Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Stela Jokic.
Citrus fruits processing results in the generation of huge amounts of citrus by-products, mainly peels, pulp, membranes, and seeds. Although they represent a major concern from both economical and environmental aspects, it is very important to emphasize that these by-products contain a rich source of value-added bioactive compounds with a wide spectrum of applications in the food, cosmetic, and pharmaceutical industries.
- citrus by-products
- citrus anatomy
- health benefits
- bioactive compounds
1. Introduction
Citrus fruits—more precisely, the genus Citrus L., which belongs to the subfamily Aurantioideae in the family Rutaceae—represent the major fruit crops commercially cultivated worldwide [1,2][1][2]. These fruits are widely known for their high health-benefiting properties, which is of great importance, since citrus fruits are the most widely consumed fruits globally [3,4,5,6][3][4][5][6]. The cultivation of Citrus genus includes the species such as lemon (C. limon (L.) Osbeck), sweet orange (C. sinensis (L.) Osbeck), mandarin (C. reticulata Blanco), grapefruit (C. paradisi Macfad.), pomelo (C. maxima (Burm.) Merr.), citron (C. medica L.), lime (C. aurantiifolia (Christm.) Swingle), and bergamot (C. × bergamia Risso & Poiteau) [4,5,7,8,9][4][5][7][8][9]. Although a significant part of the industrially processed citrus fruits is used to produce essential oils and juice, citrus-based candies, jellies, and extracts production also represent key factors for the food industry [5]. A large quantity of waste and by-products yearly produced during citrus processing has become a fundamental concern from both economical and environmental aspects. Citrus processing generates over 15 million tons of residue, mostly in the form of peels, seeds, and membranes [10,11][10][11]. Therefore, citrus waste valorization and proper industrial waste management are highly encouraged, being also a high-research priority topic for the scientific community recently. There have been many scientific reports related to the potential and beneficial utilization of industrial by-products, mainly dealing with innovative extraction methods for obtaining extracts rich in bioactive compounds [1,2,3,4,10,11,12][1][2][3][4][10][11][12]. These extracts have shown versatile health beneficial activities, due to the high content of biologically active compounds naturally present in the extracts [13,14][13][14]. Numerous studies have considered citrus extracts as a natural source of bioactive components exhibiting beneficial activities, including antioxidant [15,16,17[15][16][17][18][19],18,19], antibacterial [16,18[16][18][19][20][21],19,20,21], antidiabetic [17[17][22][23][24],22,23,24], neuroprotective [22,25[22][25][26][27],26,27], and anti-inflammatory [28,29,30,31][28][29][30][31] activities, as well as antitumor [32,33,34,35][32][33][34][35] potential. Also, citrus by-products are considered a valuable source of phytochemicals (such as d-limonene, essential oils, phenolic acids, carotenoids, vitamins, minerals, and flavonoids), which, isolated or in the form of mixtures/extracts, could exhibit versatile biological activities especially beneficial for the food industry [36,37,38][36][37][38]. Citrus-based essential oils exhibit significant antimicrobial activity against foodborne bacteria and also antioxidant activity to prevent the effects of oxidation; hence, citrus-based essential oils could act as natural preservatives [39,40,41][39][40][41].
2. Structural and Chemical Characteristics of Citrus Fruits By-Products
Citrus fruits, like many other agricultural products, are characterized by their agricultural biodiversity [62][42]. Their physicochemical characteristics, as well as a diversity of chemical compounds, depend on a variety of factors and environmental conditions, such as soil, fertilization, age, position on the tree, maturity, and others [62,63,64,65][42][43][44][45]. It is interesting that all varieties of citrus fruits, by means of microscopic and macroscopic views, have similar structural and anatomical characteristics. A schematic view of the anatomical characteristics and structural compositions of citrus fruits is presented in Figure 1.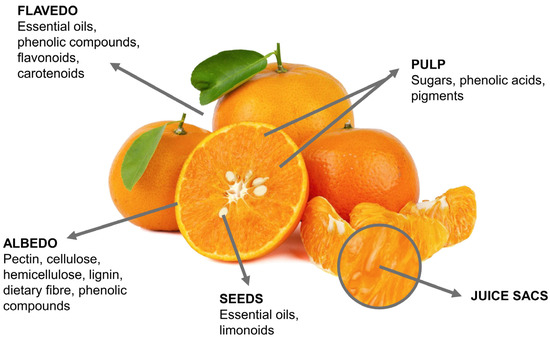
Figure 1.
Anatomical and structural characteristics of citrus fruits.
