Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jason Zhu and Version 1 by Gopal Avashthi.
Heterocyclic compounds are significant lead drug candidates based on their various structure–activity relationships (SAR), and their use in pharmaceutics is constantly developing. Benzimidazole (BnZ) is synthesized by a condensation reaction between benzene and imidazole. The BnZ structure consists of two nitrogen atoms embedded in a five-membered imide ring which is fused with a benzene ring.
- benzimidazole
- heterocyclic derivatives
- biological activity
1. Synthesis of Benzimidazole
BnZ is a bicyclic heterocyclic aromatic compound which is made up of benzene and imidazole rings bonded at 4- and 5-positions. Ortho-phenylene derivatives of BnZ such as methyl-o-phenylenediamine are known as benzoglyoxalines. Recently, several researchers have described various techniques for synthesizing 1- or 1, 2-disubstituted BnZ derivatives by employing various moieties in various reaction environments [4][1]. Initially, BnZ was synthesized as a 2,5-dimethyl-benzimidazole (III) in 1872 by Hoebrecker [5][2] through the reduction of 2-nitro-4-methylacetanilide (I) using tin (Sn) and hydrochloric acid (HCl), followed by the dehydration of 2-nitro-4-methylacetanilide(II), as explained in Scheme 1. Details regarding the specific reaction conditions are provided elsewhere [6][3].
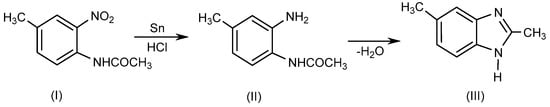
Scheme 1.
Hoebrecker method.
BnZ synthesis proceeds by choosing benzene derivatives that have nitrogen-containing functionalities at ortho positions to each other, e.g., orthophenylenediamine (Figure 1). It is evident that the starting material must have the functionality to enable the preparation of BnZ using a variety of techniques. Ortho-phenylenediamine and its derivatives undergo a condensation process with carboxylic acids or aldehydes. Therefore, several BnZ synthesis processes have been categorized based on the basic nature of o-phenylenediamine [7][4]. The direct condensation of orthophenylenediamine with nitriles has much use in the synthesis of benzimidazoles as well as in the manufacture of benzimidazole-related agricultural and pharmaceuticals chemicals because nitriles are easily accessible due to their broad supply as commodity chemicals [8][5].
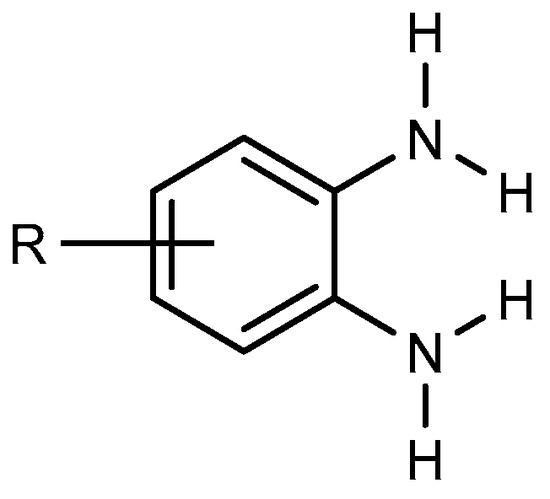
Figure 1.
Orthophenylenediamine compound.
The most typical synthetic approach for producing several BnZ derivatives is known as Phillip’s method, which entails the condensation of o-phenylenediamine (IV) with carboxylic acids (V) itself or its derivatives, and which involves heating the reagents in the presence of concentrated HCl (Scheme 2).
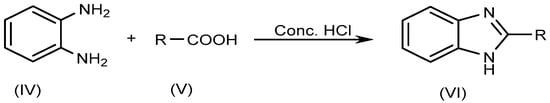
Scheme 2.
Phillip’s method.
A number of new modified BnZ derivatives have been synthesized, having a furan substituent at the second position and an alkyl/aryl substituent at the first position, which further was tested by an in silico study [9][6].
Several synthetic tactics have been successfully implemented, which are described in Figure 2 for the production of BnZ [A]. A combination of ortho-substituted aniline [B] and bifunctional o-esters produced a BnZ derivative in 2012, according to Bastug et al. [10][7]. Utilizing heterocyclic aromatic compounds like H2NC6H4NO2 with CH2O2, iron powder, and ammonium chloride, proceeded by the one pot reduction of NO2 followed by imidazole cyclisation [C] into bicyclic 2-H BnZ, was one method used by Hanan et al. (2010) [11][8]. Yang et al. developed a new method for the one-step synthesis of 2-substituted -N-H, -N-alkyl, and -N-aryl BnZ derivatives in the presence of aldehydes and sodium dithionite through the reduction of o-nitroanilines [D], succeeding in synthesizing BnZ [12][9]. Another approach for the synthesis of 2-substituted BnZ was published by Cui et al. in 2012 for producing 1,2-phenylenediamines and triacyloxyborane intermediates, which were produced via the interaction between carboxylic acids [E] and borane-THF, to yield 2-substituted BnZ [13][10]. N-methyl-1,2-phenylenediamine, sodium hydride, and carbonitriles [F] were used as the starting ingredients by Sluiter and Christoffers in 2009 [14][11]. Wray synthesized [1][12] 1H-indazoles from common arylamino oximes in 2010 in the presence of several bases, but the synthesis of BnZ was only improved by one base, i.e., triethylamine [G] [15][13]. Cuprous oxide, potassium carbonate, (CH3NH)2C2H4, and water [H] were used by Peng et al. to synthesize cost-effective and environmentally friendly BnZ [16][14]. Ortho-bromoaryl compounds are cyclized intramolecularly using cuprous oxide nanoparticles like a catalyst [I]. Saha et al. were able to synthesize substituted BnZ and 2-aminobenzimidazole. The catalyst might also be restored without altering its activity, in addition, with this method [17][15]. CuI/l-proline was applied by Diao et al. as a catalyst to condense 2-iodoacetanilides and aqueous ammonia for cyclization to create substituted 1H-BnZ by applying heat under acidic circumstances [18][16]. Kim et al. produced BnZ derivatives by condensation as well as the formation of C-N bonds. Sodium azide, RCHO [J] and 2-haloanilines are active as initial ingredients in one-pot synthesis [19][17]. Tao Zhang initially presented a model for the reaction of BnZ with [K] (10 mmol) and benzonitrile (NC-R) in a mixture of 20 mL PPA (polyphosphoric acid) and 10 mL H3PO4 under microwave irradiation (MC 275 W, 15 min), with a yield of 92% [20][18].
