simple sugars, lignocellulosic biomass, or gaseous streams remains to become feasible at an industrial scale. Efforts to reduce fermentation costs have been attempted by using low-cost fermenting media and supplements [54][55][56].
Cleaning syngas is essential for avoiding the cessation of microbial growth during fermentation. The type of gasifier employed, gasification conditions, and biomass quality greatly influence the type of impurities and syngas cleaning requirements. Therefore, a global optimization of the process is needed [238] to attain low levels of contaminants without the requirement of costly cleaning processes.
Cleaning syngas is essential for avoiding the cessation of microbial growth during fermentation. The type of gasifier employed, gasification conditions, and biomass quality greatly influence the type of impurities and syngas cleaning requirements. Therefore, a global optimization of the process is needed [57] to attain low levels of contaminants without the requirement of costly cleaning processes.
H
2
and CO/CO
2 can be converted into methane by a sequence or reactions involving water-gas shift reaction, acetogenesis, hydrogenotrophic methanation, carboxydotrophic methanation, and acetoclastic methanation [244]. Multiple reviews on this subject highlight the various benefits of this fermentation [222,245,246], and several studies report on high conversion rates when using membrane reactors, tricking beds, and high-pressure systems capable of attaining high cell concentration and productivity [247,248,249].
can be converted into methane by a sequence or reactions involving water-gas shift reaction, acetogenesis, hydrogenotrophic methanation, carboxydotrophic methanation, and acetoclastic methanation [58]. Multiple reviews on this subject highlight the various benefits of this fermentation [39][59][60], and several studies report on high conversion rates when using membrane reactors, tricking beds, and high-pressure systems capable of attaining high cell concentration and productivity [61][62][63].
A final remark worth mentioning is the scarce information regarding ethanol recovery from low-concentrated streams. This stage is fundamental to attaining process feasibility since the ethanol concentration greatly affects the performance of recovery stages and energy demand [250]. However, this fermentation suffers from product inhibition. Thus, simultaneous removal of products during the fermentation stage may become an option for increasing reactor productivity, as already proposed for ABE fermentation [251,252,253]. Technical feasibility evaluated by Michailos et al. [254] clearly summarized the disadvantages of this technology, indicating that conventional ethanol biorefineries have a better performance in terms of sustainability. The challenges to be overcome have been widely exposed: an increase in ethanol yields and mass transfer improvement of the gaseous substrate without causing metabolic shifts would benefit the mass and energy balance of the fermentation system. The integration with a biomass gasification stage requires solving the well-known tar formation problem along with removing specific toxic trace contaminants at a low cost.
A final remark worth mentioning is the scarce information regarding ethanol recovery from low-concentrated streams. This stage is fundamental to attaining process feasibility since the ethanol concentration greatly affects the performance of recovery stages and energy demand [64]. However, this fermentation suffers from product inhibition. Thus, simultaneous removal of products during the fermentation stage may become an option for increasing reactor productivity, as already proposed for ABE fermentation [65][66][67]. Technical feasibility evaluated by Michailos et al. [68] clearly summarized the disadvantages of this technology, indicating that conventional ethanol biorefineries have a better performance in terms of sustainability. The challenges to be overcome have been widely exposed: an increase in ethanol yields and mass transfer improvement of the gaseous substrate without causing metabolic shifts would benefit the mass and energy balance of the fermentation system. The integration with a biomass gasification stage requires solving the well-known tar formation problem along with removing specific toxic trace contaminants at a low cost.
References
- Munasinghe, P.C.; Khanal, S.K. Biomass-derived syngas fermentation into biofuels: Opportunities and challenges. Bioresour. Technol. 2010, 101, 5013–5022.
- European Commission: Circular Economy. The EU Aims to Transition to a Circular Economy to Make Europe Cleaner and More Competitive. Available online: https://environment.ec.europa.eu/topics/circular-economy_en (accessed on 8 August 2023).
- Hu, J.; Yu, F.; Lu, Y. Application of Fischer–Tropsch Synthesis in Biomass to Liquid Conversion. Catalysts 2012, 2, 303–326.
