Due to their potent immunoregulatory and angio-modulatory properties, mesenchymal stem cells (MSCs) and their exosomes (MSC-Exos) have emerged as potential game-changers in regenerative ophthalmology, particularly for the personalized treatment of inflammatory diseases. NLRP3 inflammasome, composed of Nod-like receptor family pyrin domain-containing protein 3 (NLRP3), apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (CARD) (ASC), and pro-caspase-1 is dysregulated in almost all inflammatory eye disease. Accordingly, a large number of experimental findings suggested that this multi-protein complex could be considered an important intracellular target for MSC-based therapy of inflammatory eye disorders.
1. Introduction
Inflammatory eye diseases encompass a diverse group of conditions characterized by inflammation in various structures of the eye
[1]. These conditions can affect different parts of the eye, including the conjunctiva (conjunctivitis), cornea (keratitis), uvea (anterior/intermediate/posterior uveitis, pan-uveitis), retina (retinitis, retinal vasculitis), and optic nerve (optic neuritis)
[1]. Inflammatory eye diseases may be caused by microbial pathogens, trauma, chemical irritants, and allergens, or could develop as a consequence of autoimmune reactions, due to immune cell-driven tissue damage
[1]. Dry eyes represent the main symptom of Sjögren’s syndrome; bulging eyes and double vision are frequently observed in patients suffering from Graves’ disease; and redness, pain, sensitivity to light, and blurred vision are the main ocular manifestations of rheumatoid arthritis and systemic lupus erythematosus (SLE)
[1].
Therapy for inflammatory and autoimmune eye diseases focuses on managing the underlying condition and reducing inflammation in the eyes
[2]. Treatment plans vary depending on the specific condition and severity of the disease
[2]. Eye drops or ointments containing corticosteroids, non-steroidal anti-inflammatory drugs (NSAIDs), or immunosuppressive agents are usually applied directly to the eye to reduce mild inflammation and alleviate conjunctivitis and keratitis-related symptoms
[3]. In more severe cases, oral medications such as corticosteroids, immunosuppressants, or biologics may be used to suppress detrimental immune responses and to prevent the progression of ongoing eye inflammation
[3]. For certain conditions, such as severe uveitis, injections of corticosteroids or immunosuppressive agents may be administered directly into the eye. In some cases, surgical procedures such as vitrectomy or cataract surgery may be necessary to restore vision and to treat complications caused by inflammatory or autoimmune eye diseases
[3]. Although therapeutic strategies for the treatment of inflammatory eye diseases have evolved significantly, there are still some issues that limit the therapeutic efficacy of these approaches
[2][3][2,3]. Prolonged use of corticosteroids may increase the risk of cataracts or glaucoma while long-term use of immunosuppressive drugs may inhibit the immune system throughout the body, increasing the risk of infections. Since inflammatory and autoimmune eye diseases manifest differently in each individual, identifying the most suitable treatment approach often requires a personalized approach
[2][3][2,3]. Finally, achieving complete remission or long-term control of the underlying autoimmune or inflammatory disease can be challenging. Some patients may require ongoing treatment and monitoring to manage their symptoms and prevent relapses. Accordingly, a large number of experimental and clinical studies are continuously conducted in order to resolve limits and to improve therapeutic options for the treatment of inflammatory eye diseases
[2][3][2,3].
Due to their potent immunoregulatory and angio-modulatory properties, mesenchymal stem cells (MSCs) have emerged as potential game-changers in regenerative ophthalmology, particularly in the field of the treatment of inflammatory diseases
[4]. MSC-based therapy targets the underlying cause of inflammation rather than just managing disease-related symptoms
[4]. MSCs have been shown to exert their therapeutic effects via several mechanisms. Firstly, MSCs suppress detrimental immune responses in the eyes and alleviate ongoing inflammation in ocular tissues by modulating the phenotype and function of all immune cells that play pathogenic roles in the development and progression of inflammatory and autoimmune eye diseases (monocytes/macrophages, dendritic cells (DCs), natural killer (NK), natural killer T (NKT) cells, and T and B lymphocytes)
[4].
Additionally, MSCs possess potent anti-angiogenic properties, which can be beneficial in the treatment of neovascularization-associated inflammatory eye diseases, such as diabetic retinopathy and age-related macular degeneration
[5]. By inhibiting the formation of abnormal blood vessels in the retina, MSCs can help preserve vision and prevent further damage to the ocular tissues
[5].
2. Activation of NLRP3 Inflammasome in Eye-Infiltrated Immune Cells as an Initial Step in the Development of Inflammatory Eye Disease
The activation of the NLRP3 inflammasome in eye-infiltrated immune cells leads to the enhanced production of pro-inflammatory cytokines (interleukin-1β (IL-1β) and IL-18), which elicit inflammatory processes in ocular structures
[6][10].