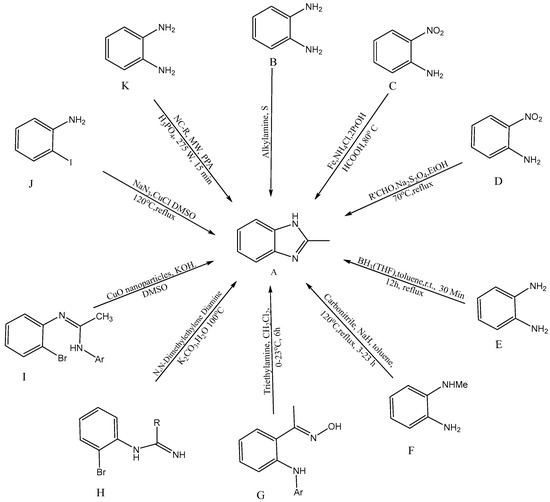
Figure 2.
Synthetic approaches for BnZ.
Several synthetic tactics have been successfully implemented, which are described in Figure 2 for the production of BnZ [A]. A combination of ortho-substituted aniline [B] and bifunctional o-esters produced a BnZ derivative in 2012, according to Bastug et al. [10][7]. Utilizing heterocyclic aromatic compounds like H2NC6H4NO2 with CH2O2, iron powder, and ammonium chloride, proceeded by the one pot reduction of NO2 followed by imidazole cyclisation [C] into bicyclic 2-H BnZ, was one method used by Hanan et al. (2010) [11][8]. Yang et al. developed a new method for the one-step synthesis of 2-substituted -N-H, -N-alkyl, and -N-aryl BnZ derivatives in the presence of aldehydes and sodium dithionite through the reduction of o-nitroanilines [D], succeeding in synthesizing BnZ [12][9]. Another approach for the synthesis of 2-substituted BnZ was published by Cui et al. in 2012 for producing 1,2-phenylenediamines and triacyloxyborane intermediates, which were produced via the interaction between carboxylic acids [E] and borane-THF, to yield 2-substituted BnZ [13][10]. N-methyl-1,2-phenylenediamine, sodium hydride, and carbonitriles [F] were used as the starting ingredients by Sluiter and Christoffers in 2009 [14][11]. Wray synthesized [1][12] 1H-indazoles from common arylamino oximes in 2010 in the presence of several bases, but the synthesis of BnZ was only improved by one base, i.e., triethylamine [G] [15][13]. Cuprous oxide, potassium carbonate, (CH3NH)2C2H4, and water [H] were used by Peng et al. to synthesize cost-effective and environmentally friendly BnZ [16][14]. Ortho-bromoaryl compounds are cyclized intramolecularly using cuprous oxide nanoparticles like a catalyst [I]. Saha et al. were able to synthesize substituted BnZ and 2-aminobenzimidazole. The catalyst might also be restored without altering its activity, in addition, with this method [17][15]. CuI/l-proline was applied by Diao et al. as a catalyst to condense 2-iodoacetanilides and aqueous ammonia for cyclization to create substituted 1H-BnZ by applying heat under acidic circumstances [18][16]. Kim et al. produced BnZ derivatives by condensation as well as the formation of C-N bonds. Sodium azide, RCHO [J] and 2-haloanilines are active as initial ingredients in one-pot synthesis [19][17]. Tao Zhang initially presented a model for the reaction of BnZ with [K] (10 mmol) and benzonitrile (NC-R) in a mixture of 20 mL PPA (polyphosphoric acid) and 10 mL H3PO4 under microwave irradiation (MC 275 W, 15 min), with a yield of 92% [20][18].
2. Metal-Catalyzed Derivatives of Benzimidazole
Metal catalysts have a few features, including the fact that they can be recovered. Catalysts have surface efficiency for catalyzing reactions and further increase overall reaction rate. Indium [III]triflate was used as a reusable catalyst by De et al., in 2006 which described the synthesis of MSBs (IX-a) with high yields. The synthesis of 2-substituted BnZ without solvent occurs by the fusion of o-phenylenediamine (VII) with aldehydes [where R = benzaldehyde (VIII)] utilizing a catalytic quantity of In(OTf)3. VII and VIII were combined in a 1:1.1 molar ratio and heated up to atmospheric temperature for 30 minutes to perform the reactions. They also used various catalysts, such as Copper [II]triflate, Lutetium[III]trifluoromethanesulfonate, Indium[III]trifluoromethanesulfonate and La(OTf)3. Among these, the most recently used catalysts gave the highest output. The process is depicted in Scheme 3 [21][19].
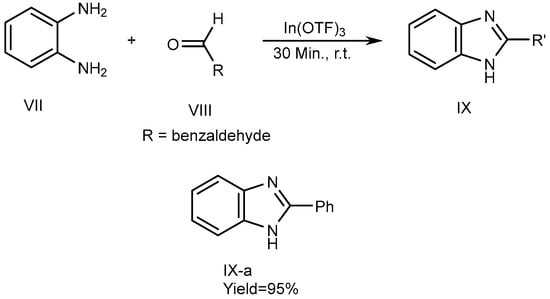
Scheme 3.
Metal-catalyzed reduction of BnZ.
Fu et al., described the method for the synthesis of 2-substituted 1H-BnZ (XIII a-b) in dimethyl sulfoxide using cesium carbonate as a base and 10 mol % Copper[I] bromide as a catalyst. The optimized yield of 2-substituted 1H-BnZ (XIII-c) using this approach was obtained by coupling, hydrolysis, and the intra-molecular cyclization of o-haloacetanilide (X) derivatives with amidines (XI). o-iodoacetanilide is required for the N-arylation of amidines at 60 °C to yield XIII-a, whereas o-bromoacetanilide required a high temperature of 90 °C to yield XIII-b and XIII-c., which is displayed in Scheme 4 [22][20].
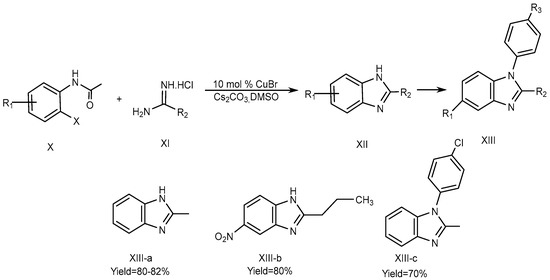
Scheme 4.
CuBr-catalyzed reactions.
In 2013, Nguyen et al. described a process using iron-sulfur as a catalyst to synthesize the BnZ derivative (XVI). For the synthesis of XVI, 4-picoline (XV) and substituted o-nitroanilines (XIV) were used as reactants, and the reaction was carried out at 150 °C under solvent-free conditions using an equimolecular amount of an Fe/S catalyst. Fe/S played a crucial role for promoting the cyclization process. Pyridine and quinoline derivatives possess a methyl group at the 2- or 4-position (XV) and are coupled with XIV to form derivative MSBs (XVI a-c), where all other suitable substrates in the reaction along with o-nitroaniline synthesize the corresponding benzimidazole products XVI-a-b with yields of 83-91%, if o-Nitroanilines have electron-donating and withdrawing substituents on its benzene ring. There are methyl groups of picolines in elemental Chloride (XVI-c), as depicted in Scheme 5 [23][21].
