Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Alfred Zheng and Version 1 by Zhenna Chen.
Selenium plays important roles in human health, but both selenium deficiency and excess can cause severe harm to humans and animals. Studies have shown that the effects of selenium supplementation depend on its dosage and species. Therefore, it is important to maintain a balanced and scientifical selenium supplementation. In recent years, natural and artificial selenium-enriched foods have been developed as the major sources of dietary selenium supplementation, and various selenium-enriched foods have been demonstrated to exhibit different physiological functions in vitro and in vivo, including antioxidant, anticancer, and anti-inflammatory effects, etc.
- selenium
- selenium-enriched foods
1. Introduction
Selenium is an essential micronutrient and plays important roles in the normal physiological activities of a living organism [1]. In human organisms, selenium is metabolized as 25 identified selenoproteins, which have various biological functions such as antioxidant and anticancer effects, and improvement of fertility and reproduction [2]. Recent studies have shown that selenium deficiency can lead to various chronic diseases [3,4,5][3][4][5], and approximately one billion people are suffering from selenium deficiency [6]. Therefore, a daily intake of selenium is recommended to maintain human health [7]. It is worth noting that the bioactivity and bioavailability of selenium in humans depend on its chemical species, which include inorganic selenium (e.g., Se(VI) and Se(IV)) and organic selenium (e.g., methylselenocysteine (MeSeCys), selenocysteine (SeCys), selenocystine (SeCys2), and selenomethionine (SeMet)) [8]. Organic selenium is less toxic, and has greater bioactivity and higher bioavailability [8]. To address selenium deficiency, selenium-enriched foods containing organic selenium have been widely developed in recent years. Selenium-enriched foods mainly include selenium-enriched plants [9], animals [10], and microorganisms [11]. Numerous studies have demonstrated the health benefits of selenium-enriched foods in overcoming selenium deficiency [12,13][12][13]. Additionally, to fully establish the links between health benefits and specific selenium species, various analytical techniques have also been established for the analysis of total selenium content and its species in selenium-enriched foods [14].
2. Selenium and Human Health
Selenium, as one of the essential trace elements, significantly impacts the normal physiological metabolism of humans. It is taken in and accumulated in the human body through the daily diet.
2.1. Physiological Role of Selenium
Selenium, as an essential trace element, has a high nutritional value in the human body. Due to the low selenium content in the food chain, selenium deficiency can lead to various diseases such as Keshan disease [3], Kashin–Beck disease [3], myocardial infarction [4], Alzheimer’s disease [5], and chronic pancreatitis [1]. Conversely, excessive selenium intake also can lead to toxicity, resulting in symptoms like hair loss and skin lesions [15]. Recent studies have also shown that an excess of selenium may also cause type 2 diabetes [16,17][16][17] and serious intestinal diseases [18]. In extreme cases, acute poisoning can lead to heart attack, kidney failure, and even death [19]. As can be seen, both selenium deficiency and excess have negative effects on human health. The recommended dietary allowance for selenium intake depends on certain parameters like age, pregnancy, and breastfeeding.
Within a narrow nutritional concentration range, selenium exhibits various biological activities in humans, including antioxidant and anticancer effects, detoxification, and others [21,22][20][21]. For example, selenium can act as an antioxidant by being synthesized into glutathione peroxidase (GPxs), which helps scavenge free radicals and protect cell membranes [23,24][22][23]. As an anticancer agent, selenium can be metabolized into selenocysteine, which inhibits protein synthesis, thereby suppressing cancer cell proliferation and causing cancer cell apoptosis [25][24]. Selenium, as a negatively charged nonmetallic ion, can also interact with positively charged metal ions (e.g., cadmium ions and mercury ions) to form metal–selenium–protein complexes, reducing the toxicity of metals [26][25]. Additionally, selenium can improve immunity by enhancing the bactericidal ability of macrophages [27][26]. Selenium has also been found to improve male fertility by increasing sperm concentration, motility, and seminal antioxidant capacity [28][27]. Lastly, a selenium-sufficient diet can prevent or treat some diseases such as cardiovascular and cerebrovascular diseases, Keshan disease, Kashin–Beck disease, and liver diseases [29][28].