3. Converting Waste into Treasure—Utilization of Citrus By-Products
Recently, waste management has become one of the great concerns globally, and its valorization has created more sustainable and smart waste management solutions. Primarily, waste valorization includes employing different technologies toward obtaining value-added products with a wide spectrum of potential applications. Citrus by-products have been extensively studied due to their rich-bioactive properties, and their valorization enables beneficial gains from both economical and environmental points of view [96,97][67][68]. The scientific focus has been placed on innovative extraction methods for obtaining high-quality citrus essential oils [98,99,100,101][69][70][71][72] and enriched extracts in general [11,94,102,103][11][73][74][75]. Solid citrus waste can be also utilized for the production of animal food. Due to its good nutritional composition containing dietary fibers, lipids, flavonoids, enzymes, vitamins, and carotenoids, citrus waste represents a promising by-product for the production of livestock feeds [105][76]. The literature reports that citrus pulp (the main residue after juice extraction), citrus molasses (produced by concentrating on the press liquor of citrus peel residue with a high content of sugars), citrus peel liquor (similar to molasses but not as concentrated), and citrus-activated sludge (produced from liquid waste) could be considered as by-product feedstuffs [106][77]. The nutrient content of citrus by-products mainly depends on the source and variety of citrus fruits, as well as on the type of processing [88][78]. The main issues in the utilization of citrus by-products for the production of feedstuffs are the low nitrogen content and poor storage, which can lead to the development of mycotoxins [88][78]. Another valuable utilization of citrus waste is the production of packaging films that meets all the standards of sustainable and biodegradable forms of biopolymers [10,107][10][79]. Conventional packaging films are considered an environmental concern due to their poor biodegradable properties, and therefore, new innovative and sustainable solutions are welcomed [107][79]. An important advantage of using biopolymers derived from plant materials is that those raw materials naturally contain significant amounts of bioactive components that exhibit antioxidant and antimicrobial properties. The matrix of the citrus-based package is pectin, which enables solid support for the production of active packaging films [10]. The application of plant-based and phenolic extracts in the food industry is highly limited due to their poor bioaccessibility, low water, and liquid solubility, while it is well known that bioactive phenolic compounds are extremely sensitive to light, oxidants, and changes in pH conditions and temperatures [47,97,109][68][80][81]. The main challenge presents as overcoming the limiting incorporation of low water-soluble compounds into aqueous-based foods, which directly limits the proper gastrointestinal bioaccessibility. In order to overcome these issues, encapsulation has been employed more frequently to protect bioactive compounds [47,110,111][80][82][83]. The spray-drying and freeze-drying techniques are commonly used methods for obtaining stable encapsulated functional substances, while extrusion methods, coacervation, and emulsification methods have been also applied [110][82].4. Bioactivities of the Individual Groups of Compounds Present in Citrus By-Products
4.1. Waxes and Carotenoids
Cuticular wax plays an important role in fruit preservation and proper storage, and it is well known that it acts as a natural barrier that protects plants from biological and non-biological stress [73,74][54][55]. Also, the structural characteristics, content, and composition of cuticular wax have been found to affect the postharvest storage quality against fruit water loss and softening and could be responsible for the resistance to fruit diseases. Waxes are comprised of long-chain fatty acids and their derivates, esters, aldehydes, ketones, primary and secondary alcohols, and triterpenoids [115][84]. Most of the studies related to the topic of citrus cuticular waxes focused on the synthesis and transcriptional regulation of cuticular wax in citrus fruits. The investigation of the carotenoid content separated from C. reticulata by-products and its influence on the immuneoxidative status of broiler chickens was carried out by Mavrommatis et al. [118][85]. The carotenoid-rich extract was prepared, and the chickens were fed a supplemented diet consisting of a freeze-dried formulation containing carotenoid extract and soluble starch. It was demonstrated that carotenoid-supplemented feed exerted inhibitory activity against Gram-positive (Staphylococcus (S.) aureus), as well as Gram-negative (Klebsiella (K.) oxytoca, Escherichia (E.) coli, and Salmonella (S.) typhimurium), bacteria. The implementation of the carotenoid content in the supplementation led to alanine aminotransferase and breast muscle malondialdehyde, and the activity of superoxide dismutase increased. Also, several parameters were downregulated, such as catalase, NADPH oxidase 2, interleukin 1β, and tumor necrosis factor.4.2. Aromatic Compounds—Essential Oils
Citrus essential oils are known as a fragrant mixture of chemical compounds exhibiting versatile activities used in the food, cosmetical, and pharmaceutical industries, as well as in aromatherapy [79][60]. The involvement of nanotechnology has provided new solutions for developing essential oil-based nanosystems with the aim of bioaccessibility enhancement. Interestingly, the formulation of C. lemon essential oil in nanohexosomes was prepared by the group of authors Sedeek et al. [120][86] for the purpose of antifungal activity investigation. Firstly, C. lemon, C. aurantifolia, C. maxima, and C. sinensis essential oils were extracted using hydrodistillation in a Clevenger’s apparatus from powdered peels. The hexosomal dispersions loaded with oils were prepared by the hot emulsification method reported by Abdel-Bar et al. [121][87]. Furthermore, the antimicrobial efficacy of citrus-based essential oils against foodborne pathogen Listeria monocytogenes (L. monocytogenes) was demonstrated in the study by Guo et al. [126][88]. Gram-positive bacteria L. monocytogenes is a highly adaptable pathogen that causes listeriosis, a life-threatening infection, and it is especially dangerous if the central nervous system is affected [141][89]. The essential oil from the C. Changshan-huyou Y.B. Chang (Huyou) species was extracted from peels by the steam distillation procedure using water as the solvent. The results of the antimicrobial activity of Huyou essential oil against L. monocytogenes showed dose-dependant antimicrobial activity when comparing treatments of pathogens with the 1xMIC (minimum inhibitory concentration), 0.25xMIC, and 0.125 MIC. The study also discussed the changes in the physical morphology of L. monocytogenes biofilms when treated with 1xMIC for 8, 16, and 24 h by using scanning electron microscopy (SEM) and confocal laser scanning microscopy (CLSM) analyses.4.3. Pectins