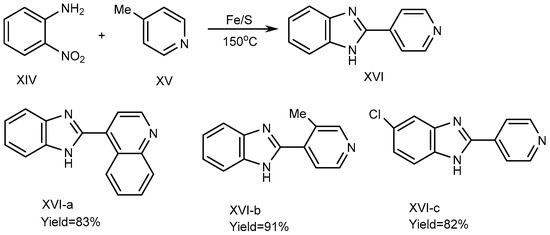
Scheme 5.
Fe/S-catalyzed reaction of BnZ.
Sun et al. (2017) described a procedure for the feasible condensation of phenylhydrosilicon, o-phenylenediamine (XVII) and dimethylformamide (XVIII). According to this scheme, hydrosilicon is utilized as a reaction catalyst, activating the carbonyl group of di-methylformamide to make an Si–O bond at 120 °C for 12 hours. An amine-group in o-phenylenediamine was coupled with activated DMF-hydrosilicon mixture, and further undergoes cyclization, succeeding in the excellent synthesis of BnZ (XIX), as shown in Scheme 6 [24][22].
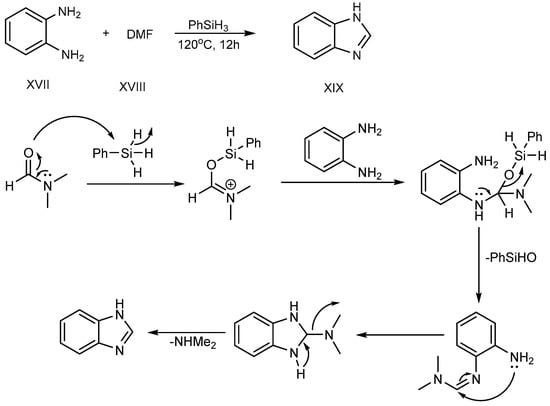
Scheme 6.
Cyclization of o-phenylenediamines with DMF.
A dehydrogenation reaction, using cobalt-pincer complexes (XXII) as a catalyst, was carried out at 150 °C for 24 hours by Milstein et al. (2017). Primary alcohol (XXI) and o-phenylenediamine (XX) were combined to synthesize the 2-MSB derivative (XXIII) (Scheme 7) [25][23].
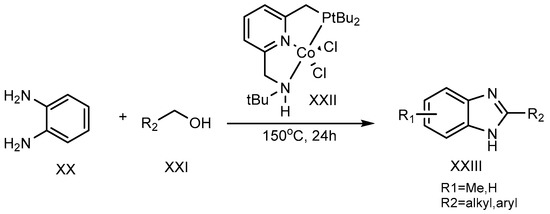
Scheme 7.
Co-complex catalyst-mediated reaction.
3. Metal-Free Catalyzed Derivatives of BnZ
A one-pot synthesis with an exceptional yield was reported by Nguyen et al. in 2012 under dry and metal-free conditions to obtain XXX–XXXIV. In this method, the reaction was completed with trialkyl amine (triethylamine) (XXVIII), benzene-1,2-diamine (XXIX), and derivatives of phenylmethanamines (XXIV–XXVII). Sulphur atoms helped to proceed the reaction for generating C–N bonds, and the researchers succeeded in its cyclization (Scheme 8) [26][24].
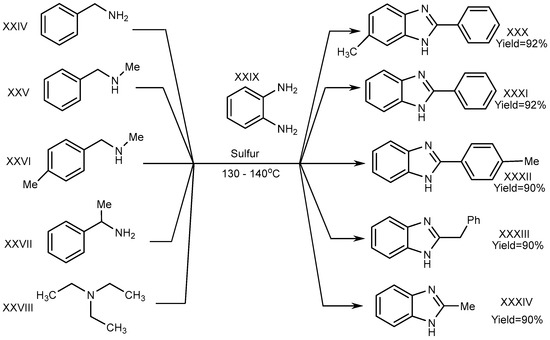
Scheme 8.
Cross–coupling reaction of o–phenylenediamines with aliphatic amines (S).
Shanmugam et al. described the efficient metal-free synthesis of aryl-substituted BnZ (XXXVII-a) through the use of acetic acid as a catalyst under microwave heating conditions. This synthesis involves the reaction of o-phenylenediamine (XXXVI) with a substituted aromatic aldehyde (XXXV). Shown in Scheme 9, the reaction is entirely free from metals, as well as using environmentally friendly green solvent [27][25].
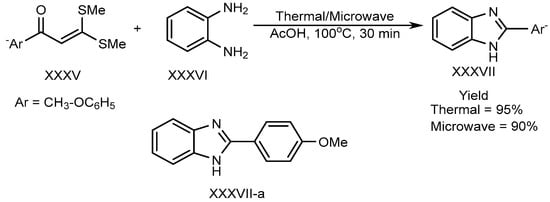
Scheme 9.
Thermal/microwave-assisted BnZ synthesis.
Liu et al. described a metal–free process for the preparation of N-substituted BnZ (XL) under microwave irradiation. The improved yield is produced when fluoro-aryl formamidines (XXXVIII) with primary amines (XXXIX) undergo an SN–Ar reaction [28][26]. (Scheme 10).
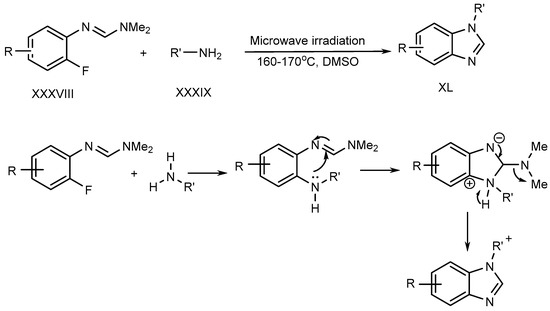
Scheme 10.
Synthesis of BnZ under metal-free conditions with amines.
Mostafavi et al. published a method in 2018 for BnZ biosynthesis. Hexamethyldisilazane regulated the reaction at 120 °C, and transformed o-phenylenediamine (XLI) and N,N-Dimethylformamide into BnZ. There is no need for any acid, transition metal, or fluid for proceeding the reaction to achieve good yields XLIII (Scheme 11) [29][27].
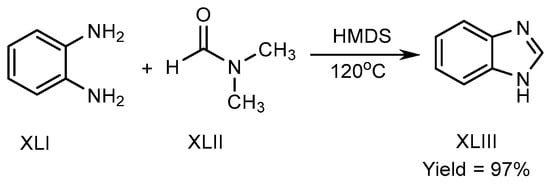
Scheme 11.
Metal-free BnZ biosynthesis.