2.2. Metabolism of Selenium
The biological functions of selenium are primarily achieved through its metabolism into 25 selenoproteins in an organism [30][29]. The biological activity of selenium in foods depends upon its chemical species [8]. Generally, the existing species of selenium in foods are mainly divided into inorganic selenium (e.g., Se(IV) and Se(VI)) and organic selenium (e.g., SeCys2, MeSeCys, SeMet, and SeCys). Figure 1 shows the metabolism of different selenium species in organisms. Inorganic selenium, such as Se(IV) and Se(VI), is absorbed via passive diffusion and co-transport [31][30], while organic selenium with a higher absorption rate is absorbed via the active absorption pathway of amino acid [32][31]. In organisms, Se(VI) is reduced to Se(IV), which is further reduced into selenodiglutathione (GSSeSG), selenenylsulfide (GSSeH), and hydrogen selenide (H2Se) [31][30]. SeMet participates in the synthesis of selenium protein instead of methionine or is metabolized to SeCys via trans-sulfurization [33][32]. SeCys2 is reduced to SeCys by glutathione and glutathione reductase, and SeCys is converted to H2Se under the action of selenocysteine β-lyase [33,34][32][33]. In addition, MeSeCys is cleaved to methylselenol by cystathionine β-lyase, and further converted to H2Se under the demethylation reaction of methylselenol demethylase [35][34]. As can be seen, different selenium species are eventually metabolized to H2Se, which is involved in selenoprotein synthesis after activation to selenophosphoric acid [36][35]. The excess H2Se in tissues is further metabolized into methylselenol, dimethylselenide, and selenosugars, which are mainly excreted in urine and breath [33,34,35][32][33][34].
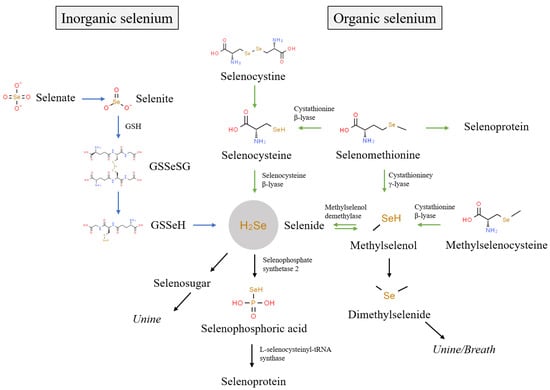
Figure 1.
Metabolic pathways of different selenium species in organism.
3. Physiological Functions of Selenium-Enriched Foods
Several researchers have revealed that selenium combined with other active nutrients such as zinc and vitamin E in food could initiate synergistic health effects on various biological activities [12]. Figure 2 illustrates the diverse physiological functions of selenium-enriched foods, including antioxidant, anti-inflammatory, and anticancer effects; detoxification; improvement of male fertility; and others [13].
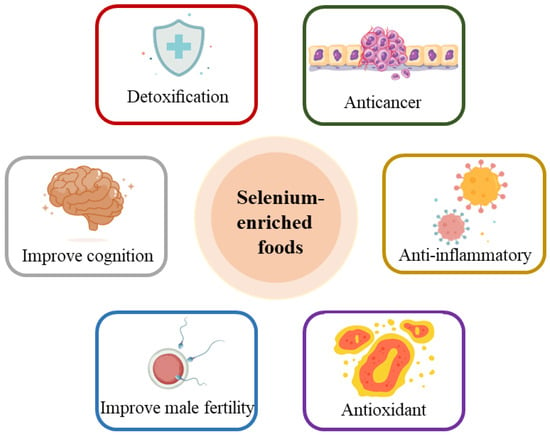
Figure 2.
Physiological functions of selenium-enriched foods.