- Böhme, C. BASF Develops Process for Climate-Friendly Methanol. Available online: https://www.basf.com/global/en/media/news-releases/2019/05/p-19-218.html (accessed on 12 August 2023).
- Vogt, C.; Monai, M.; Kramer, G.J.; Weckhuysen, B.M. The renaissance of the Sabatier reaction and its applications on Earth and in space. Nat. Catal. 2019, 2, 188–197.
- Shadle, L.J.; Berry, D.A.; Syamlal, M. Coal Conversion Processes, Gasification. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley Online Library: Hoboken, NJ, USA, 2002.
- IGCC PROJECT EXAMPLES. Available online: https://www.netl.doe.gov/research/coal/energy-systems/gasification/gasifipedia/project-examples (accessed on 12 August 2023).
- Wabash River Generating Station. Available online: https://www.gem.wiki/Wabash_River_Generating_Station#Unit_Retirements (accessed on 12 August 2023).
- Puertollano IGCC Power Station. Available online: https://www.gem.wiki/Puertollano_IGCC_power_station (accessed on 12 August 2023).
- Ward, C.; Goldstein, H.; Maurer, R.; Thimsen, D.; Sheets, B.J.; Hobbs, R.; Isgrigg, F.; Steiger, R.; Revay Madden, D.; Porcu, A.; et al. Making coal relevant for small scale applications: Modular gasification for syngas/engine CHP applications in challenging environments. Fuel 2020, 267, 117303.
- Stoll, I.K.; Boukis, N.; Sauer, J. Syngas Fermentation to Alcohols: Reactor Technology and Application Perspective. Chem. Ing. Tech. 2020, 92, 125–136.
- Drake, H.L.; Gößner, A.S.; Daniel, S.L. Old Acetogens, New Light. Ann. N. Y. Acad. Sci. 2008, 1125, 100–128.
- Ahuja, V.; Bhatt, A.K.; Ravindran, B.; Yang, Y.; Bhatia, S.K. A Mini-Review on Syngas Fermentation to Bio-Alcohols: Current Status and Challenges. Sustainability 2023, 15, 3765.
- Schiel-Bengelsdorf, B.; Dürre, P. Pathway engineering and synthetic biology using acetogens. FEBS Lett. 2012, 586, 2191–2198.
- Shanmugasundaram, T.; Wood, H.G. Interaction of ferredoxin with carbon monoxide dehydrogenase from Clostridium thermoaceticum. J. Biochem. Chem. 1992, 267, 897–900.
- Henstra, A.M.; Sipma, J.; Rinzema, A.; Stams, A.J.M. Microbiology of synthesis gas fermentation for biofuel production. Curr. Opin. Biotechnol. 2007, 18, 200–206.
- Daniell, J.; Köpke, M.; Simpson, S.D. Commercial Biomass Syngas Fermentation. Energies 2012, 5, 5372–5417.
- Phillips, J.R.; Huhnke, R.L.; Atiyeh, H.K. Syngas Fermentation: A Microbial Conversion Process of Gaseous Substrates to Various Products. Fermentation 2017, 3, 28.
- Kennes, D.; Abubackar, H.N.; Diaz, M.; Veiga, M.C.; Kennes, C. Bioethanol production from biomass: Carbohydrate vs syngas fermentation. J. Chem. Technol. Biotechnol. 2016, 91, 304–317.
- Mohammadi, M.; Younesi, H.; Najafpour, G.; Mohamed, A.R. Sustainable ethanol fermentation from synthesis gas by Clostridium ljungdahlii in a continuous stirred tank bioreactor. J. Chem. Technol. Biotechnol. 2012, 87, 837–843.
- Calvo, D.C.; Luna, H.J.; Arango, J.A.; Torres, C.I.; Rittmann, B.E. Determining global trends in syngas fermentation research through a bibliometric analysis. J. Environ. Manag. 2022, 307, 114522.
- He, P.; Han, W.; Shao, L.; Lü, F. One-step production of C6–C8 carboxylates by mixed culture solely grown on CO. Biotechnol. Biofuels Bioprod. 2018, 11, 4.