In the presence of 5ml of dilute hydrochloric acid solution, o-phenylenediamine (XLV) and Ibuprofen (XLIV) are used to produce a BnZ variant XLVI. Further, the mixture was heated in a water bath at 100 °C for two hours with the slow addition of 10% sodium hydroxide, as shown in Scheme 12. The researchers discovered that BnZs have very strong antimicrobial properties [30][28].
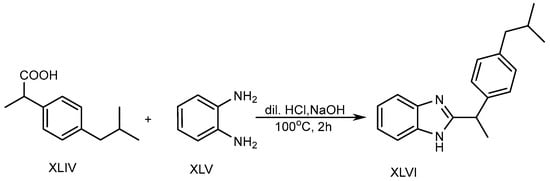
Scheme 12.
Base-catalyzed synthesis of BnZ.
4. Green Synthesis of Benzimidazole
Most chemical organizations or industries, including pharma-enterprises, are now coping with mild ecological issues, like excessive solvent, chemicals waste, and the use of catalysts in the production of BnZ. Green synthesis mediates solvent-free production by using environmentally friendly catalysts like C3H6O3 or B(OH)3 under microwave condition, which is an effective way to get rid of these issues.
XLVII and aldehydes (XLVIII) undergo cyclic condensation in an equal amount of a bioabsorbable solution like ethanol and lactic acid for 4-6 hours at normal temperature. Song et al. (2016) disclosed the one-pot synthesis of disubstituted-benzimidazole (DSBs) (XLIX-a) derivatives. The optimized result is obtained in lactic acid (Scheme 13) [31][29].
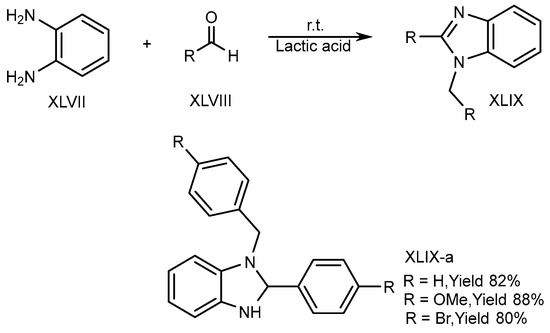
Scheme 13.
Synthesis of XLIX-a in green solvent.
Khunt et al. (2014) described a strategy for the preparation of a BnZ candidate (LII) via the interaction of 1,2-diaminobenzene (L) with aldehydes (LI) by use of an ecofriendly solvent such as polyethylene glycol (PEG)-400. Above 80–85 °C, the process produces the highest yield of PEG-400. The targeted molecule was produced through a new unique synthetic pathway, in which glycerol/water is used (Scheme 14) [32][30].
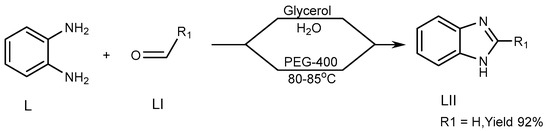
Scheme 14.
Synthesis of BnZ in solution instead of PEG.
Kathirvelan et al. discovered the one-pot synthesis of a monosubstituted benzimidazole (MSB) (LV a-b) scaffold in 2013 (Scheme 15) by the coupling of benzene-1,2-diamine (LIII) and aldehydes (LIV) using an ecofriendly feasible catalyst, i.e., ammonium chloride in ethanol at 80–90 °C [33][31].
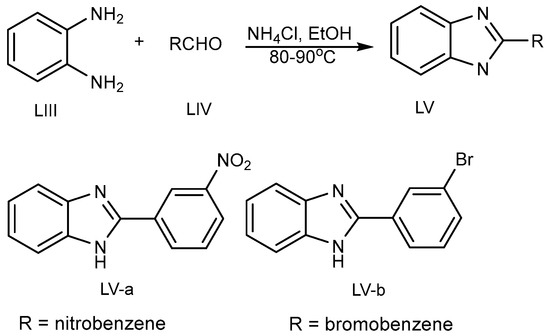
Scheme 15.
BnZ synthesis using ammonium chloride.
Kidwai et al. (2010) described a unique approach using ceric ammonium nitrate (CAN) catalyst for the synthesis of BnZ scaffold (LVIII) in a polyethylene glycol at 50 °C for 2 h. Benzene-1,2-diamine (LVI) combined with aldehyde (LVII) in CAN catalyst tends to lead to exceptional PEG yields (LVIII-a) (Scheme 16) [34][32].
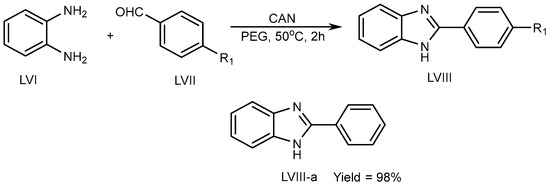
Scheme 16.
CAN-catalyzed polyethylene glycol–MSB reaction.
Azarifar et al. (2010) suggested an eco-friendly microwave-assisted reflux reaction of o-phenylenediamine (LIX) with an RCHO derivative (LX) at 80 °C for the synthesis of MSBs and DSBs (LXI, LXII) (Scheme 17) [35][33].
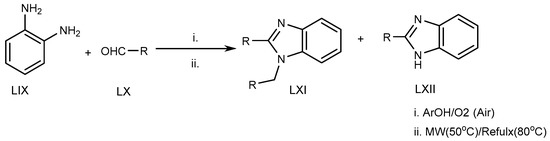
Scheme 17.
Microwave-irradiation-mediated BnZ synthesis.
5. Multifaceted Efficacy of Benzimidazole
The significance of chiral BnZs has led to a relatively new sector of drug discovery for various biological applications; therefore, its synthesis has received special attention [36][34]. Additionally, chiral BnZs have been utilized as organocatalysts for the enantioselective chlorination, Diels–Alder reactions, asymmetric Michael additions, and asymmetric aldol-type reactions. However, Rh- and Pd-based BnZ complexes are synthesized using a Mizoroki–Heck reaction [37][35]. The reduction processes is performed using Suzuki–Miyaura coupling reactions [38][36]. Earlier surveys demonstrate that the BnZ scaffold is crucial for its therapeutic usage, in addition to as optical sensors for use in nanomaterial properties [39][37]. These properties can apparently benefit the numerous sensing devices, as well as its cost effectiveness and operational flexibility; therefore, it seems to have specific usage in medicine, geology, and industrial innovation (Figure 3). Another use of BnZ and its derivatives is in supra-molecular assemblies with intriguing features for various uses, such as thermostable polymers, adsorbent materials, nano-containers, and liquid crystals for electronic conduction. For the last few years, it has been seen in a lot of research in the area of polybenzimidazole (PBI) derivatives, such as for solid electrolytes of fuel cells [40,41,42,43,44][38][39][40][41][42], fiber [45][43], thin coatings [46][44], advanced protective coverings [47][45], or to remove palladium, Th, and U from a watery medium [48][46]. PBI-based technologies exist in Charlotte, North Carolina, used in the pioneering industrial applications for firefighter safety in Europe, United States, and Middle East, with 32 years of expertise. PBI materials are famous for their tested protection against heat and flames, and shield the firemen in a various fire services [49][47].