3.1. The Function of Reducing Oxidative Stress
In recent years, a variety of selenium-enriched foods have been confirmed to exhibit antioxidant properties. For example, Ma et al. [66][36] prepared selenium-enriched polysaccharides from Pleurotus ostreatus using hot water extraction, and the in vitro study results indicated that selenium-enriched polysaccharides exhibited high antioxidant capacity and reduced hydrogen peroxide-induced oxidative stress in murine skeletal muscle cells. Guo et al. [67][37] discovered that selenium-enriched yeast protein hydrolysate reduced ultraviolet B radiation-induced oxidative stress by increasing glutathione peroxidase and catalase activities in vivo. Li et al. [68][38] investigated the antioxidant effect of selenium-enriched G. frondosa on cyclophosphamide-treated mice, and found that selenium-enriched G. frondosa displayed stronger antioxidant activity through the MAPKs signaling pathways. Furthermore, healthy women supplemented with selenium-enriched rice experienced an increase in serum selenium levels and GPx-activity [69][39].
3.2. The Function of Inhibiting Inflammation
At present, selenium-enriched foods have been found to exert anti-inflammatory effects, mainly through the NF-κB/MAPKs signaling pathway. Chomchan et al. [70][40] found that selenium-enriched ricegrass juice extracts promoted macrophage cell proliferation and reduced nitric oxide levels in LPS-induced RAW264.7 cells, and the foremost bioactive components were identified as flavone glycosides by UHPLC-MS. Similarly, RAW264.7 cell assay also indicated that both selenium-enriched brown rice protein hydrolysates [71][41] and selenium-enriched oolong tea extract [72][42] exhibited excellent anti-inflammatory functions via the NF-κB/MAPKs signaling pathway. Furthermore, selenium-enriched Cordyceps militaris exhibited an anti-inflammatory effect in LPS-injured mice by inhibiting pro-inflammatory mediator production and increasing anti-inflammatory cytokine levels [73][43].
3.3. The Function of Inhibiting Cancer
Numerous medical studies have demonstrated the excellent anticancer activity of selenium-enriched foods. For instance, Zhang et al. [13] found that selenium-enriched polysaccharide fraction obtained by Pleurotus ostreatus induced the apoptosis of various cancer cells by inhibiting the epithelial-to-mesenchymal transition, without a significant effect on normal cells. Luo et al. [74][44] confirmed that selenium-enriched Cordyceps militaris inhibited the viability of NCI-H292 and A549 cells, and induced cancer cell apoptosis by altering the expression of apoptotic and cell cycle regulatory proteins. Daniela et al. [75][45] evaluated selenium-enriched chickpea sprouts and found that they inhibited cancer tumor growth through the overexpression of Fas protein in vivo.
3.4. The Function of Alleviating the Toxicity of Heavy Metals
Studies have indicated that selenium-enriched foods can alleviate the biological toxicity of heavy metals such as cadmium, mercury, and lead. For instance, Su et al. [76][46] found that selenium-enriched rice significantly alleviated injury in mice with cadmium poisoning by overexpressing antioxidant genes (e.g., Nrf-2, GPX1, TrxR2, and TNF-2). Shang et al. [77][47] elucidated that selenium-enriched probiotics had a detoxification effect on brain injury caused by cadmium poisoning through MAPK, calcium, and PI3K-Akt signaling pathways. Additionally, Shang et al. [78][48] demonstrated that selenium-enriched Bacillus subtilis protected carp from mercury-induced inflammation, effectively reducing mercury toxicity. Zhu et al. [79][49] investigated that selenium-enriched rice protein hydrolysates reduced lead-induced cytotoxicity via slowing the accumulation of lead in cells.
3.5. The Function of Improving Male Fertility
The ability of selenium-enriched foods to improve male fertility have also been reported. At the cellular level, a mouse testicular cell assay indicated that selenium-enriched green tea inhibited the chromosomal aberrations induced by mitomycin C [80][50]. As for animal experiments, selenium-enriched probiotic supplementation alleviated the adverse effects of hyperlipidemia in male mice by reducing testicular tissue damage, increasing serumal testosterone levels, and improving sperm indexes [81][51]. Selenium-enriched yeast also exhibited a significant effect on the improvement of male fertility of roosters [82][52]. Additionally, selenium-enriched Spirulina observably protected the reproductive systems of male zebrafish exposed to Beta-cypermethrin by enhancing antioxidant enzyme activity and androgen secretion [83][53]. These findings suggest that selenium-enriched foods may have potential benefits for improving male fertility in humans.
