Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Emanuela Ribichini and Version 2 by Lindsay Dong.
Strenuous exercise can be associated with “Exercise Induced Gastrointestinal Syndrome” (Ex-GIS), a clinical condition characterized by a series of gastrointestinal (GI) disturbances that may impact the physical and psychological performance of athletes. The pathophysiology comprises multi-factorial interactions between the GI tract and the circulatory, immune, enteric, and central nervous systems.
- gastrointestinal symptoms
- endurance sport
- diet
- IBS
1. Introduction
Physical activity has many positive effects on health, especially on the musculoskeletal, cardiovascular, and gastrointestinal (GI) systems. Its effect depends on the intensity, duration, and modality of physical activity. Mild- to moderate-intensity exercise with a regular duration (e.g., between 3.5 and 4 h per week) plays a protective role against colon cancer, diverticular disease, gallstones, and constipation [1][2][1,2]. On the other hand, strenuous exercise and endurance sports may cause exercise-induced gastrointestinal symptoms (Ex-GISs) in up to 70% of athletes, and they can manifest as upper symptoms (e.g., regurgitation, upper abdominal bloating, belching, epigastric pain, and heartburn) and lower symptoms (e.g., flatulence, urge to defecate, lower abdominal bloating, abdominal pain, abnormal defecation including loose water stools, diarrhea, and fecal blood loss) [3]. Endurance exercise is considered a sport aimed at improving the ability to sustain intense physical activity over time, without significant loss of performance. Endurance includes different physical activities with different levels of intensity and thus workouts can differ greatly according to the disciplines practiced. Cycling, swimming, marathons, triathlons, running, mountain biking, and climbing are some of those considered endurance sports simply because they require an expenditure of energy for a long time [4]. Ex-GIS might result from responses to exercise that compromise gastrointestinal integrity and function and may even be the reason why some stop sports participation [3]. The mechanisms leading to GI discomfort during exercise are not yet fully understood [5]. Exercise responses may involve two different pathways: a circulatory–gastrointestinal pathway [6] and a neuroendocrine–gastrointestinal pathway [7]. The combination of splanchnic hypoperfusion and altered enteric nervous system activity may result in a compromised GI system. The loss of epithelial integrity observed during strenuous physical exercise leads to increased intestinal permeability with bacterial translocation and inflammation. This alteration may negatively impact exercise performance and post-exercise recovery due to abdominal distress and impairment in the uptake of fluid, electrolytes, and nutrients. Exercise may also have a substantial impact on gut microbiota (GM) composition and structure, but the role of the microbiota in exercise adaptation remains unknown [8].
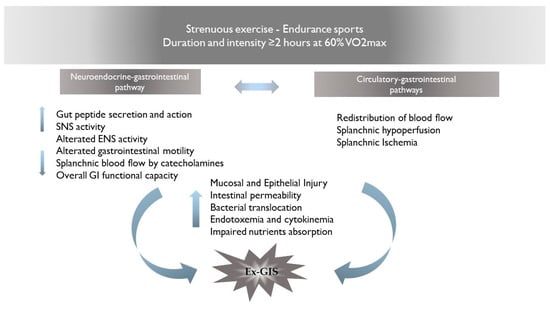
2. Pathophysiology of Ex-GIS: Proposed Mechanisms for GI Distress
The pathophysiology of Ex-GIS includes two primary pathways: (I) the neuroendocrine–gastrointestinal pathway, involving an increase in sympathetic activation, reducing overall GI functional capacity [3] and (II) the circulatory–gastrointestinal pathway, involving the redistribution of blood flow to working muscles and peripheral circulation, subsequently reducing total splanchnic perfusion and nutrient absorption [9][10][10,11]. It is still under debate whether the neuroendocrine pathway may affect the circulatory–gastrointestinal one and, in cascade, reduce the total splanchnic perfusion, or whether the splanchnic hypoperfusion in response to the intensity/duration of muscle activity may influence the neuroendocrine activation. It is plausible that the combination of the altered enteric nervous system activity and the splanchnic hypoperfusion may result in GI symptoms and/or in acute or chronic health complications [5]. The proposed mechanisms for gastrointestinal discomfort are summarized in Figure 1.
Figure 1. Proposed mechanisms for Ex-GIS pathophysiology. Abbreviations: Ex-GIS: exercise-induced gastrointestinal symptoms; VO2max: maximal oxygen consumption; SNS: sympathetic nervous system; ENS: enteric nervous system.