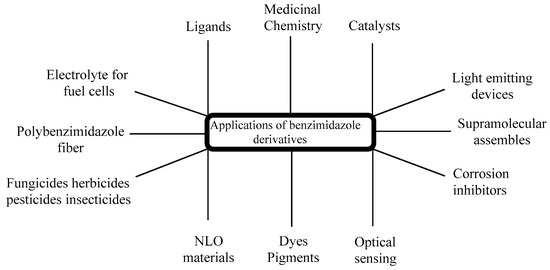
Figure 3.
Applications of BnZ derivatives.
Another use of poly-Benzimidazole, as a combined matrix, shows extraordinarily strong evaporation property due to its high porosity and selectivity [50][48]. Additionally, this is the most recommended material for another photonic technology in organic frames. This has led to the study of a number of small molecules of BnZ derivatives like 2-mercaptobenzimidazole, 2-phenylbenzimidazole, and 2-hydroxybenzimidazole, with excellent non-linear optical (NLO) properties [51][49] and highly intricate structures [52][50]. Benlate and Carbendazim both used BnZ as fungicides with minimal cytotoxicity at lower concentrations and no risk for carcinogenic or genetic effects [53][51]. There is documentation regarding the utilization of BnZ as fertilizers and insecticides, which also specifies their uses [54][52]. BnZ is an organic molecule, recently highlighted as a corrosion inhibitor for copper, iron, or zinc in acidic circumstances [55,56][53][54] because of its properties of BnZ-bonded electron donating and accepting substituents. Scientists have demonstrated that BnZ is a flexible and necessary chromophore for natural dyes, with excellent photophysical, electrochemical, and photovoltaic characteristics [57][55]. Benzimidazol-2-one is highly considerable because of its durability and light-resistance properties. It has been used for the last three decades to produce a wide variety of subtleties in water for color painting and electro-photographic toner [58][56]. BnZ has proven to be a vital molecule in organic light-emitting devices (OLEDs), with excellent phosphorescence, thermal features, and morphological stabilities [59][57]. For innovative research with a new mode of action, heterocyclic compounds are frequently used. Aryl aziridines is a heterocyclic compound [60][58] and BnZ derivatives play a significant role because of the vast range of biological actions they exhibit. According to a literature survey, BnZ and its derivatives have physiological and pharmacological activities. These are able to treat a broad range of disorders, particularly epilepsy, diabetes, and pregnancy. Such derivatives exhibit a variety of bioactivities like antiallergic [61][59], antihistamine [62][60], anti-ulcerative, antiproliferative [63][61], antioxidant [64[62][63],65], antifungal, antibacterial, antitubercular [66][64], antihypertensive [67][65], anti-inflammatory, analgesic, antiamoebic [68][66], antitumor [69[67][68][69],70,71], antimalarial [72][70], and anti-kinase activity [73][71]. Several BnZ derivatives are also being investigated as cholinesterase inhibitors and anti-parasitic drugs [74,75,76,77][72][73][74][75].
6. Benzimidazole-Derivative-Based Biological Activities
Numerous derivatives have been explored in individual investigations, for various therapeutic treatments. These include the antiemetic, KB-R-6933; the anti-cancer, Bendastumide; the antiviral, l Hoechst 33342; the antihistamine, Clemizole; the anti-ulcerative, Omeprazole; and the anti-hypertensive, Telmisartan. Additionally, Thiabendazol, an anti-fungal agent, has been employed to examine its effects (Figure 4) [78][76].
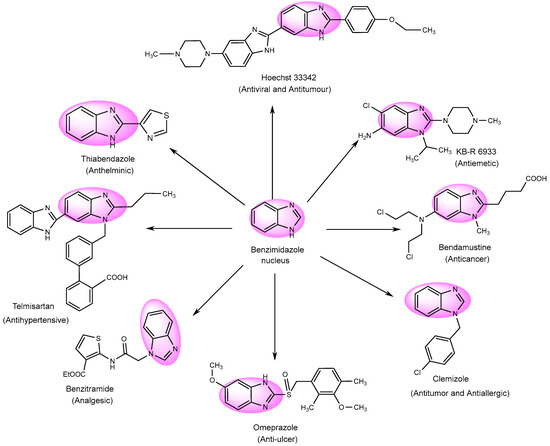
Figure 4.
Multifunctional examples of BnZ derivatives.
References
- wright, J.B. The Chemistry of the Benzimidazoles. Chem. Rev. 1951, 48, 397–541.
- Hoebrecker, F. Benzimidazole. Ber 1872, 5, 920–926.
- Fei, F.; Zhou, Z. New Substituted Benzimidazole Derivatives: A Patent Review (2010–2012). Expert Opin. Ther. Pat. 2013, 23, 1157–1179.
- Azeez, S.; Sureshbabu, P.; Chaudhary, P.; Sabiah, S.; Kandasamy, J. Tert-Butyl Nitrite Catalyzed Synthesis of Benzimidazoles from o-Phenylenediamine and Aldehydes at Room Temperature. Tetrahedron Lett. 2020, 61, 151735.
- Zhang, J.; Yao, R.; Chen, J.; Li, T.; Xu, Y. Hydrogenative Coupling of Nitriles with Diamines to Benzimidazoles Using Lignin-Derived Rh2P Catalyst. iScience 2021, 24, 103045.
- Vijayalakshmi, P.B.; Haridasan, A.P. Pharmacognostic and Preliminary Phytochemical Evaluation of Nelamuchchala (Gymnostachyum Febrifugum Benth.). Int. J. Res. Ayush Pharm. Sci. 2017, 1, 1–6.
- Bastug, G.; Eviolitte, C.; Markó, I.E. Functionalized Orthoesters as Powerful Building Blocks for the Efficient Preparation of Heteroaromatic Bicycles. Org. Lett. 2012, 14, 3502–3505.
- Hanan, E.J.; Chan, B.K.; Estrada, A.A.; Shore, D.G.; Lyssikatos, J.P. Mild and General One-Pot Reduction and Cyclization of Aromatic and Heteroaromatic 2-Nitroamines to Bicyclic 2 H-Imidazoles. Synlett 2010, 18, 2759–2764.
- Yang, D.; Fokas, D.; Li, J.; Yu, L.; Baldino, C.M. A Versatile Method for the Synthesis of Benzimidazoles from O-Nitroanilines and Aldehydes in One Step via a Reductive Cyclization. Synthesis 2005, 1, 47–56.