3.6. Other Functions
In addition to the aforementioned effects, selenium-enriched foods have other physiological effects such as improving cognition, regulating the balance of intestinal bacteria, and protecting the liver. For example, Yu et al. [84][54] found that the crude polysaccharides prepared from selenium-enriched C. militaris had positive antiobesity and gut microbiota modulatory effects. Jia et al. [85][55] found that selenium-enriched radish sprouts improved the antioxidant capacity and alleviated liver damage in mice treated with carbon tetrachloride.
References
- Kieliszek, M. Selenium-fascinating microelement, properties and sources in food. Molecules 2019, 24, 1298.
- Schweizer, U.; Fradejas-Villar, N. Why 21? The significance of selenoproteins for human health revealed by inborn errors of metabolism. FASEB J. 2016, 30, 3669–3681.
- Hartikainen, H. Biogeochemistry of selenium and its impact on food chain quality and human health. J. Trace Elem. Med. Biol. 2005, 18, 309–318.
- Shahid, M.; Niazi, N.K.; Khalid, S.; Murtaza, B.; Bibi, I.; Rashid, M.I. A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ. Pollut. 2018, 234, 915–934.
- Pillai, R.; Uyehara-Lock, J.H.; Bellinger, F.P. Selenium and selenoprotein function in brain disorders. IUBMB Life 2014, 66, 229–239.
- Nothstein, A.K.; Eiche, E.; Riemann, M.; Nick, P.; Winkel, L.H.E.; Göttlicher, J.; Steininger, R.; Brendel, R.; Brasch, M.V.; Konrad, G.; et al. Tracking Se assimilation and speciation through the rice plant-nutrient competition, toxicity and distribution. PLoS ONE 2016, 26, e0152081.
- Wang, N.; Tan, H.; Li, S.; Xu, Y.; Guo, W.; Feng, Y.B. Supplementation of micronutrient selenium in metabolic diseases: Its role as an antioxidant. Oxidative Med. Cell. Longev. 2017, 2017, 7478523.
- Zhang, K.; Zhao, Q.Y.; Zhan, T.F.; Han, Y.S.; Tang, C.H.; Zhang, J.M. Effect of different selenium sources on growth performance, tissue selenium content, meat quality, and selenoprotein gene expression in finishing pigs. Biol. Trace Elem. Res. 2020, 196, 463–471.
- Ari, B.; Oz, E.; Can, S.Z.; Bakirdere, S. Bioaccessibility and bioavailability of selenium species in Se-enriched leeks (Allium Porrum) cultivated by hydroponically. Food Chem. 2022, 372, 131314.
- Zhang, K.; Guo, X.Q.; Zhao, Q.Y. Development and application of a HPLC-ICP-MS method to determine selenium speciation in muscle of pigs treated with different selenium supplements. Food Chem. 2020, 302, 125371.
- Wu, G.J.; Liu, F.; Sun, X.W.; Lin, X.G.; Zhan, F.; Fu, Z.H. Preparation of selenium-enriched yeast by re-using discarded saccharomyces cerevisiae from the beer industry for Se-supplemented fodder applications. Appl. Sci. 2019, 9, 3777.
- Tangjaidee, P.; Swedlund, P.; Xiang, J.; Yin, H.Q.; Quek, S.Y. Selenium-enriched plant foods: Selenium accumulation, speciation, and health functionality. Front. Nutr. 2022, 9, 962312.
- Zhang, Z.M.; Zhang, Y.S.; Liu, H.; Wang, J.H.; Wang, D.; Deng, Z.W.; Li, T.H.; He, Y.; Yang, Y.J.; Zhong, S.A. A water-soluble selenium-enriched polysaccharide produced by Pleurotus ostreatus: Purification, characterization, antioxidant and antitumor activities in vitro. Int. J. Biol. Macromol. 2021, 168, 356–370.
- Infante, H.J.; Hearn, R.; Catterick, T. Current mass spectrometry strategies for selenium speciation in dietary sources of high-selenium. Anal. Bioanal. Chem. 2005, 382, 957–967.