The importance of pectins in the food industry is widely known; however, this group of polysaccharides has found their place in a variety of human applications, such as in the pharmaceuticals, cosmetics, drug delivery, and biomedical fields [86,141,142][64][89][90]. The versatile application of pectic biopolymers is enabled due to their structural diversity and chemical complexities, as well as the possibility of structural modifications [143][91]. In addition, Zhang et al. [145][92] obtained pectin oligosaccharide fractions by the controlled degradation of citrus peel pectin. Three different oligosaccharides were prepared by adjusting the concentration of trifluoroacetic acid or H2O2 at the appropriate pH value, producing pectin oligosaccharides of variable molecular weight ranging from <2000 Da, 2000 to 3000 Da, and 3000 to 4000 Da. The results demonstrated a high prebiotic activity (pectic oligosaccharides obtained by H2O2 oxidation; 3543 Da) for Bifidobacterium (B.) bifidum and moderate activity against the Lactobacillus (L.) paracasei bacterial species. Thise study showed the enormous prebiotic potential of citrus-based pectic oligosaccharides; however, the greatest challenge remains to be overcome, as the human gastrointestinal tract includes complex pH-dependent processes and the presence of different enzymes that could affect in vivo digestion and bioaccessibility.4.4. Phenolic Compounds
Natural phenolic compounds have been studied extensively for their essential role in plant protection, as well as for their beneficial effects on human health. It is well known that citrus by-products contain substantial contents of different phenolic compounds in the forms of acids and flavonoids, which have recently become the great subject of studies as natural antioxidants [156,157][93][94]. The representative bioactive compounds for the citrus family are flavanone aglycones (hesperetin, naringenin, and eriodictyol); flavone and flavonol aglycones (kaempferol, quercetin, apigenin, and diosmetin); flavanone-7-O-glycosides (eriocitrin, hesperidin, naringin, narirutin, poncirin, and didymin); and polymethoxyflavones (PMFs; nobiletin, tangeretin, and sinensetin) [62,91,158][42][66][95].5. Citrus By-Products Formulations with Enhanced Bioactivities
The biomass-derived compounds are known for their health-promoting properties, and there is a rising trend in waste and by-product valorization to obtain value-added products with a wide spectrum of applications [2,11,194,195][2][11][96][97]. The beneficial effects of different citrus by-products on human health are not disputable; however, poor bioaccessibility is a crucial and limiting factor for successful in vivo applications. Therefore, new and innovative ideas with the implementation of nanotechnology brought about some new solutions in bioaccessibility and bioactivity enhancements. The preparation of silver nanoparticles (AgNPs) from citrus (C. tangerina, C. sinensis, and C. limon) peel extract was reported by Niluxsshun et al. [196][98]. Firstly, citrus peel extracts were prepared by boiling peels in hot water, and afterward, a solution of AgNO3 was added to the flask when a golden colloidal suspension was formed. The structural and morphological analyses confirmed the presence of AgNPs in sizes of 10–70 nm, containing different morphological characteristics of nanoparticles. The presence of natural antioxidants, flavonoids, phenolic acids, and other phenolic compounds could act as a reducing agent, leading to the formation of silver nanoparticles. The AgNPs were investigated for antimicrobial activity against the Gram-negative bacteria E. coli and the Gram-positive bacteria S. aureus, and the results showed the superior antimicrobial activity of orange-based AgNPs on both bacteria strains. Also, it is expected that the bioactivity of nanoparticles is dose- and size-dependent [197][99], and it is assumed that silver potentially interacts with thiol groups of proteins on cell membranes, causing respiration blocking, which leads to cell death. Another example of AgNP synthesis by using citrus by-products for the purposes of antimicrobial investigation was reported by Alkhulaifi et al. [198][100]. In thise study, C. limon peels were used for the synthesis of AgNPs, which were formed by the addition of a AgNO3 solution. Again, a possible explanation for the AgNPs formation was the reduction of Ag+ ions to silver nanoparticles in the presence of phenolic compounds, and the AgNPs demonstrated spherical- and rod-like-shaped morphologies. The antimicrobial activity investigation was carried out on Acinetobacter (A.) baumannii, S. typhimurium, E. coli, Pseudomonas (P.) aeruginosa, S. aureus, and Proteus (P.) vulgaris human pathogenic bacteria. The results indicated the good performance of AgNPs against the Gram-negative (E. coli, S. typhimurium, and P. aeruginosa) and Gram-positive (S. aureus) bacteria.References
- Khan, U.M.; Sameen, A.; Aadil, R.M.; Shahid, M.; Sezen, S.; Zarrabi, A.; Ozdemir, B.; Sevindik, M.; Kaplan, D.N.; Selamoglu, Z.; et al. Citrus Genus and Its Waste Utilization: A Review on Health-Promoting Activities and Industrial Application. Evid.-Based Complement. Altern. Med. 2021, 2021, 2488804.
- Lv, X.; Zhao, S.; Ning, Z.; Zeng, H.; Shu, Y.; Tao, O.; Xiao, C.; Lu, C.; Liu, Y. Citrus Fruits as a Treasure Trove of Active Natural Metabolites That Potentially Provide Benefits for Human Health. Chem. Cent. J. 2015, 9, 68.
- Liu, S.; Lou, Y.; Li, Y.; Zhang, J.; Li, P.; Yang, B.; Gu, Q. Review of Phytochemical and Nutritional Characteristics and Food Applications of Citrus L. Fruits. Front. Nutr. 2022, 9, 968604.
- Russo, C.; Maugeri, A.; Lombardo, G.E.; Musumeci, L.; Barreca, D.; Rapisarda, A.; Cirmi, S.; Navarra, M. The Second Life of Citrus Fruit Waste: A Valuable Source of Bioactive Compounds. Molecules 2021, 26, 5991.
- Ben Hsouna, A.; Sadaka, C.; Generalić Mekinić, I.; Garzoli, S.; Švarc-Gajić, J.; Rodrigues, F.; Morais, S.; Moreira, M.M.; Ferreira, E.; Spigno, G.; et al. The Chemical Variability, Nutraceutical Value, and Food-Industry and Cosmetic Applications of Citrus Plants: A Critical Review. Antioxidants 2023, 12, 481.
- Rao, M.J.; Zuo, H.; Xu, Q. Genomic Insights into Citrus Domestication and Its Important Agronomic Traits. Plant Commun. 2021, 2, 100138.