- Kucek, L.A.; Spirito, C.M.; Angenent, L.T. High n-caprylate productivities and specificities from dilute ethanol and acetate: Chain elongation with microbiomes to upgrade products from syngas fermentation. Energy Environ. Sci. 2016, 9, 3482–3494.
- Gavala, H.N.; Grimalt-Alemany, A.; Asimakopoulos, K.; Skiadas, I.V. Gas Biological Conversions: The Potential of Syngas and Carbon Dioxide as Production Platforms. Waste Biomass Valoriz. 2021, 12, 5303–5328.
- Phillips, J.R.; Atiyeh, H.K.; Tanner, R.S.; Torres, J.R.; Saxena, J.; Wilkins, M.R.; Huhnke, R.L. Butanol and hexanol production in Clostridium carboxidivorans syngas fermentation: Medium development and culture techniques. Bioresour. Technol. 2015, 190, 114–121.
- Kundiyana, D.K.; Huhnke, R.L.; Wilkins, M.R. Syngas fermentation in a 100-L pilot scale fermentor: Design and process considerations. J. Biosci. Bioeng. 2010, 109, 492–498.
- Ramachandriya, K.D.; Kundiyana, D.K.; Sharma, A.M.; Kumar, A.; Atiyeh, H.K.; Huhnke, R.L.; Wilkins, M.R. Critical Factors Affecting the Integration of Biomass Gasification and Syngas Fermentation Technology. Available online: https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1719&context=biosysengfacpub (accessed on 18 August 2023).
- Liakakou, E.T.; Infantes, A.; Neumann, A.; Vreugdenhil, B.J. Connecting gasification with syngas fermentation: Comparison of the performance of lignin and beech wood. Fuel 2021, 290, 120054.
- Dahmen, N.; Abeln, J.; Eberhard, M.; Kolb, T.; Leibold, H.; Sauer, J.; Stapf, D.; Zimmerlin, B. The bioliq process for producing synthetic transportation fuels. WIREs Energy Environ. 2017, 6, e236.
- Thunman, H.; Seemann, M.; Berdugo Vilches, T.; Maric, J.; Pallares, D.; Ström, H.; Berndes, G.; Knutsson, P.; Larsson, A.; Breitholtz, C.; et al. Advanced biofuel production via gasification—Lessons learned from 200 man-years of research activity with Chalmers’ research gasifier and the GoBiGas demonstration plant. Energy Sci. Eng. 2018, 6, 6–34.
- Frilund, C.; Tuomi, S.; Kurkela, E.; Simell, P. Small- to medium-scale deep syngas purification: Biomass-to-liquids multi-contaminant removal demonstration. Biomass Bioenergy 2021, 148, 106031.
- Calì, G.; Deiana, P.; Bassano, C.; Meloni, S.; Maggio, E.; Mascia, M.; Pettinau, A. Syngas Production, Clean-Up and Wastewater Management in a Demo-Scale Fixed-Bed Updraft Biomass Gasification Unit. Energies 2020, 13, 2594.
- Hai, I.U.; Sher, F.; Zarren, G.; Liu, H. Experimental investigation of tar arresting techniques and their evaluation for product syngas cleaning from bubbling fluidized bed gasifier. J. Clean. Prod. 2019, 240, 118239.
- Lanzillo, F.; Ruggiero, G.; Raganati, F.; Russo, M.E.; Marzocchella, A. Batch Syngas Fermentation by Clostridium carboxidivorans for Production of Acids and Alcohols. Processes 2020, 8, 1075.
- Kim, J.; Kim, K.-Y.; Ko, J.K.; Lee, S.-M.; Gong, G.; Kim, K.H.; Um, Y. Characterization of a Novel Acetogen Clostridium sp. JS66 for Production of Acids and Alcohols: Focusing on Hexanoic Acid Production from Syngas. Biotechnol. Bioprocess Eng. 2022, 27, 89–98.
- Hurst, K.M.; Lewis, R.S. Carbon monoxide partial pressure effects on the metabolic process of syngas fermentation. Biochem. Eng. J. 2010, 48, 159–165.