2.1. Neuroendocrine–Gastrointestinal Pathway
The alteration of the enteric nervous system (ENS) activity, through a cascade of events, results in clinical complications and GI symptoms known as Ex-GIS. Physical activity induces sympathetic activation, which is considered the main cause of altered ENS activity [11][12][12,13]. The digestive system is controlled by the bidirectional activity of the central nervous system (CNS) and the ENS, both of which participate in the regulation of the various functions of the intestines. ENS can independently regulate GI functions without central input. Indeed, the ENS is considered a quasi-autonomous part of the nervous system including several neural circuits that control motor functions, local blood flow, and mucosal transport and secretions and modulate immune and endocrine functions [13][14]. Enteroendocrine cells (EECs), which are basal-granulated cells dispersed in the gut epithelium, represent the endocrine elements of the intestine and release gut peptides, such as cholecystokinin (CCK), glucagon-like peptide-1 (GLP-1), and peptide YY (PYY), all having an anorectic effect. Ghrelin, on the other hand, is an orexigenic peptide produced by the enteroendocrine cells in the oxyntic glands of the stomach and upper intestine; thus, its plasma levels are high before meals and are suppressed in response to food intake [14][15]. These neuropeptides act either in a paracrine fashion on both intestinal and neural cells in proximity or enter the bloodstream and can have peripheral effects, such as change in gastric emptying and gut motility [15][16]. Briefly, CCK is synthesized and released from I cells of the upper intestine in response to food intake. It slows down gastric emptying and stimulates pancreatic and gallbladder secretions. CCK exerts its satiety action primarily through the activation of vagal afferent fibers innervating both the stomach and the upper intestine [16][17][17,18]. CCK levels rapidly increase after food ingestion, present a peak a few minutes after meal initiation, and decline to baseline levels with meal termination [18][19]. In contrast, the other gut peptides have patterns of release and actions that are consistent with effects beyond the meal. Indeed, plasma levels of both PYY and GLP-1, which are synthesized and released from L cells located primarily in the distal intestine, occur more slowly, not peaking until after meal termination and remaining high for several hours after a meal [19][20][20,21]. Both PYY and GLP-1 inhibit food intake. Few data are currently available regarding the modification of gut peptide levels in response to physical activity. Various animal and cell models have demonstrated that the activation of adenosine receptors induces the release of GLP-1 and PYY from EECs [21][22][22,23], and concentrations of both are increased during moderate- and high-intensity exercise. Moreover, exercise induces the vago-vagal reflex from the brain back to the gut [23][24], which plays a role in the ability of CCK to regulate gastric emptying and intestinal motility [24][25].
2.2. Circulatory–Gastrointestinal Pathways
The splanchnic vasculature is a system with an extraordinary capacity to adapt to physiological stressors affecting vasodilation or constriction in response to neuroendocrine, humoral, and paracrine mechanisms [25][42]. During strenuous exercise, the release of norepinephrine generates splanchnic vasoconstriction, thereby raising total splanchnic vascular resistance [26][43]. Blood is rapidly redistributed from the splanchnic vasculature to the periphery for use in tissues with increased activity during exercise, such as the heart, lungs, active muscles, and skin [9][10]. As a result, the splanchnic blood flow (SBF) can be depleted by up to 80%, leading to significant gastrointestinal tract hypoperfusion and damage [27][44]. SBF hypoperfusion can be assessed through gastric tonometry [28][45], a functional test that measures the accumulated mucosal carbon dioxide (CO2). In response to inadequate tissue perfusion and tissue hypoxia, the gap between gastric and systemic PCO2 reflects the adequacy of splanchnic perfusion [29][46]. The application of tonometry during exercise shows the most pronounced change in splanchnic perfusion during the first 10 min of strenuous exercise [10][11], indicating a rapid response of the splanchnic vascular bed. In addition, tonometry shows that splanchnic hypoperfusion can be aggravated by younger age, exercise intensity and duration, dehydration, and high environmental temperature [3]. The reductions in SBF are less conspicuous in the elderly (mean age 64 years) compared with younger people [30][47]. SBF hypoperfusion despoils enterocytes of oxygen and adenosine triphosphate (ATP), leading to cell damage and loss of epithelial integrity [31][48], which may be responsible for the mucosal erosions and GI bleeding observed during endoscopy after strenuous endurance running [32][49]. Loss of epithelial