- Goh, R.M.V.; Pua, A.; Luro, F.; Ee, K.H.; Huang, Y.; Marchi, E.; Liu, S.Q.; Lassabliere, B.; Yu, B. Distinguishing Citrus Varieties Based on Genetic and Compositional Analyses. PLoS ONE 2022, 17, e0267007.
- Conti, G.; Xoconostle-Cázares, B.; Marcelino-Pérez, G.; Hopp, H.E.; Reyes, C.A. Citrus Genetic Transformation: An Overview of the Current Strategies and Insights on the New Emerging Technologies. Front. Plant Sci. 2021, 12, 768197.
- Wu, G.A.; Terol, J.; Ibanez, V.; López-García, A.; Pérez-Román, E.; Borredá, C.; Domingo, C.; Tadeo, F.R.; Carbonell-Caballero, J.; Alonso, R.; et al. Genomics of the Origin and Evolution of Citrus. Nature 2018, 554, 311–316.
- Suri, S.; Singh, A.; Nema, P.K. Current Applications of Citrus Fruit Processing Waste: A Scientific Outlook. Appl. Food Res. 2022, 2, 100050.
- Maqbool, Z.; Khalid, W.; Atiq, H.T.; Koraqi, H.; Javaid, Z.; Alhag, S.K.; Al-Shuraym, L.A.; Bader, D.M.D.; Almarzuq, M.; Afifi, M.; et al. Citrus Waste as Source of Bioactive Compounds: Extraction and Utilization in Health and Food Industry. Molecules 2023, 28, 1636.
- Hussien Abou Baker, D.; Ahmed Ibrahim, E.; Abd El-Rhaman Salama, Z. Citrus Peels as a Source of Bioactive Compounds with Industrial and Therapeutic Applications. In Biochemistry; Badria, F.A., Ed.; IntechOpen: London, UK, 2022; Volume 26, ISBN 978-1-83969-346-5.
- Li, P.; Yao, X.; Zhou, Q.; Meng, X.; Zhou, T.; Gu, Q. Citrus Peel Flavonoid Extracts: Health-Beneficial Bioactivities and Regulation of Intestinal Microecology in Vitro. Front. Nutr. 2022, 9, 888745.
- Matsuzaki, K.; Nakajima, A.; Guo, Y.; Ohizumi, Y. A Narrative Review of the Effects of Citrus Peels and Extracts on Human Brain Health and Metabolism. Nutrients 2022, 14, 1847.
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic Composition, Antioxidant Potential and Health Benefits of Citrus Peel. Food Res. Int. 2020, 132, 109114.
- Guo, C.; Shan, Y.; Yang, Z.; Zhang, L.; Ling, W.; Liang, Y.; Ouyang, Z.; Zhong, B.; Zhang, J. Chemical Composition, Antioxidant, Antibacterial, and Tyrosinase Inhibition Activity of Extracts from Newhall Navel Orange (Citrus sinensis Osbeck Cv. Newhall) Peel. J. Sci. Food Agric. 2020, 100, 2664–2674.
- Ali, A.M.; Gabbar, M.A.; Abdel-Twab, S.M.; Fahmy, E.M.; Ebaid, H.; Alhazza, I.M.; Ahmed, O.M. Antidiabetic Potency, Antioxidant Effects, and Mode of Actions of Citrus reticulata Fruit Peel Hydroethanolic Extract, Hesperidin, and Quercetin in Nicotinamide/Streptozotocin-Induced Wistar Diabetic Rats. Oxidative Med. Cell. Longev. 2020, 2020, 1730492.
- Lin, X.; Cao, S.; Sun, J.; Lu, D.; Zhong, B.; Chun, J. The Chemical Compositions, and Antibacterial and Antioxidant Activities of Four Types of Citrus Essential Oils. Molecules 2021, 26, 3412.
- Shehata, M.G.; Awad, T.S.; Asker, D.; El Sohaimy, S.A.; Abd El- Aziz, N.M.; Youssef, M.M. Antioxidant and Antimicrobial Activities and UPLC-ESI-MS/MS Polyphenolic Profile of Sweet Orange Peel Extracts. Curr. Res. Food Sci. 2021, 4, 326–335.
- Hou, H.-S.; Bonku, E.M.; Zhai, R.; Zeng, R.; Hou, Y.-L.; Yang, Z.-H.; Quan, C. Extraction of Essential Oil from Citrus Reticulate Blanco Peel and Its Antibacterial Activity against Cutibacterium acnes (Formerly Propionibacterium acnes). Heliyon 2019, 5, e02947.
- Degirmenci, H.; Erkurt, H. Chemical Profile and Antioxidant Potency of Citrus aurantium L. Flower Extracts with Antibacterial Effect against Foodborne Pathogens in Rice Pudding. LWT 2020, 126, 109273.
- Duletić-Laušević, S.; Oalđe, M.; Alimpić-Aradski, A. In vitro Evaluation of Antioxidant, Antineurodegenerative and Antidiabetic Activities of Ocimum basilicum L., Laurus nobilis L. Leaves and Citrus reticulata Blanco Peel Extracts. Lek. Sirovine 2019, 39, 60–68.
- Ani, P.N.; Ochu, K.E. Anti-Diabetic, Anti-Hyperlipidemic and Hepatoprotective Potential of Shaddock (Citrus maxima) Peel Extract. Acta Sci. Pol. Technol. Aliment. 2020, 19, 271–278.
- Benayad, O.; Bouhrim, M.; Tiji, S.; Kharchoufa, L.; Addi, M.; Drouet, S.; Hano, C.; Lorenzo, J.M.; Bendaha, H.; Bnouham, M.; et al. Phytochemical Profile, α-Glucosidase, and α-Amylase Inhibition Potential and Toxicity Evaluation of Extracts from Citrus aurantium (L) Peel, a Valuable By-Product from Northeastern Morocco. Biomolecules 2021, 11, 1555.