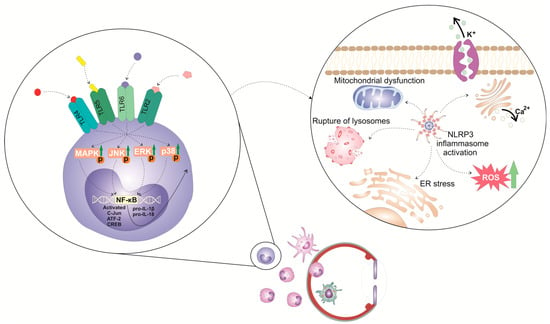
The activation of the NLRP3 inflammasome in immune cells is a tightly regulated, multi-step process (
Figure 1). It requires two steps: priming and activation. Since some immune cells do not naturally express pro-IL-1 and have inadequate amounts of NLRP3 for inflammasome activation, one of the main functions of the priming step is to stimulate the transcriptional production of NLRP3, pro-IL-1β, and pro-IL-18. This initial step involves the activation of transcriptional factors (the nuclear factor kappa B (NF-κB), c-Jun, ATF-2, and CREB) that, upon activation, translocate to the nucleus to induce the enhanced transcription of pro-inflammatory genes, resulting in the increased synthesis of NLRP3 and pro-IL-1β. The priming step of NLRP3 inflammasome activation is elicited by the activation of pattern recognition receptors (PRRs), including Toll-like receptors (TLR). These membrane-bound receptors are expressed on immune cells where recognized pathogen-associated molecular patterns (PAMPs) of microbial antigens or damage-associated molecular patterns (DAMPs) are released from injured cells
[6][7][10,11]. Signals generated from activated PRRs, particularly TLR-2, TLR-4, TLR-5, and TLR-6, initiate phosphorylation and the consequent activation of extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 mitogen-activated protein kinase (MAPK), which, in turn, phosphorylate c-Jun, ATF-2, and CREB transcription factors that bind to the promoter regions of NLRP3 and pro-IL-1β, leading to their transcriptional upregulation
[6][7][10,11]. Additionally, upon binding to TLRs, TLR ligands elicit the MyD88-driven activation of IL-1R-associated kinase 1 (IRAK-1), which, in a TNFR-associated factor 6 (TRAF6)-dependent manner, induces the activation of transforming growth factor-beta-activated kinase 1 (TAK-1), a key protein kinase responsible for the optimal activation of NF-κB. Activated TAK-1 phosphorylates and activates the IκB kinase (IKK) complex, which consists of IKKα, IKKβ, and IKKγ (also known as NEMO). The activated IKK complex phosphorylates the Inhibitor of κB (IκB), which, within cytosol, binds to NF-κB and prevents its nuclear translocation. The phosphorylation of IκB leads to its degradation via the ubiquitin–proteasome pathway. This degradation releases NF-κB, allowing it to translocate into the nucleus. Once in the nucleus, NF-κB binds to specific DNA sequences called κB sites, promoting the transcription of NF-κB-dependent genes, such as NLRP3, pro-IL-1β, and pro-IL-18, which are necessary for inflammasome activation.
Figure 1. Molecular mechanisms and signaling pathways involved in the activation of NLRP3 inflammasome in eye-infiltrated immune cells. The activation of the NLRP3 inflammasome in immune cells is a tightly regulated, multi-step process. It requires 2 steps: priming and activation. The priming step of NLRP3 inflammasome activation is elicited by the activation of pattern recognition receptors (PRRs), including Toll-like receptors (TLR). These membrane-bound receptors are expressed on immune cells that recognize pathogen-associated molecular patterns (PAMPs) of microbial antigens or damage-associated molecular patterns (DAMPs) released from injured cells. Signals generated from activated TLR-2, TLR-4, TLR-5, and TLR-6 initiate phosphorylation and consequent activation of extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 mitogen-activated protein kinase (MAPK), which, in turn, phosphorylate c-Jun, ATF-2, and CREB transcription factors that bind to the promoter regions of NLRP3 and pro-IL-1β, leading to their transcriptional upregulation.