integrity, including disruption of the tight junctions interconnecting the intestinal epithelial cells, is associated with increased GI permeability, intestinal inflammation, and bacterial translocation [33][51]. Bacterial translocation induces endotoxemia, which is characterized by the presence of circulating bacterial lipopolysaccharides (LPSs). Loss of tight junction integrity and/or enterocyte damage cause passage of LPSs into the circulation, which in turn triggers the activation of T lymphocytes, monocytes, and tissue macrophages. The local immune response induces the release of proinflammatory cytokines such as tumor necrosis factor and interleukin-1, interferon-γ, and nitric oxide (NO) [34][52], which can generate a vicious cycle that promotes greater intestinal barrier dysfunction through production of these mediators [35][53]. Brock-Utne and colleagues [36][54] provided the initial evidence that the intestinal barrier was being compromised during prolonged, strenuous exercise. In that study, 81% of the 89 marathon runners (89.4 km) were found to be endotoxemic [36][54]. The translocation of endotoxic microorganisms into circulation may also be dependent on the presence of indigenous bacterial species within the GI tract, such as Enterobacteriaceae, Proteobacteria, Firmicutes, Bacteroides, and Actinobacteria [37][40]. The impact of exercise on GM composition and structure needs to be mentioned in this context. Remarkable differences have been described between competing athletes and sedentary people, related to a huge microbiota Ⲁ-diversity in athletes—mostly associated with dietary patterns and protein consumption. Ⲁ-diversity is an index to describe the quality of the GM and is expected to decrease in disturbed conditions such as disease and poor health [38][39][40][60,61,62].3. Gastrointestinal Symptoms during Exercise
If an athlete experiences GI symptoms during exercise, it should be ascertained that they are not a sign of underlying disease. Once medical causes have been excluded, Ex-GIS can be considered. The vascular and neuroendocrine alterations that occur during endurance physical activity led to the occurrence of GI symptoms that can affect the athlete’s physical performance and psychophysical well-being [5]. There is a great variability in the severity of symptoms amongst individuals, and these may range from minor discomfort to significant health disturbances that impair their ability to compete in races and even lead to hospitalization in some cases [5]. Symptoms may appear hours to days after exposure and range from GI (e.g., abdominal bloating, loose stool, abdominal pain) to extraintestinal symptoms including fatigue, headaches, and cognitive difficulties. Several of these symptoms can be confused with irritable bowel syndrome (IBS) [5], a functional disorder of gut–brain interaction (DGBI) [41][68]. IBS and EX-GIS share similar pathogenesis in some respects, including alterations in the GM, intestinal permeability, gut immune function, motility, visceral sensation, brain–gut interactions, and psychosocial status [42][69]. Hungin et al., who reported an IBS prevalence of 14.1% overall with 10.8% undiagnosed meeting either the Manning or Rome (I or II) diagnostic criteria [43][71]. Underdiagnosed IBS may occur in those athletes who have not consulted a medical professional regarding their symptoms and are attempting to manage their symptoms through various strategies. In theory, if these strategies are not effective in managing their symptoms, these athletes will not be considered as IBS sufferers. Regarding Ex-GIS, an estimated 30–90% of endurance athletes engaging in marathons, triathlons, and running report experiencing GI symptoms during exercise [1]. To investigate the prevalence of Ex-GIS, Ter Steege and colleagues included 2076 athletes competing in a long-distance run to assess the prevalence, risk factors, and timing of GI disturbances [44][72]. They received a questionnaire where the reported GI complaints were related to variables such as age, gender, distance, fluid, and food ingestion, running experience, and environmental conditions. Three athletes dropped out because of GI complaints, 45% had at least one GI complaint during running, 11% referred to serious GI complaints, and 2.7% had complaints during the first 24 h after the run [44][72]. Concerning other GI symptoms, side ache, stitch, and subcostal pain, commonly referred to as exercise-induced transient abdominal pain (ETAP), are common during exercise. ETAP was reported in 18% of the competitors in a recreational run, whereas 4% reported severe abdominal pain, but the incidence of ETAP is influenced by the type of sport [45][78]. Finally, the lower-GI-tract symptoms, such as abdominal pain, flatulence, cramping, the urge to defecate, diarrhea, and rectal bleeding, are more severe compared to upper-GI disturbances, having the potential to impair performance [46][80]. The incidence of severe lower GI symptoms during a recreational run is up to 30%, but the percentage may increase up to 50% in cyclists and to 70% in competitive long-distance runners [47][81]. Recreational athletes are least likely to report symptoms. Usually, they are competing at lower intensities and thus have fewer symptoms, as GI symptoms are reported to increase with distance and exercise intensity [48][82].4. Nutritional and Behavior Strategies to Reduce Ex-GIS