- Jang, A.; Choi, G.; Kim, Y.; Lee, G.; Hyun, K. Neuroprotective properties of ethanolic extract of Citrus unshiu Markovich peel through NADPH oxidase 2 inhibition in chemotherapy-induced neuropathic pain animal model. Phytother. Res. 2021, 35, 6918–6931.
- Furukawa, Y.; Okuyama, S.; Amakura, Y.; Sawamoto, A.; Nakajima, M.; Yoshimura, M.; Igase, M.; Fukuda, N.; Tamai, T.; Yoshida, T. Isolation and Characterization of Neuroprotective Components from Citrus Peel and Their Application as Functional Food. Chem. Pharm. Bull. 2021, 69, 2–10.
- Eddin, L.B.; Jha, N.K.; Meeran, M.F.N.; Kesari, K.K.; Beiram, R.; Ojha, S. Neuroprotective Potential of Limonene and Limonene Containing Natural Products. Molecules 2021, 26, 4535.
- Malik, A.; Najda, A.; Bains, A.; Nurzyńska-Wierdak, R.; Chawla, P. Characterization of Citrus nobilis Peel Methanolic Extract for Antioxidant, Antimicrobial, and Anti-Inflammatory Activity. Molecules 2021, 26, 4310.
- Lala, M.; Modak, D.; Paul, S.; Sarkar, I.; Dutta, A.; Kumar, A.; Bhattacharjee, S.; Sen, A. Potent Bioactive Methanolic Extract of Wild Orange (Citrus macroptera Mont.) Shows Antioxidative, Anti-Inflammatory, and Antimicrobial Properties in in Vitro, in Vivo, and in Silico Studies. Bull. Natl. Res. Cent. 2020, 44, 81.
- Roko, O.G.; Dougnon, V.; Hounkpatin, A.; Klotoé, J.R.; Baba-Moussa, L. Anti-Inflammatory, Analgesic and Antipyretic Properties of Ethanolic Extracts of Three Plants of Beninese’s Pharmacopoeia: Euphorbia hirta, Citrus aurantifolia and Heterotis rotundifolia. AJOB 2020, 8, 1–8.
- Matuka, T.; Oyedeji, O.; Gondwe, M.; Oyedeji, A. Chemical Composition and In Vivo Anti-Inflammatory Activity of Essential Oils from Citrus sinensis (L.) Osbeck Growing in South Africa. J. Essent. Oil Bear. Plants 2020, 23, 638–647.
- Ho, Y.; Suphrom, N.; Daowtak, K.; Potup, P.; Thongsri, Y.; Usuwanthim, K. Anticancer Effect of Citrus hystrix DC. Leaf Extract and Its Bioactive Constituents Citronellol and, Citronellal on the Triple Negative Breast Cancer MDA-MB-231 Cell Line. Pharmaceuticals 2020, 13, 476.
- El-Kersh, D.M.; Ezzat, S.M.; Salama, M.M.; Mahrous, E.A.; Attia, Y.M.; Ahmed, M.S.; Elmazar, M.M. Anti-Estrogenic and Anti-Aromatase Activities of Citrus Peels Major Compounds in Breast Cancer. Sci. Rep. 2021, 11, 7121.
- Ko, Y.-C.; Choi, H.S.; Liu, R.; Kim, J.-H.; Kim, S.-L.; Yun, B.-S.; Lee, D.-S. Inhibitory Effects of Tangeretin, a Citrus Peel-Derived Flavonoid, on Breast Cancer Stem Cell Formation through Suppression of Stat3 Signaling. Molecules 2020, 25, 2599.
- Wen, S.; Sun, L.; An, R.; Zhang, W.; Xiang, L.; Li, Q.; Lai, X.; Huo, M.; Li, D.; Sun, S. A Combination of Citrus reticulata Peel and Black Tea Inhibits Migration and Invasion of Liver Cancer via PI3K/AKT and MMPs Signaling Pathway. Mol. Biol. Rep. 2020, 47, 507–519.
- Caggia, C.; Palmeri, R.; Russo, N.; Timpone, R.; Randazzo, C.L.; Todaro, A.; Barbagallo, S. Employ of Citrus By-Product as Fat Replacer Ingredient for Bakery Confectionery Products. Front. Nutr. 2020, 7, 46.
- Magalhães, D.; Vilas-Boas, A.A.; Teixeira, P.; Pintado, M. Functional Ingredients and Additives from Lemon By-Products and Their Applications in Food Preservation: A Review. Foods 2023, 12, 1095.
- Taghavi Kevij, H.; Salami, M.; Mohammadian, M.; Khodadadi, M.; Emam-Djomeh, Z. Mechanical, Physical, and Bio-Functional Properties of Biopolymer Films Based on Gelatin as Affected by Enriching with Orange Peel Powder. Polym. Bull. 2021, 78, 4387–4402.
- Bhandari, D.P.; Poudel, D.K.; Satyal, P.; Khadayat, K.; Dhami, S.; Aryal, D.; Chaudhary, P.; Ghimire, A.; Parajuli, N. Volatile Compounds and Antioxidant and Antimicrobial Activities of Selected Citrus Essential Oils Originated from Nepal. Molecules 2021, 26, 6683.
- Manzur, M.; Luciardi, M.C.; Blázquez, M.A.; Alberto, M.R.; Cartagena, E.; Arena, M.E. Citrus sinensis Essential Oils an Innovative Antioxidant and Antipathogenic Dual Strategy in Food Preservation against Spoliage Bacteria. Antioxidants 2023, 12, 246.