- Cui, W.; Kargbo, R.B.; Sajjadi-Hashemi, Z.; Ahmed, F.; Gauuan, J.F. Efficient One-Pot Synthesis of 2-Substituted Benzimidazoles from Triacyloxyborane Intermediates. Synlett 2012, 2, 247–250.
- Sluiter, J.; Christoffers, J. Synthesis of 1-Methylbenzimidazoles from Carbonitriles. Synlett 2009, 1, 63–66.
- Rao, G.E.; Babu, P.S.; Koushik, O.S.; Sharmila, R.; Vijayabharathi, M.; Maruthikumar, S.; Prathyusha, R.; Pavankumar, P. A Review on Chemistry of Benzimidazole Nucleus and its Biological Significance. Int. J. Pharm. Chem. Biol. Sci. 2016, 6, 227–232.
- Wray, B.C.; Stambuli, J.P. Synthesis of N-Arylindazoles and Benzimidazoles from a Common Intermediate. Org. Lett. 2010, 12, 4576–4579.
- Peng, J.; Ye, M.; Zong, C.; Hu, F.; Feng, L.; Wang, X.; Wang, Y.; Chen, C. Copper-Catalyzed Intramolecular C-N Bond Formation: A Straightforward Synthesis of Benzimidazole Derivatives in Water. J. Org. Chem. 2011, 76, 716–719.
- Saha, P.; Ramana, T.; Purkait, N.; Ali, M.A.; Paul, R.; Punniyamurthy, T. Ligand-Free Copper-Catalyzed Synthesis of Substituted Benzimidazoles, 2-Aminobenzimidazoles, 2-Aminobenzothiazoles, and Benzoxazoles. J. Org. Chem. 2009, 74, 8719–8725.
- Diao, X.; Wang, Y.; Jiang, Y.; Ma, D. Assembly of Substituted 1H-Benzimidazoles and 1,3-Dihydrobenzimidazol-2- Ones via CuI/L-Proline Catalyzed Coupling of Aqueous Ammonia with 2-Iodoacetanilides and 2-Iodophenylcarbamates. J. Org. Chem. 2009, 74, 7974–7977.
- Kim, Y.; Kumar, M.R.; Park, N.; Heo, Y.; Lee, S. Copper-Catalyzed, One-Pot, Three-Component Synthesis of Benzimidazoles by Condensation and C-N Bond Formation. J. Org. Chem. 2011, 76, 9577–9583.
- Zhang, T.; Huang, L.-Z.; Wu, J.; Lu, D.; Ma, B.-L.; Du, Z.-T. Microwave-Assisted Syntehsis of 2-Substituted 1H-Benzoimidazoles and Their Antifungal Activities in vitro. Heterocycles 2013, 87, 1545–1552.
- Trivedi, R.; De, S.K.; Gibbs, R.A. A Convenient One-Pot Synthesis of 2-Substituted Benzimidazoles. J. Mol. Catal. A Chem. 2006, 245, 8–11.
- Yang, D.; Fu, H.; Hu, L.; Jiang, Y.; Zhao, Y. Copper-Catalyzed Synthesis of Benzimidazoles via Cascade Reactions of o-Haloacetanilide Derivatives with Amidine Hydrochlorides. J. Org. Chem. 2008, 73, 7841–7844.
- Nguyen, T.B.; Ermolenko, L.; Al-mourabit, A. Iron Sulfide Catalyzed Redox/Condensation Cascade Reaction between 2-Amino/Hydroxy Nitrobenzenes and Activated Methyl Groups: A Straightforward Atom Economical Approach to 2-Hetaryl-Benzimidazoles and -Benzoxazoles. J. Am. Chem. Soc. 2013, 135, 118–121.
- Zhu, J.; Zhang, Z.; Miao, C.; Liu, W.; Sun, W. Synthesis of Benzimidazoles from O-Phenylenediamines and DMF Derivatives in the Presence of PhSiH3. Tetrahedron 2017, 73, 3458–3462.
- Daw, P.; Ben-David, Y.; Milstein, D. Direct Synthesis of Benzimidazoles by Dehydrogenative Coupling of Aromatic Diamines and Alcohols Catalyzed by Cobalt. ACS Catal. 2017, 7, 7456–7460.
- Nguyen, T.B.; Ermolenko, L.; Dean, W.A.; Al-Mourabit, A. Benzazoles from Aliphatic Amines and o-Amino/Mercaptan/Hydroxyanilines: Elemental Sulfur as a Highly Efficient and Traceless Oxidizing Agent. Org. Lett. 2012, 14, 5948–5951.
- Dhanalakshmi, P.; Thimmarayaperumal, S.; Shanmugam, S. Metal Catalyst Free One-Pot Synthesis of 2-Arylbenzimidazoles from α-Aroylketene Dithioacetals. RSC Adv. 2014, 4, 12028–12036.
- Liu, X.; Cao, H.; Bie, F.; Yan, P.; Han, Y. C A N Bond Formation and Cyclization: A Straightforward and Metal-Free Synthesis of N -1-Alkyl-2-Unsubstituted Benzimidazoles. Tetrahedron Lett. 2019, 60, 1057–1059.
- Mostafavi, H.; Islami, M.R.; Ghonchepour, E.; Tikdari, A.M. Synthesis of 1H-1,3-Benzimidazoles, Benzothiazoles and 3H-ImidazoPyridine Using DMF in the Presence of HMDS as a Reagent under the Transition-Metal-Free Condition. Chem. Pap. 2018, 72, 2973–2978.
- Jain, P.; Tiwari, M. Synthesis and Antimicrobial Activity of Some Benzimidazole and 2-Methylbenzimidazole Derivatives. Asian J. Chem. 2017, 29, 838–842.
- Yu, Z.Y.; Zhou, J.; Fang, Q.S.; Chen, L.; Song, Z.B. Chemoselective Synthesis of 1,2-Disubstituted Benzimidazoles in Lactic Acid without Additive. Chem. Pap. 2016, 70, 1293–1298.
- Khunt, M.D.; Kotadiya, V.C.; Viradiya, D.J.; Baria, B.H. Easy, Simplistic and Green Synthesis of Various Benzimidazole and Benzoxazole Derivatives Using PEG 400 as a Green Solvent. Int. Lett. Chem. Phys. Astron. 2014, 25, 61–68.
- Kathirvelan, D.; Yuvaraj, P.; Babu, K.; Nagarajan, A.S.; Reddy, B.S.R. A Green Synthesis of Benzimidazoles Benzimidazole Moieties Are a Very Important Class of Heterocyclic Compounds That Have Many Applications in Pharmaceutical Chemistry. Indian J. Chem. 2013, 52, 1152–1156.
- Kidwai, M.; Jahan, A.; Bhatnagar, D. Polyethylene Glycol: A Recyclable Solvent System for the Synthesis of Benzimidazole Derivatives Using CAN as Catalyst. J. Chem. Sci. 2010, 122, 607–612.