- Sieblist, C.; Hägeholz, O.; Aehle, M.; Jenzsch, M.; Pohlscheidt, M.; Lübbert, A. Insights into large-scale cell-culture reactors: II. Gas-phase mixing and CO2 stripping. Biotechnol. J. 2011, 6, 1547–1556.
- Yan, X.; Jia, Y.; Wang, L.; Cao, Y. Drag coefficient fluctuation prediction of a single bubble rising in water. Chem. Eng. J. 2017, 316, 553–562.
- González, R.; Cabeza, I.O.; Casallas-Ojeda, M.; Gómez, X. Biological Hydrogen Methanation with Carbon Dioxide Utilization: Methanation Acting as Mediator in the Hydrogen Economy. Environments 2023, 10, 82.
- Munasinghe, P.C.; Khanal, S.K. Syngas fermentation to biofuel: Evaluation of carbon monoxide mass transfer coefficient (kLa) in different reactor configurations. Biotechnol. Prog. 2010, 26, 1616–1621.
- Ungerman, A.J.; Heindel, T.J. Carbon Monoxide Mass Transfer for Syngas Fermentation in a Stirred Tank Reactor with Dual Impeller Configurations. Biotechnol. Prog. 2007, 23, 613–620.
- Monir, M.U.; Aziz, A.A.; Khatun, F.; Yousuf, A. Bioethanol production through syngas fermentation in a tar free bioreactor using Clostridium butyricum. Renew. Energy 2020, 157, 1116–1123.
- De Tissera, S.; Köpke, M.; Simpson, S.D.; Humphreys, C.; Minton, N.P.; Dürre, P. Syngas Biorefinery and Syngas Utilization. In Biorefineries; Advances in Biochemical Engineering/Biotechnology; Wagemann, K., Tippkötter, N., Eds.; Springer: Cham, Switzerland, 2017; Volume 166, pp. 247–280.
- Orgill, J.J.; Atiyeh, H.K.; Devarapalli, M.; Phillips, J.R.; Lewis, R.S.; Huhnke, R.L. A comparison of mass transfer coefficients between trickle-bed, hollow fiber membrane and stirred tank reactors. Bioresour. Technol. 2013, 133, 340–346.
- Devarapalli, M.; Lewis, R.S.; Atiyeh, H.K. Continuous Ethanol Production from Synthesis Gas by Clostridium ragsdalei in a Trickle-Bed Reactor. Fermentation 2017, 3, 23.
- Kantarci, N.; Borak, F.; Ulgen, K.O. Bubble column reactors. Process Biochem. 2005, 40, 2263–2283.
- Hsu, Y.C.; Duduković, M.P. 18 Gas holdup and liquid recirculation in gas-lift reactors. Chem. Eng. Sci. 1980, 35, 135–141.
- Ham, P.; Bun, S.; Painmanakul, P.; Wongwailikhit, K. Effective Analysis of Different Gas Diffusers on Bubble Hydrodynamics in Bubble Column and Airlift Reactors towards Mass Transfer Enhancement. Processes 2021, 9, 1765.
- Elisiário, M.P.; De Wever, H.; Van Hecke, W.; Noorman, H.; Straathof, A.J.J. Membrane bioreactors for syngas permeation and fermentation. Crit. Rev. Biotechnol. 2022, 42, 856–872.
- Sauer, M. Industrial production of acetone and butanol by fermentation—100 years later. FEMS Microbiol. Lett. 2016, 363, fnw134.
- Amiri, H. Recent innovations for reviving the ABE fermentation for production of butanol as a drop-in liquid biofuel. Biofuel Res. J. 2020, 7, 1256–1266.
- Schüler, M.A.; Stegmann, B.A.; Poehlein, A.; Daniel, R.; Dürre, P. Genome sequence analysis of the temperate bacteriophage TBP2 of the solvent producer Clostridium saccharoperbutylacetonicum N1-4 (HMT, ATCC 27021). FEMS Microbiol. Lett. 2020, 367, fnaa103.
- Liberato, V.; Benevenuti, C.; Coelho, F.; Botelho, A.; Amaral, P.; Pereira, N.; Ferreira, T. Clostridium sp. As Bio-Catalyst for Fuels and Chemicals Production in a Biorefinery Context. Catalysts 2019, 9, 962.