- Randazzo, W.; Jiménez-Belenguer, A.; Settanni, L.; Perdones, A.; Moschetti, M.; Palazzolo, E.; Guarrasi, V.; Vargas, M.; Germanà, M.A.; Moschetti, G. Antilisterial Effect of Citrus Essential Oils and Their Performance in Edible Film Formulations. Food Control 2016, 59, 750–758.
- Berk, Z. Citrus Fruit Processing; Academic Press: London, UK, 2016; ISBN 978-0-12-803133-9.
- Martínez-Nicolas, J.J.; Núñez-Gómez, D.; Lidón, V.; Martínez-Font, R.; Melgarejo, P.; Hernández, F.; Legua, P. Physico-Chemical Attributes of Lemon Fruits as Affected by Growing Substrate and Rootstock. Foods 2022, 11, 2487.
- Diering, N.L.; Ulrich, A.; Scapini, T.; Müller, C.; Gasparetto, I.G.; Júnior, F.W.R.; Treichel, H.; Mossi, A.J. Microbial Natural Bioactive Formulations in Citrus Development. Biotechnol. Rep. 2022, 34, e00718.
- Nieto, G.; Fernández-López, J.; Pérez-Álvarez, J.A.; Peñalver, R.; Ros-Berruezo, G.; Viuda-Martos, M. Valorization of Citrus Co-Products: Recovery of Bioactive Compounds and Application in Meat and Meat Products. Plants 2021, 10, 1069.
- Andrade, M.A.; Barbosa, C.H.; Shah, M.A.; Ahmad, N.; Vilarinho, F.; Khwaldia, K.; Silva, A.S.; Ramos, F. Citrus By-Products: Valuable Source of Bioactive Compounds for Food Applications. Antioxidants 2022, 12, 38.
- Casas Cardoso, L.; Cejudo Bastante, C.; Mantell Serrano, C.; Martínez de la Ossa, E.J. Application of Citrus By-Products in the Production of Active Food Packaging. Antioxidants 2022, 11, 738.
- Petracek, P.D. Peel Morphology and Fruit Blemishes. In Citrus Flowering & Fruiting Short Course; Citrus Research and Education Center: Lake Alfred, FL, USA, 1997.
- Inthachat, W.; Temviriyanukul, P.; On-Nom, N.; Kanoongon, P.; Thangsiri, S.; Chupeerach, C.; Suttisansanee, U. Optimization of Phytochemical-Rich Citrus Maxima Albedo Extract Using Response Surface Methodology. Molecules 2023, 28, 4121.
- Nomura, R.; Ohata, J.; Otsugu, M.; Okawa, R.; Naka, S.; Matsumoto-Nakano, M.; Nakano, K. Inhibitory Effects of Flavedo, Albedo, Fruits, and Leaves of Citrus unshiu Extracts on Streptococcus mutans. Arch. Oral. Biol. 2021, 124, 105056.
- Ahmed, M.; Saeid, A. Citrus Fruits: Nutritive Value and Value-Added Products. In Citrus—Research, Development and Biotechnology; Sarwar Khan, M., Ahmad Khan, I., Eds.; IntechOpen: London, UK, 2021; ISBN 978-1-83968-723-5.
- Wang, J.; Hao, H.; Liu, R.; Ma, Q.; Xu, J.; Chen, F.; Cheng, Y.; Deng, X. Comparative Analysis of Surface Wax in Mature Fruits between Satsuma Mandarin (Citrus unshiu) and ‘Newhall’ Navel Orange (Citrus sinensis) from the Perspective of Crystal Morphology, Chemical Composition and Key Gene Expression. Food Chem. 2014, 153, 177–185.
- Pradeep, M.; Kruszka, D.; Kachlicki, P.; Mondal, D.; Franklin, G. Uncovering the Phytochemical Basis and the Mechanism of Plant Extract-Mediated Eco-Friendly Synthesis of Silver Nanoparticles Using Ultra-Performance Liquid Chromatography Coupled with a Photodiode Array and High-Resolution Mass Spectrometry. ACS Sustain. Chem. Eng. 2022, 10, 562–571.
- Romero, P.; Lafuente, M.T. Abscisic Acid Deficiency Alters Epicuticular Wax Metabolism and Morphology That Leads to Increased Cuticle Permeability during Sweet Orange (Citrus sinensis) Fruit Ripening. Front. Plant Sci. 2020, 11, 594184.
- Pashova, S. Application of Plant Waxes in Edible Coatings. Coatings 2023, 13, 911.
- García-Coronado, H.; Tafolla-Arellano, J.C.; Hernández-Oñate, M.Á.; Burgara-Estrella, A.J.; Robles-Parra, J.M.; Tiznado-Hernández, M.E. Molecular Biology, Composition and Physiological Functions of Cuticle Lipids in Fleshy Fruits. Plants 2022, 11, 1133.
- Lado, J.; Alós, E.; Manzi, M.; Cronje, P.J.R.; Gómez-Cadenas, A.; Rodrigo, M.J.; Zacarías, L. Light Regulation of Carotenoid Biosynthesis in the Peel of Mandarin and Sweet Orange Fruits. Front. Plant Sci. 2019, 10, 1288.
- Kato, M. Mechanism of Carotenoid Accumulation in Citrus Fruit. J. Jpn. Soc. Hort. Sci. 2012, 81, 219–233.
- Rodrigo, M.J.; Alquézar, B.; Alós, E.; Lado, J.; Zacarías, L. Biochemical Bases and Molecular Regulation of Pigmentation in the Peel of Citrus Fruit. Sci. Hortic. 2013, 163, 46–62.