- Azarifar, D.; Pirhayati, M.; Maleki, B.; Sanginabadi, M.; Yami, R.N. Acetic Acid-Promoted Condensation of o-Phenylenediamine with Aldehydes into 2-Aryl-1-(Arylmethyl)-1H-Benzimidazoles under Microwave Irradiation. J. Serbian Chem. Soc. 2010, 75, 1181–1189.
- Khose, V.N.; John, M.E.; Pandey, A.D.; Karnik, A.V. Chiral Benzimidazoles and Their Applications in Stereodiscrimination Processes. Tetrahedron Asymmetry 2017, 28, 1233–1289.
- Said, N.R.; Mustakim, M.A.; Sani, N.N.M.; Baharin, S.N.A. Heck Reaction Using Palladium-Benzimidazole Catalyst: Synthesis, Characterisation and Catalytic Activity. IOP Conf. Ser. Mater. Sci. Eng. 2018, 458, 012019.
- Günnaz, S.; Gökçe, A.G.; Türkmen, H. Synthesis of Bimetallic Complexes Bridged by 2,6-Bis(Benzimidazol-2-Yl) Pyridine Derivatives and Their Catalytic Properties in Transfer Hydrogenation. Dalt. Trans. 2018, 47, 17317–17328.
- Horak, E.; Kassal, P.; Murković Steinberg, I. Benzimidazole as a Structural Unit in Fluorescent Chemical Sensors: The Hidden Properties of a Multifunctional Heterocyclic Scaffold. Supramol. Chem. 2018, 30, 838–857.
- Agarwal, R.A.; Aijaz, A.; Ahmad, M.; Sañudo, E.C.; Xu, Q.; Bharadwaj, P.K. Two New Coordination Polymers with Co(II) and Mn(II): Selective Gas Adsorption and Magnetic Studies. Cryst. Growth Des. 2012, 12, 2999–3005.
- Nath, I.; Chakraborty, J.; Verpoort, F. Metal Organic Frameworks Mimicking Natural Enzymes: A Structural and Functional Analogy. Chem. Soc. Rev. 2016, 45, 4127–4170.
- Tan, S.; Wei, B.; Liang, T.; Yang, X.; Wu, Y. Anhydrous Proton Conduction in Liquid Crystals Containing Benzimidazole Moieties. RSC Adv. 2016, 6, 34038–34042.
- Yuan, S.; Guo, X.; Aili, D.; Pan, C.; Li, Q.; Fang, J. Poly(Imide Benzimidazole)s for High Temperature Polymer Electrolyte Membrane Fuel Cells. J. Membr. Sci. 2014, 454, 351–358.
- Jiang, J.-J.; Pan, M.; Liu, J.-M.; Wang, W.; Su, C.-Y. Assembly of Robust and Porous Hydrogen-Bonded Coordination Frameworks: Isomorphism, Polymorphism, and Selective Adsorption. Inorg. Chem. 2010, 49, 10166–10173.
- Yin, C.; Dong, J.; Zhang, Z.; Zhang, Q.; Lin, J. Structure and Properties of Polyimide Fibers Containing Benzimidazole and Amide Units. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 183–191.
- Nabavian, S.; Naderi, R.; Asadi, N. Determination of Optimum Concentration of Benzimidazole Improving the Cathodic Disbonding Resistance of Epoxy Coating. Coatings 2018, 8, 471.
- Iqbal, H.M.S.; Bhowmik, S.; Benedictus, R. Performance Evaluation of Polybenzimidazole Coating for Aerospace Application. Prog. Org. Coat. 2017, 105, 190–199.
- VijayaKumar, V.; Ramesh Kumar, C.; Suresh, A.; Jayalakshmi, S.; Kamachi Mudali, U.; Sivaraman, N. Evaluation of Polybenzimidazole-Based Polymers for the Removal of Uranium, Thorium and Palladium from Aqueous Medium. R. Soc. Open Sci. 2018, 5, 171701.
- Mandal, S.; Song, G. Characterizing Thermal Protective Fabrics of Firefighters’ Clothing in Hot Surface Contact. J. Ind. Text. 2018, 47, 622–639.
- Akhtar, F.H.; Kumar, M.; Villalobos, L.F.; Vovusha, H.; Shevate, R.; Schwingenschlögl, U.; Peinemann, K.V. Polybenzimidazole-Based Mixed Membranes with Exceptionally High Water Vapor Permeability and Selectivity. J. Mater. Chem. A 2017, 5, 21807–21819.
- Muthuraja, A.; Kalainathan, S. A Study on Growth, Optical, Mechanical, and NLO Properties of 2-Mercaptobenzimidazole, 2-Phenylbenzimidazole and 2-Hydroxy Benzimidazole Single Crystals: A Comparative Investigation. Mater. Technol. 2017, 32, 335–348.
- Tayade, R.P.; Sekar, N. Benzimidazole-Thiazole Based NLOphoric Styryl Dyes with Solid State Emission—Synthesis, Photophysical, Hyperpolarizability and TD-DFT Studies. Dye. Pigment. 2016, 128, 111–123.
- Gupta, P.K. Toxicity of Fungicides, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018.
- Ermler, S.; Scholze, M.; Kortenkamp, A. Seven Benzimidazole Pesticides Combined at Sub-Threshold Levels Induce Micronuclei in Vitro. Mutagenesis 2013, 28, 417–426.
- Eldebss, T.M.A.; Farag, A.M.; Shamy, A.Y.M. Synthesis of Some Benzimidazole-Based Heterocycles and Their Application as Copper Corrosion Inhibitors. J. Heterocycl. Chem. 2019, 56, 371–390.
- Anastasiou, E.; Lorentz, K.O.; Stein, G.J.; Mitchell, P.D. Prehistoric Schistosomiasis Parasite Found in the Middle East. Lancet Infect. Dis. 2014, 14, 553–554.
- Saltan, G.M.; Dinçalp, H.; Kiran, M.; Zafer, C.; Erbaş, S.Ç. Novel Organic Dyes Based on Phenyl-Substituted Benzimidazole for Dye Sensitized Solar Cells. Mater. Chem. Phys. 2015, 163, 387–393.
- Mamedov, V.A.; Khafizova, E.A.; Syakaev, V.V.; Gubaidullin, A.T.; Samigullina, A.I.; Algaeva, N.E.; Latypov, S.K. The Rearrangement of 1H,1′H-Spiro-2,3′ (3H,4′H)-Diones—A New and Efficient Method for the Synthesis of 4-(Benzimidazol-2-Yl)Quinolin-2(1H)-Ones. Tetrahedron 2018, 74, 6544–6557.
- Zhao, Y.; Wu, C.; Qiu, P.; Li, X.; Wang, Q.; Chen, J.; Ma, D. New Benzimidazole-Based Bipolar Hosts: Highly Efficient Phosphorescent and Thermally Activated Delayed Fluorescent Organic Light-Emitting Diodes Employing the Same Device Structure. ACS Appl. Mater. Interfaces 2016, 8, 2635–2643.