In addition to the priming step, a second step, often referred to as the “activation signal” or the “danger signal” is necessary for the full and optimal activation of the NLRP3 inflammasome. This second step is driven by NLRP3 agonists, which activate NLRP3 to cause inflammasome assembly and mature IL-1 production. It can be triggered by a variety of stimuli, including microbial components, extracellular matrix breakdown products, and environmental antigens
[6][7][8][10,11,12]. The exact mechanisms by which these stimuli activate the NLRP3 inflammasome are still not fully understood, but proposed mechanisms include alterations in potassium (K
+) efflux, lysosomal rupture, mitochondrial dysfunction, and enhanced reactive oxygen species (ROS) production
[6][7][10,11]. Increased intracellular levels of ATP, massive accumulation of uric acid crystals, microbial antigens, and environmental irritants can induce potassium (K
+) efflux from the immune cell, leading to decreased intracellular potassium levels. NLRP3 contains a potassium (K
+)-sensing domain that undergoes conformational changes in response to altered potassium levels
[6][7][10,11]. Accordingly, a decrease in intracellular potassium levels leads to conformational changes in the NLRP3 protein, allowing its oligomerization and consequent association with adaptor ASC protein made via homotypic interactions between the pyrin domains of these two molecules. NLRP3:ASC interaction leads to the formation of a large protein complex called the inflammasome
[6][7][10,11]. NIMA-related kinase 7 (NEK7), an important component of the NLRP3 inflammasome complex, stabilizes the NLRP3 protein and promotes its interaction with ASC protein. Once the NLRP3 inflammasome complex is formed, the CARD domain of ASC protein interacts with the CARD domain of pro-caspase-1, facilitating its recruitment to the inflammasome complex
[8][12]. Afterward, pro-caspase-1 undergoes self-cleavage and activation, resulting in the formation of active caspase-1. Active caspase-1 then cleaves pro-IL-1β and pro-IL-18 into their mature forms, IL-1β and IL-18. These two cytokines are important inflammatory mediators that crucially contribute to the generation and propagation of all inflammatory eye diseases
[6][7][10,11].
In addition to cytokine release, caspase-1 activation also leads to the cleavage of gasdermin D (GSDMD), an effector protein involved in the execution of pyroptosis, a highly inflammatory form of programmed cell death, which has an important pathogenic role in the development and progression of ocular inflammation
[9][13]. NLRP3 and caspase-1-dependent cleavage of GSDMD results in the formation of two distinct fragments of GSDMD protein: the N-terminal (GSDMD-N) and the C-terminal domains (GSDMD-C). Once released from the C-terminal domain, GSDMD-N oligomerizes and inserts into the lipid bilayer of the plasma membrane, leading to the formation of large pores called gasdermin pores
[9][13]. Gasdermin pores disrupt the integrity of the plasma membrane, causing the efflux of intracellular ions, cytoplasmic proteins, and pro-inflammatory cytokines (IL-1β and IL-18) into the extracellular space. IL-1β and IL-18 propagate and aggravate inflammation initially elicited by the activation of NLRP3 inflammasome and importantly contribute to the creation of a “positive inflammatory loop” in inflamed eyes that finally results in enhanced activation and the increased accumulation of inflammatory immune cells in inflamed eyes
[9][13].
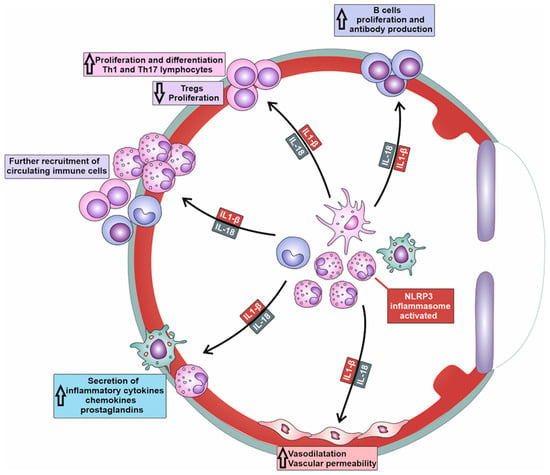
3. Molecular Mechanisms Responsible for NLRP3/IL-1β/IL-18-Dependent Generation of Detrimental Immune Response in Inflamed Eyes
NLRP3/IL-1β/IL-18-dependent recruitment of immune cells is a complex process involving the activation of endothelial cells (ECs), the release of chemotactic factors, the modulation of adhesion molecules, and changes in vascular dynamics (
Table 1)
[10][9]. NLRP3-generated IL-1β and IL-18 induce increased expression of adhesion molecules (intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), E and P-selectins) on the membrane of ECs
[10][9]. Additionally, the activation of NLRP3 inflammasome in immune cells enhances the affinity and avidity of integrin–ligand interactions and facilitates firm adhesion of immune cells to ECs. Precisely, the NLRP3/IL-1β/IL-18 axis upregulates the expression of integrins (lymphocyte function-associated antigen-1 (LFA-1) and very late antigen-4 (VLA-4)) on immune cells, which interact with their ligands on ECs, further promoting the adhesion and subsequent transmigration of immune cells into the inflamed tissues of affected eyes
[10][9].
Table 1. The role of inflammatory cytokines and chemokines in the development and progression of NLRP3 inflammasome-driven eye inflammation.