- Agarwal, P.; Sebghatollahi, Z.; Kamal, M.; Dhyani, A.; Shrivastava, A.; Singh, K.K.; Sinha, M.; Mahato, N.; Mishra, A.K.; Baek, K.-H. Citrus Essential Oils in Aromatherapy: Therapeutic Effects and Mechanisms. Antioxidants 2022, 11, 2374.
- Van den Bruinhorst, A.; Kouris, P.; Timmer, J.; De Croon, M.; Kroon, M. Exploring Orange Peel Treatment with Deep Eutectic Solvents and Diluted Organic Acids. Nat. Prod. Chem. Res. 2016, 4, 1–5.
- Singhal, S.; Swami Hulle, N.R. Citrus Pectins: Structural Properties, Extraction Methods, Modifications and Applications in Food Systems—A Review. Appl. Food Res. 2022, 2, 100215.
- Sharma, P.; Dadwal, V.; Rahmatkar, S.N.; Gupta, M.; Singh, D. Flavonoid Composition and Antioxidant Efficacy of Citrus Peels: An Integrated in Vitro and in Silico Approach toward Potential Neuroprotective Agents. J. Sci. Ind. Res. 2022, 81, 445–454.
- Chandel, V.; Biswas, D.; Roy, S.; Vaidya, D.; Verma, A.; Gupta, A. Current Advancements in Pectin: Extraction, Properties and Multifunctional Applications. Foods 2022, 11, 2683.
- Addi, M.; Elbouzidi, A.; Abid, M.; Tungmunnithum, D.; Elamrani, A.; Hano, C. An Overview of Bioactive Flavonoids from Citrus Fruits. Appl. Sci. 2021, 12, 29.
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.-S. Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes. Antioxidants 2022, 11, 239.
- Sharma, P.; Vishvakarma, R.; Gautam, K.; Vimal, A.; Kumar Gaur, V.; Farooqui, A.; Varjani, S.; Younis, K. Valorization of Citrus Peel Waste for the Sustainable Production of Value-Added Products. Bioresour. Technol. 2022, 351, 127064.
- Chavan, P.; Singh, A.K.; Kaur, G. Recent Progress in the Utilization of Industrial Waste and By-products of Citrus Fruits: A Review. J. Food Process Eng. 2018, 41, e12895.
- Martínez-Abad, A.; Ramos, M.; Hamzaoui, M.; Kohnen, S.; Jiménez, A.; Garrigós, M.C. Optimisation of Sequential Microwave-Assisted Extraction of Essential Oil and Pigment from Lemon Peels Waste. Foods 2020, 9, 1493.
- Tunç, M.T.; Odabaş, H.İ. Single-Step Recovery of Pectin and Essential Oil from Lemon Waste by Ohmic Heating Assisted Extraction/Hydrodistillation: A Multi-Response Optimization Study. Innov. Food Sci. Emerg. Technol. 2021, 74, 102850.
- Sandhu, H.K.; Sinha, P.; Emanuel, N.; Kumar, N.; Sami, R.; Khojah, E.; Al-Mushhin, A.A.M. Effect of Ultrasound-Assisted Pretreatment on Extraction Efficiency of Essential Oil and Bioactive Compounds from Citrus Waste By-Products. Separations 2021, 8, 244.
- Teigiserova, D.A.; Tiruta-Barna, L.; Ahmadi, A.; Hamelin, L.; Thomsen, M. A Step Closer to Circular Bioeconomy for Citrus Peel Waste: A Review of Yields and Technologies for Sustainable Management of Essential Oils. J. Environ. Manag. 2021, 280, 111832.
- Zayed, A.; Badawy, M.T.; Farag, M.A. Valorization and Extraction Optimization of Citrus Seeds for Food and Functional Food Applications. Food Chem. 2021, 355, 129609.
- Mahato, N.; Sinha, M.; Sharma, K.; Koteswararao, R.; Cho, M.H. Modern Extraction and Purification Techniques for Obtaining High Purity Food-Grade Bioactive Compounds and Value-Added Co-Products from Citrus Wastes. Foods 2019, 8, 523.
- Panwar, D.; Panesar, P.S.; Chopra, H.K. Recent Trends on the Valorization Strategies for the Management of Citrus By-Products. Food Rev. Int. 2021, 37, 91–120.
- Idamokoro, E.M.; Hosu, Y.S. Out-Look on Worldwide Trends of Related Studies on Citrus Waste as Feed for Livestock Production: A Scientometric Analysis. Front. Res. Metr. Anal. 2022, 7, 869974.
- Mamma, D.; Christakopoulos, P. Biotransformation of Citrus By-Products into Value Added Products. Waste Biomass Valor. 2014, 5, 529–549.
- Bampidis, V.A.; Robinson, P.H. Citrus By-Products as Ruminant Feeds: A Review. Anim. Feed. Sci. Technol. 2006, 128, 175–217.
- Yun, D.; Liu, J. Recent Advances on the Development of Food Packaging Films Based on Citrus Processing Wastes: A Review. J. Agric. Food Res. 2022, 9, 100316.
- Caballero, S.; Li, Y.O.; McClements, D.J.; Davidov-Pardo, G. Encapsulation and Delivery of Bioactive Citrus Pomace Polyphenols: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 8028–8044.
- Spigno, G.; Donsì, F.; Amendola, D.; Sessa, M.; Ferrari, G.; De Faveri, D.M. Nanoencapsulation Systems to Improve Solubility and Antioxidant Efficiency of a Grape Marc Extract into Hazelnut Paste. J. Food Eng. 2013, 114, 207–214.
- Jones, D.; Caballero, S.; Davidov-Pardo, G. Bioavailability of Nanotechnology-Based Bioactives and Nutraceuticals. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2019; Volume 88, pp. 235–273. ISBN 978-0-12-816073-2.