We believe that in the framework of Ex-GIS, risk factors screening should be recommended. Exercise modality, state of dehydration, environmental temperature, concomitant therapies, and diet should be evaluated, and if risk elements are present, an attempt should be made to modify them. Since Ex-GIS may also be linked to food-related reactions, the habitual diet should be investigated. Food intolerance appears to be on the rise among athletes, but unvalidated food intolerance tests and self-reported incidence do not allow an accurate estimate of true intolerances [49][91]. Given the multifaced food intolerances or malabsorption manifestation, there is a tendency for athletes to self-diagnose intolerances and subsequently restrict foods or food groups. Lactose and fructose malabsorption, which result from insufficient enzyme and functional capability of the transporter, respectively, are the most-reported food intolerances [50][92]. To ensure adequate energy intake, current guidelines recommend a carbohydrate intake of about 60 g for exercise lasting for up to 2 h. When the exercise lasts 2 h, slightly greater amounts of carbohydrates (90 g/h) would be recommended, and generally, these carbohydrates should consist of a mix of multiple transportable carbohydrates, e.g., glucose and fructose or maltodextrin and fructose [51][93]. Finally, food choice pre-exercise has a significant impact on the gut’s tolerance to running, and athletes self-manage their diet to reduce Ex-GIS. Dietary elements including high fiber, fat, and protein intake, as well as concentrated carbohydrate loads, have been reported to trigger GI symptoms in triathletes [52][83]. Dark chocolate needs to be mentioned in this paragraph, since it has been proposed as an ergogenic aid via increased nitric oxide [53][94], but caution should be advised for chocolate since it has been described as a food that provokes GI disturbance, particularly constipation [54][95]. GI effects may depend on the concentration of cocoa or other biologically active compounds, including caffeine and fat, which may aggravate EX-GIS [55][96]. This evidence may explain why morning caffeine intake has been associated with increased lower GI symptoms in triathletes [56][97]. On the other hand, competitive athletes and longer-distance runners were less likely to avoid coffee or tea, and this may be due to the potential ergogenic effects of caffeine in endurance exercise [57][98]. To assess dietary restrictions pre-racing and GI symptoms, Jill A. Parnell et al. [52][83] designed a questionnaire and administered it to 388 runners. Their analysis showed the foods regularly avoided were meat (32%), milk products (31%), fish/seafood (28%), poultry (24%), and high-fiber foods (23%). Caffeinated beverages were commonly avoided in events 10 km or less, while high-fiber foods were avoided in females. Rates of food avoidance were elevated in younger and more competitive runners. Interestingly, athletes did not identify the consumption of high-osmolarity carbohydrate supplements as a risk factor for the development of symptoms. In fact, symptoms may depend on the quantity and quality of carbohydrates ingested before exercise. Another strategy to manage Ex-GIS was proposed by van Wijck et al. [9][10]. Since the perfusion of the gut is implicated in EX-GIS pathogenesis, upregulating intestinal nitric oxide (NO) production could be a way to reduce symptoms. Manipulation of intestinal NO can be obtained through nitric-oxide-synthase-dependent (glutamine–arginine–citrulline) and nitric-oxide-synthase-independent (nitrate–nitrite) supplementations or by increasing the dietary nitrate intake [9][10].56. Efficacy of Specific Diets Applied by Endurance Athletes to Avoid Ex-GIS
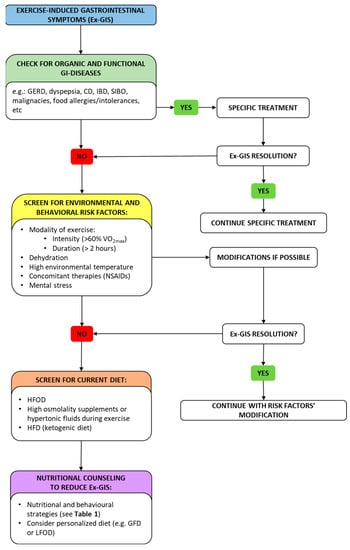
Endurance athletes need a regular nutrition program to fill their energy stores before training/racing and to provide nutritional support during training/racing and effective recovery after training/racing. Some nutritional regimens may benefit the performance of athletes but somehow have a negative impact on the development of symptoms [58][59][60][61][102,103,104,105]. An example is represented by the vegetarian diet; it has been suggested that a vegetarian diet may improve endurance performance by increasing exercise capacity and performance, modulating exercise-induced oxidative stress [62][106] and inflammatory processes, including anti-inflammatory, immunologic responses [63][107], and upper-respiratory-tract infections (URTIs) [64][108], finally providing better cardiovascular