- Navarro-Alarcon, M.; Cabrera-Vique, C. Selenium in food and the human body: A review. Sci. Total Environ. 2008, 400, 115–141.
- Cardoso, B.R.; Braat, S.; Graham, R.M. Selenium status is associated with insulin resistance markers in adults: Findings from the 2013 to 2018 national health and nutrition examination survey (NHANES). Front. Nutr. 2021, 8, 696024.
- Casanova, P.; Monleon, D. Role of selenium in type 2 diabetes, insulin resistance and insulin secretion. World J. Diabetes 2023, 14, 147–158.
- Tortelly, V.C.; Melo, D.F.; Matsunaga, A.M. The relevance of selenium to alopecias. Int. J. Trichol. 2018, 10, 92–93.
- Vinceti, M.; Mandrioli, J.; Borella, P.; Michalke, B.; Tsatsakis, A.; Finkelstein, Y. Selenium neurotoxicity in humans: Bridging laboratory and epidemiologic studies. Toxicol. Lett. 2014, 230, 295–303.
- Brigelius-Flohe, R.; Arner, E.S.J. Selenium and selenoproteins in (redox) signaling, diseases, and animal models-200 year anniversary issue. Free Radic. Biol. Med. 2018, 127, 1–2.
- Wang, L.; Sagada, G.; Wang, R.L.; Li, P.W.; Xu, B.Y.; Zhang, C.; Qiao, J.L.; Yan, Y.Z. Different forms of selenium supplementation in fish feed: The bioavailability, nutritional functions, and potential toxicity. Aquaculture 2022, 549, 737819.
- Zhang, Y.; Roh, Y.J.; Han, S.J.; Park, I.; Lee, H.M.; Ok, Y.S.; Lee, B.C.; Lee, S.R. Role of selenoproteins in redox regulation of signaling and the antioxidant system: A review. Antioxidants 2020, 9, 383.
- do Nascimento da Silva, E.; Aureli, F.; Amato, M.; Raggi, A.; Cadore, S.; Cubadda, F. Selenium bioaccessibility and speciation in selenium-enriched lettuce: Investigation of the selenocompounds liberated after in vitro simulated human digestion using two-dimensional HPLC-ICP-MS. J. Agric. Food Chem. 2017, 65, 3031–3038.
- Gangadoo, S.; Stanley, D.; Hughes, R.J.; Moore, R.J.; Chapman, J. The synthesis and characterisation of highly stable and reproducible selenium nanoparticles. Inorg. Nano-Met. Chem. 2017, 47, 1568–1576.
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Exogenous selenium pretreatment protects rapeseed seedlings from cadmium-induced oxidative stress by upregulating antioxidant defense and methylglyoxal detoxification systems. Biol. Trace Elem. Res. 2012, 149, 248–261.
- Kursvietiene, L.; Mongirdiene, A.; Bernatoniene, J.; Sulinskiene, J.; Staneviciene, I. Selenium anticancer properties and impact on cellular redox status. Antioxidants 2020, 9, 80.
- Ghafarizadeh, A.A.; Vaezi, G.; Shariatzadeh, M.A.; Malekirad, A.A. Effect of in vitro selenium supplementation on sperm quality in asthenoteratozoospermic men. Aadrologia 2018, 50, e12869.
- Han, M.Q.; Liu, K.L. Selenium and selenoproteins: Their function and development of selenium-rich foods. Int. J. Food Sci. Technol. 2022, 57, 7026–7037.
- Nicholson, J.L.; Toh, P.; Alfulaij, N.; Berry, M.J.; Torres, D.J. New insights on selenoproteins and neuronal function. Free Radic. Biol. Med. 2022, 190, 55–61.
- Thiry, C.; Ruttens, A.; Pussemier, L.; Schneider, Y.J. An in vitro investigation of species-dependent intestinal transport of selenium and the impact of this process on selenium bioavailability. Br. J. Nutr. 2013, 109, 2126–2134.
- Longchamp, M.; Castrec-Rouelle, M.; Biron, P.; Bariac, T. Variations in the accumulation, localization and rate of metabolization of selenium in mature Zea mays plants supplied with selenite or selenate. Food Chem. 2015, 182, 128–135.