- Benevenuti, C.; Botelho, A.; Ribeiro, R.; Branco, M.; Pereira, A.; Vieira, A.C.; Ferreira, T.; Amaral, P. Experimental Design to Improve Cell Growth and Ethanol Production in Syngas Fermentation by Clostridium carboxidivorans. Catalysts 2020, 10, 59.
- Sun, X.; Thunuguntla, R.; Zhang, H.; Atiyeh, H. Biochar amended microbial conversion of C1 gases to ethanol and butanol: Effects of biochar feedstock type and processing temperature. Bioresour. Technol. 2022, 360, 127573.
- Gungormusler, M.; Azbar, N.; Keskin, T. Bioethanol production from C1 gases using alternative media by syngas fermentation. Int. J. Glob. Warm. 2022, 28, 42–59.
- You, S.; Ok, Y.S.; Tsang, D.C.W.; Kwon, E.E.; Wang, C.-H. Towards practical application of gasification: A critical review from syngas and biochar perspectives. Crit. Rev. Environ. Sci. Technol. 2018, 48, 1165–1213.
- Grimalt-Alemany, A.; Skiadas, I.V.; Gavala, H.N. Syngas biomethanation: State-of-the-art review and perspectives. Biofuels Bioprod. Biorefin. 2018, 12, 139–158.
- Paniagua, S.; Lebrero, R.; Muñoz, R. Syngas biomethanation: Current state and future perspectives. Bioresour. Technol. 2022, 358, 127436.
- Bellini, R.; Bassani, I.; Vizzarro, A.; Azim, A.A.; Vasile, N.S.; Pirri, C.F.; Verga, F.; Menin, B. Biological Aspects, Advancements and Techno-Economical Evaluation of Biological Methanation for the Recycling and Valorization of CO2. Energies 2022, 15, 4064.
- Westman, S.Y.; Chandolias, K.; Taherzadeh, M.J. Syngas Biomethanation in a Semi-Continuous Reverse Membrane Bioreactor (RMBR). Fermentation 2016, 2, 8.
- Mauerhofer, L.-M.; Zwirtmayr, S.; Pappenreiter, P.; Bernacchi, S.; Seifert, A.H.; Reischl, B.; Schmider, T.; Taubner, R.-S.; Paulik, C.; Rittmann, S.K.-M.R. Hyperthermophilic methanogenic archaea act as high-pressure CH4 cell factories. Commun. Biol. 2021, 4, 289.
- Feickert Fenske, C.; Md, Y.; Strübing, D.; Koch, K. Preliminary gas flow experiments identify improved gas flow conditions in a pilot-scale trickle bed reactor for H2 and CO2 biological methanation. Bioresour. Technol. 2023, 371, 128648.
- Janković, T.; Straathof, A.J.J.; Kiss, A.A. Advanced downstream processing of bioethanol from syngas fermentation. Sep. Purif. Technol. 2023, 322, 124320.
- Yao, P.; Xiao, Z.; Chen, C.; Li, W.; Deng, Q. Cell growth behaviors of Clostridium acetobutylicum in a pervaporation membrane bioreactor for butanol fermentation. Biotechnol. Appl. Biochem. 2016, 63, 101–105.
- Sarchami, T.; Munch, G.; Johnson, E.; Kießlich, S.; Rehmann, L. A Review of Process-Design Challenges for Industrial Fermentation of Butanol from Crude Glycerol by Non-Biphasic Clostridium pasteurianum. Fermentation 2016, 2, 13.
- Arregoitia-Sarabia, C.; González-Revuelta, D.; Fallanza, M.; Ortiz, A.; Gorri, D. PEBA/PDMS Composite Multilayer Hollow Fiber Membranes for the Selective Separation of Butanol by Pervaporation. Membranes 2022, 12, 1007.
- Michailos, S.; Parker, D.; Webb, C. Design, sustainability analysis and multiobjective optimisation of ethanol production via syngas fermentation. Waste Biomass Valoriz. 2019, 10, 865–876.
More