- Giovine, A.; Muraglia, M.; Florio, M.A.; Rosato, A.; Corbo, F.; Franchini, C.; Musio, B.; Degennaro, L.; Luisi, R. Synthesis of Functionalized Arylaziridines as Potential Antimicrobial Agents. Molecules 2014, 19, 11505–11519.
- Šindelář, Z.; Kopel, P. Bis(Benzimidazole) Complexes, Synthesis and Their Biological Properties: A Perspective. Inorganics 2023, 11, 113.
- Mirzaeian, F.; Eshghi, H. Synthesis of Benzimidazoles by Ni-Catalyzed Hydrogen Transfer Reduction of Nitroarenes with Alcohols. In Proceedings of the 21st ICS International Chemistry Congres, Mashhad, Iran, 26–28 July 2022; Available online: https://profdoc.um.ac.ir/paper-abstract-1091156.html (accessed on 1 May 2023).
- Haddadi, M.H. Benzimidazole Derivatives: A Versatile Scaffold for Drug Development against Helicobacter Pylori-Related Diseases. Fundam. Clin. Pharmacol. 2022, 36, 930–943.
- Haque, R.A.; Iqbal, M.A.; Asekunowo, P.; Majid, A.M.S.A.; Khadeer Ahamed, M.B.; Umar, M.I.; Al-Rawi, S.S.; Al-Suede, F.S.R. Synthesis, Structure, Anticancer, and Antioxidant Activity of Para-Xylyl Linked Bis-Benzimidazolium Salts and Respective Dinuclear Ag(I) N-Heterocyclic Carbene Complexes (Part-II). Med. Chem. Res. 2013, 22, 4663–4676.
- Nile, S.H.; Kumar, B.; Park, S.W. In Vitro Evaluation of Selected Benzimidazole Derivatives as an Antioxidant and Xanthine Oxidase Inhibitors. Chem. Biol. Drug Des. 2013, 82, 290–295.
- Mohanty, S.K.; Khuntia, A.; Yellasubbaiah, N.; Ayyanna, C.; Naga Sudha, B.; Harika, M.S. Design, Synthesis of Novel Azo Derivatives of Benzimidazole as Potent Antibacterial and Anti Tubercular Agents. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 646–651.
- Iqbal, H.; Verma, A.K.; Yadav, P.; Alam, S.; Mishra, D.; Khan, F.; Hanif, K.; Negi, A.S.; Chanda, D. Antihypertensive Effect of a Novel Angiotensin II Receptor Blocker Fluorophenyl Benzimidazole: Contribution of CGMP, Voltage-Dependent Calcium Channels, and BK Ca Channels to Vasorelaxant Mechanisms. Front. Pharmacol. 2021, 12, 611109.
- Bhagat, P.K.; Raj, R.; Thakur, D.N. Preparation and Characterization of Some Bivalent Metal Complexes with Bis and Studies of Their Antiamoebic and Antihelminthic Activities. Int. J. Chem. Environ. Sci. 2023, 4, 49–53.
- Hernández-Romero, D.; Rosete-Luna, S.; López-Monteon, A.; Chávez-Piña, A.; Pérez-Hernández, N.; Marroquín-Flores, J.; Cruz-Navarro, A.; Pesado-Gómez, G.; Morales-Morales, D.; Colorado-Peralta, R. First-Row Transition Metal Compounds Containing Benzimidazole Ligands: An Overview of Their Anticancer and Antitumor Activity. Coord. Chem. Rev. 2021, 439, 213930.
- Hu, J.; Cao, T.; Yuan, B.; Guo, Y.; Zhang, J.; Zhao, X.; Hou, H. Benzimidazole-Quinoline-Based Copper Complexes: Exploration for Their Possible Antitumor Mechanism. Polyhedron 2022, 211, 115563.
- Son, D.-S.; Lee, E.-S.; Adunyah, S.E. The Antitumor Potentials of Benzimidazole Anthelmintics as Repurposing Drugs. Immune Netw. 2020, 20, e29. Available online: https://immunenetwork.org/DOIx.php?id=10.4110/in.2020.20.e29 (accessed on 1 May 2023).
- Dziwornu, G.A.; Coertzen, D.; Leshabane, M.; Korkor, C.M.; Cloete, C.K.; Njoroge, M.; Gibhard, L.; Lawrence, N.; Reader, J.; van der Watt, M. Antimalarial Benzimidazole Derivatives Incorporating Phenolic Mannich Base Side Chains Inhibit Microtubule and Hemozoin Formation: Structure–Activity Relationship and in Vivo Oral Efficacy Studies. J. Med. Chem. 2021, 64, 5198–5215.
- Yadav, S.; Sinha, D.; Sanjay, K.; Singh, V.K. Novel Benzimidazole Analogs as Inhibitors of EGFR Tyrosine Kinase. Chem. Biol. Drug Des. 2012, 80, 625–630.
- Yoon, Y.K.; Ali, M.A.; Wei, A.C.; Choon, T.S.; Khaw, K.Y.; Murugaiyah, V.; Osman, H.; Masand, V.H. Synthesis, Characterization, and Molecular Docking Analysis of Novel Benzimidazole Derivatives as Cholinesterase Inhibitors. Bioorg. Chem. 2013, 49, 33–39.
- Zhu, J.; Wu, C.F.; Li, X.; Wu, G.S.; Xie, S.; Hu, Q.N.; Deng, Z.; Zhu, M.X.; Luo, H.R.; Hong, X. Synthesis, Biological Evaluation and Molecular Modeling of Substituted 2-Aminobenzimidazoles as Novel Inhibitors of Acetylcholinesterase and Butyrylcholinesterase. Bioorg. Med. Chem. 2013, 21, 4218–4224.
- Ceballos, L.; Virkel, G.; Elissondo, C.; Canton, C.; Canevari, J.; Murno, G.; Denegri, G.; Lanusse, C.; Alvarez, L. A Pharmacology-Based Comparison of the Activity of Albendazole and Flubendazole against Echinococcus Granulosus Metacestode in Sheep. Acta Trop. 2013, 127, 216–225.
- Pérez-Villanueva, J.; Hernández-Campos, A.; Yépez-Mulia, L.; Méndez-Cuesta, C.; Méndez-Lucio, O.; Hernández-Luis, F.; Castillo, R. Synthesis and antiprotozoal activity of novel 2-{sulfanyl}-1H-benzimidazole derivatives. Bioorganic Med. Chem. Lett. 2013, 23, 4221–4224.
- Narasimhan, B.; Sharma, D.; Kumar, P. Benzimidazole: A Medicinally Important Heterocyclic Moiety. Med. Chem. Res. 2012, 21, 269–283.
More