- Schrauzer, G. The nutritional significance, metabolism and toxicology of selenomethionine. Adv. Food Nutr. Res. 2003, 47, 73–112.
- Weekley, C.M.; Harris, H.H. Which form is that? The importance of selenium speciation and metabolism in the prevention and treatment of disease. Chem. Soc. Rev. 2013, 42, 8870.
- Mojadadi, A.; Au, A.; Salah, W.; Witting, P.; Ahmad, G. Role for selenium in metabolic homeostasis and human reproduction. Nutrients 2021, 13, 3256.
- Lu, J.; Zhang, J.; Jiang, C.; Deng, Y.; Ozten, N.; Bosland, M.C. Cancer chemoprevention research with selenium in the post-SELECT era: Promises and challenges. Nutr. Cancer 2016, 68, 1–17.
- Ma, L.; Zhao, Y.; Yu, J.; Ji, H.Y.; Liu, A.J. Characterization of se-enriched Pleurotus ostreatus polysaccharides and their antioxidant effects in vitro. Int. J. Biol. Macromol. 2018, 111, 421–429.
- Guo, H.K.; Guo, S.Y.; Liu, H.M. Antioxidant activity and inhibition of ultraviolet radiation-induced skin damage of Selenium-rich peptide fraction from selenium-rich yeast protein hydrolysate. Bioorg. Chem. 2020, 105, 104431.
- Li, Q.; Chen, G.; Chen, H.; Zhang, W.J.; Ding, Y.Y.; Yu, P.; Zhao, T.; Mao, G.H.; Feng, W.W.; Yang, L.Q.; et al. Se-enriched G. frondosa polysaccharide protects against immunosuppression in cyclophosphamide-induced mice via MAPKs signal transduction pathway. Carbohydr. Polym. 2018, 196, 445–456.
- Giacosa, A.; Faliva, M.A.; Perna, S.; Minoia, C.; Ronchi, A.; Rondanelli, M. Selenium fortification of an Italian rice cultivar via foliar fertilization with sodium selenate and its effects on human serum selenium levels and on erythrocyte glutathione peroxidase activity. Nutrients 2014, 6, 1251–1261.
- Chomchan, R.; Puttarak, P.; Brantner, A.; Siripongvutikorn, S. Selenium-rich ricegrass juice improves antioxidant properties and nitric oxide inhibition in macrophage cells. Antioxidants 2018, 7, 57.
- Feng, M.J.; Wang, X.Y.; Xiong, H.; Qiu, T.T.; Zhang, H.; Guo, F.H.; Jiang, L.; Sun, Y. Anti-inflammatory effects of three selenium-enriched brown rice protein hydrolysates in LPS-induced RAW264.7 macro phages via NF-kB/MAPKs signaling pathways. J. Funct. Foods 2021, 76, 104320.
- Wang, Q.; Huang, J.Q.; Zheng, Y.F.; Guan, X.F.; Lai, C.C.; Gao, H.Y.; Ho, C.T.; Lin, B. Selenium enriched oolong tea (Camellia sinensis) extract exerts anti inflammatory potential via targeting NF-kB and MAPK pathways in macrophages. Food Sci. Hum. Well. 2022, 11, 635–642.
- Wu, S.J.; Wu, Q.P.; Wang, J.; Li, Y.F.; Chen, B.; Zhu, Z.J.; Huang, R.; Chen, M.F.; Huang, A.H.; Xie, Y.Z.; et al. Novel selenium peptides obtained from selenium-enriched cordyceps militaris alleviate neuroinflammation and gut microbiota dysbacteriosis in LPS-injured mice. J. Agric. Food Chem. 2022, 70, 3179–3206.
- Luo, L.; Ran, R.; Yao, J.; Zhang, F.; Xing, M.H.; Jin, M.; Wang, L.Q.; Zhang, T. Se-enriched cordyceps militaris inhibits cell proliferation, induces cell apoptosis, and causes G2/M phase arrest in human non-small cell lung cancer cells. Oncotargets Ther. 2019, 12, 8751–8763.