- Todorović, A.; Šturm, L.; Salević-Jelić, A.; Lević, S.; Osojnik Črnivec, I.G.; Prislan, I.; Skrt, M.; Bjeković, A.; Poklar Ulrih, N.; Nedović, V. Encapsulation of Bilberry Extract with Maltodextrin and Gum Arabic by Freeze-Drying: Formulation, Characterisation, and Storage Stability. Processes 2022, 10, 1991.
- Wu, W.; Jiang, B.; Liu, R.; Han, Y.; Fang, X.; Mu, H.; Farag, M.A.; Simal-Gandara, J.; Prieto, M.A.; Chen, H.; et al. Structures and Functions of Cuticular Wax in Postharvest Fruit and Its Regulation: A Comprehensive Review with Future Perspectives. Engineering 2023, 23, 118–129.
- Mavrommatis, A.; Zografaki, M.-E.; Marka, S.; Myrtsi, E.D.; Giamouri, E.; Christodoulou, C.; Evergetis, E.; Iliopoulos, V.; Koulocheri, S.D.; Moschopoulou, G.; et al. Effect of a Carotenoid Extract from Citrus reticulata By-Products on the Immune-Oxidative Status of Broilers. Antioxidants 2022, 11, 144.
- Sedeek, M.S.; Al-Mahallawi, A.M.; Hussien, R.A.A.; Ali, A.M.A.; Naguib, I.A.; Mansour, M.K. Hexosomal Dispersion: A Nano-Based Approach to Boost the Antifungal Potential of Citrus Essential Oils against Plant Fungal Pathogens. Molecules 2021, 26, 6284.
- Abdel-Bar, H.M.; Khater, S.E.; Ghorab, D.M.; Al-mahallawi, A.M. Hexosomes as Efficient Platforms for Possible Fluoxetine Hydrochloride Repurposing with Improved Cytotoxicity against HepG2 Cells. ACS Omega 2020, 5, 26697–26709.
- Guo, J.; Gao, Z.; Li, G.; Fu, F.; Liang, Z.; Zhu, H.; Shan, Y. Antimicrobial and Antibiofilm Efficacy and Mechanism of Essential Oil from Citrus Changshan-Huyou Y. B. Chang against Listeria monocytogenes. Food Control 2019, 105, 256–264.
- Disson, O.; Lecuit, M. Targeting of the Central Nervous System by Listeria monocytogenes. Virulence 2012, 3, 213–221.
- Freitas, C.M.P.; Coimbra, J.S.R.; Souza, V.G.L.; Sousa, R.C.S. Structure and Applications of Pectin in Food, Biomedical, and Pharmaceutical Industry: A Review. Coatings 2021, 11, 922.
- Minzanova, S.; Mironov, V.; Arkhipova, D.; Khabibullina, A.; Mironova, L.; Zakirova, Y.; Milyukov, V. Biological Activity and Pharmacological Application of Pectic Polysaccharides: A Review. Polymers 2018, 10, 1407.
- Zhang, S.; Hu, H.; Wang, L.; Liu, F.; Pan, S. Preparation and Prebiotic Potential of Pectin Oligosaccharides Obtained from Citrus Peel Pectin. Food Chem. 2018, 244, 232–237.
- Rojas-Lema, S.; Torres-Giner, S.; Quiles-Carrillo, L.; Gomez-Caturla, J.; Garcia-Garcia, D.; Balart, R. On the Use of Phenolic Compounds Present in Citrus Fruits and Grapes as Natural Antioxidants for Thermo-Compressed Bio-Based High-Density Polyethylene Films. Antioxidants 2020, 10, 14.
- Kaurinovic, B.; Vastag, D. Flavonoids and Phenolic Acids as Potential Natural Antioxidants. In Antioxidants; Shalaby, E., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78923-919-5.
- Tripoli, E.; Guardia, M.L.; Giammanco, S.; Majo, D.D.; Giammanco, M. Citrus Flavonoids: Molecular Structure, Biological Activity and Nutritional Properties: A Review. Food Chem. 2007, 104, 466–479.
- Roy, P.; Mohanty, A.K.; Dick, P.; Misra, M. A Review on the Challenges and Choices for Food Waste Valorization: Environmental and Economic Impacts. ACS Environ. Au 2023, 3, 58–75.
- Nirmal, N.P.; Khanashyam, A.C.; Mundanat, A.S.; Shah, K.; Babu, K.S.; Thorakkattu, P.; Al-Asmari, F.; Pandiselvam, R. Valorization of Fruit Waste for Bioactive Compounds and Their Applications in the Food Industry. Foods 2023, 12, 556.
- Niluxsshun, M.C.D.; Masilamani, K.; Mathiventhan, U. Green Synthesis of Silver Nanoparticles from the Extracts of Fruit Peel of Citrus tangerina, Citrus sinensis, and Citrus limon for Antibacterial Activities. Bioinorg. Chem. Appl. 2021, 2021, 6695734.
- Osonga, F.J.; Akgul, A.; Yazgan, I.; Akgul, A.; Eshun, G.B.; Sakhaee, L.; Sadik, O.A. Size and Shape-Dependent Antimicrobial Activities of Silver and Gold Nanoparticles: A Model Study as Potential Fungicides. Molecules 2020, 25, 2682.
- Alkhulaifi, M.M.; Alshehri, J.H.; Alwehaibi, M.A.; Awad, M.A.; Al-Enazi, N.M.; Aldosari, N.S.; Hatamleh, A.A.; Abdel- Raouf, N. Green Synthesis of Silver Nanoparticles Using Citrus limon Peels and Evaluation of Their Antibacterial and Cytotoxic Properties. Saudi J. Biol. Sci. 2020, 27, 3434–3441.
More