parameters. On the other hand, a vegetarian diet may theoretically result in developing GI symptoms due to high fiber content, but some cross-sectional studies and available case reports have not evaluated this aspect [65][109]. Another diet applied by endurance athletes aiming to improve performance is a high-fat diet (HFD), but results of studies are conflicting. This regimen has been widely applied as a treatment option for neurological diseases such as epilepsy or as an effective dietary strategy for weight loss [66][110]. The goal of an HFD is to increase the body’s ability to use ketone bodies (KBs) and fatty acids as energy sources. The utilization of fatty acids and KBs may lead to many advantages, such as sparing muscle glycogen stores, increasing body fat mass loss, improving aerobic capacity, improving time to exhaustion and time-trial performance, and increasing cognitive performance [65][109]. In the last decade, one of the most common dietary strategies used to manage GI symptoms has been the exclusion of gluten from the diet. In recent years, the GFD has become a trendy diet among the general population, followed in about 5–10% of cases, and it is even more widespread among non-celiac athletes (NCAs), in whom the percentage rises to over 40% [67][114] since many athletes believe that gluten removal might reduce GI symptoms [68][73]. The elimination of gluten from the diet is particularly prominent within endurance athletes, likely due to their higher frequency of EX-GIS (15–30%) compared to other types of athletes [68][73]. Despite the paucity of supportive evidence, NCAs choose to adhere to a GFD for various reasons including clinically or self-diagnosed NCGS and the belief that a GFD is healthier because it reduces inflammation and gastrointestinal discomfort, or it may improve exercise performance through the reduction in fatigue [67][114]. The main clinical beneficial effect of a GFD reported by athletes is the resolution of abdominal bloating, gas, diarrhea, and fatigue [67][114]. This justifies the new dietary strategy to address the multifactorial nature of gastrointestinal disorders in athletes using a “low FODMAP” approach and not a GFD [69][127]. FODMAPs are a family of fermentable short-chain carbohydrates found in a wide assortment of foods/food constituents [70][128]. In predisposed individuals, FODMAPs are partially digested, remaining in the intestinal lumen, particularly in the colon, where they are subsequently fermented by the microbiota, releasing gas, recalling water, and leading to exacerbation of the symptoms of IBS [71][129]. The distension of the intestinal wall initiates a painful visceral response conveyed by the nerve fibers that are present in the intestine. Several controlled clinical trials and meta-analyses have demonstrated the superiority of the low-FODMAP diet (LFOD) over control diets in patients with IBS [71][129]. Figure 2 proposes a diagnostic–therapeutic algorithm to manage Ex-GIS.
Figure 2. A diagnostic–therapeutic algorithm with suggestions for managing Ex-GIS. In the framework of Ex-GIS, an initial check for organic/functional disease and risk factors is recommended. Exercise modality, state of dehydration, environmental temperature, concomitant therapies, and mental status should be evaluated, and if risk elements are present, an attempt should be made to modify them. If no resolution has been obtained, screening for current diet and nutritional counseling should be advised by a nutritionist. Abbreviation: Ex-GIS: exercise-induced gastrointestinal symptoms; GERD: gastro-esophageal reflux disease; CD: celiac disease; IBD: inflammatory bowel disease; SIBO: small intestinal bowel overgrowth; VO2max: maximal oxygen consumption; NSAIDs: non-steroidal anti-inflammatory drugs; HFOD: high-FODMAP diet; HFD: high-fat diet; GFD: gluten-free diet; LFOD: low-FODMAP diet.
67. Conclusions
Endurance exercise causes physiological and pathological disturbances that alter GI function and integrity, which eventually results in Ex-GIS. Endurance can cause acute GI symptoms even in a healthy gut through multiple pathological changes associated with hypoperfusion, ischemia, epithelial injury, impaired barrier function, endotoxemia, local and systemic inflammation, impaired nutrient absorption, and altered gastric and intestinal motility. These changes are the result of multifactorial interactions between the gastrointestinal tract and the circulatory, immune, enteric, and central nervous systems, analogous in some respects to the pathogenesis of IBS, as well as in terms of the symptomatology triggered. Ex-GIS can impact the physical and psychological performance of athletes during competitions and vice versa; the psychological stress to which they are subjected may worsen GI disturbances. Numerous nutritional and behavioral interventions have been investigated to alleviate Ex-GIS; diet is perhaps the principal one and should be personalized and planned by experts to avoid self-managed diets.