- Guardado-Felix, D.; Antunes-Ricardo, M.; Rocha-Pizana, M.R.; Martinez-Torres, A.C.; Gutierrez-Uribe, J.A.; Saldivar, S. Chickpea (Cicer arietinum L.) sprouts containing supranutritional levels of selenium decrease tumor growth of colon cancer cells xenografted in immune-suppressed mice. J. Funct. Foods 2019, 53, 76–84.
- Su, Y.; Li, L.; Farooq, M.U.; Huang, X.; Zheng, T.D.; Zhang, Y.J.; Ei, H.H.; Panhwar, F.H.; Tang, Z.C.; Zeng, R.; et al. Rescue effects of Se-enriched rice on physiological and biochemical characteristics in cadmium poisoning mice. Environ. Sci. Pollut. Res. 2021, 28, 20023–20033.
- Shang, X.; Geng, L.; Zhao, Z.; Luo, L.; Shi, X.D.; Zhang, Q.; Du, R.J.; Cong, T.F.; Xu, W. Transcriptomics reveals the mechanism of selenium-enriched Lactobacillus plantarum alleviating brain oxidative stress under cadmium stress in Luciobarbus capito. Ecotoxicol. Environ. Saf. 2022, 242, 113890.
- Shang, X.C.; Wang, B.; Sun, Q.S.; Zhang, Y.; Lu, Y.T.; Liu, S.J.; Li, Y.H. Selenium-enriched Bacillus subtilis reduces the effects of mercury-induced on inflammation and intestinal microbes in carp (Cyprinus carpio var. specularis). Fish Physiol. Biochem. 2022, 48, 215–226.
- Zhu, Y.Q.; Ding, J.; Shi, Y.; Fang, Y.; Li, P.; Fan, F.J.; Wu, J.; Hu, Q.H. Deciphering the role of selenium-enriched rice protein hydrolysates in the regulation of Pb2+-induced cytotoxicity: Anin vitroCaco-2 cell model study. Int. J. Food Sci. Technol. 2021, 56, 420–428.
- Li, F.; Xu, J.; Zhou, J.; Zhao, L.Y.; Sheng, J.C.; Sun, G.J.; Hu, Q.H. Inhibition of mitomycin C-induced chromosomal aberrations by micrometer powder of selenium-enriched green tea in mice spermatocytes. Mutat. Res. Genet. Toxicol. Environ. 2009, 675, 11–16.
- Ibrahim, H.; Zhu, Y.; Wu, C.; Lu, C.H.; Ezekwe, M.O.; Liao, S.F.; Haung, K.H. Selenium-enriched probiotics improves murine male fertility compromised by high fat diet. Biol. Trace Elem. Res. 2012, 147, 251–260.
- Razieh, S.; Ahmad, Z.; Mahdi, Z.; Yousefi, A.R.; Rafieian, H.R. Effects of dietary supplementation of different sources and levels of selenium on the semen quality and reproductive performance in aged broiler breeder roosters. Poultry Sci. 2022, 101, 101908.
- Zhang, Y.; Zhou, Y.; Tang, Q.; Hu, F.; Feng, L.X.; Shen, J.L.; Huang, B. The protective effects of selenium-enriched spirulina on the reproductive system of male zebrafish (Danio rerio) exposed to beta-cypermethrin. Food Funct. 2018, 9, 5791–5804.
- Yu, M.; Yue, J.; Hui, N.; Zhi, Y.E.; Hayat, K.; Yang, X.J.; Zhang, D.; Chu, S.H.; Zhou, P. Anti-hyperlipidemia and gut microbiota community regulation effects of selenium-rich Cordyceps militaris Polysaccharides on the high-fat diet-fed mice model. Foods 2021, 10, 2252.
- Jia, L.; Wang, T.; Sun, Y.; Zhang, M.R.; Tian, J.Y.; Chen, H.; Shen, Z.G.; Abro, H.K.; Su, N.N.; Cui, J. Protective effect of selenium-enriched red radish sprouts on carbon tetrachloride-induced liver injury in mice. J. Food Sci. 2019, 84, 3027–3036.
More
