Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Fanny Huang and Version 1 by SEYED ALIREZA VALI.
One way to store the energy content of methane is through its conversion to methanol. Methanol is a liquid under ambient conditions, easy to transport, and, apart from its use as an energy source, it is a chemical platform that can serve as a starting material for the production of various higher-value products.
- catalysis
- methane
- methanol
1. Introduction
Global warming has raised many concerns during the last few decades. Undoubtedly, the major cause is the release of greenhouse gases into the air [1,2][1][2]. Among such gases, methane and carbon dioxide make the biggest contribution to the global problem. Furthermore, when compared by mass, methane has around 25 times more effect on global warming than carbon dioxide. Hence, scientists have given a sharper focus on the conversion of methane to more beneficial chemicals, that is, higher hydrocarbons or liquid fuels [3].
The production of methanol, formaldehyde, propanol, and other compounds through various methods has been gaining more interest to unlink its production from non-renewable sources. So far, diverse studies have been carried out for the catalytic conversion of methane to syngas and methanol on different transition metals, including Ir, Pt, Rh, and Ru [4,5[4][5][6],6], perovskites [7,8][7][8] and single metal atoms incorporated in supports such as graphene [8], metal-organic frameworks [9,10][9][10] and metal oxides [11,12][11][12]. The conversion of methane into methanol is normally carried out through direct and indirect pathways. While through an indirect route, via a two-step procedure, methanol is formed by a catalytic reaction from syngas (CO + H2), which is produced via oxidation or steam reforming of methane, methane can also be directly converted to methanol through a direct route. Since steam reforming is a thermodynamically unfavorable reaction due to its intrinsic endothermic nature and therefore is immensely energy intensive, the indirect route may not be the best option, especially when it comes to industrial applications.
Thus, direct conversion of methane under mild conditions has recently become the main objective of researchers’ studies. Common direct pathways so far have been partial oxidation of methane (POM) to methanol and acetic acid, conversion of methane to olefins and aromatics through a non-oxidative route (NOCM), and oxidative coupling of methane (OCM). Whereas through OCM and NOCM routes other products rather than methanol are generated, the path that leads to a high methanol yield is stated to be partial oxidation of methane (POM), which is a thermodynamically favorable process since the change in Gibbs free energy for such reactions is negative using oxygen as the oxidant [13,14,15,16][13][14][15][16].
In general, there are several challenges to the direct conversion of methane to methanol. One is the strong C-H bond in methane, which requires severe conditions such as high temperatures to be cleaved. Due to high costs, this issue questions the industrial applicability of the process. Moreover, it causes the overoxidation of the produced methanol to produce more thermodynamically favorable products such as carbon monoxide and dioxide. The reason for this phenomenon is that the dissociation energy of the C-H bond in methanol is lower than that of methane. In other words, as the temperature increases, methanol is more susceptible to oxidation than methane. Consequently, the selectivity for the formation of methanol will decrease due to the generation of other products. In this respect, a catalyst that may activate the C-H bond of methane and simultaneously impede methanol oxidation would be of significant value [17,18][17][18]. In fact, methane monooxygenase enzymes existing in aerobic methanotrophic bacteria are naturally capable of converting methane to methanol under ambient conditions thanks to their intrinsic catalytic system [19]. Hence, emulating such a natural catalytic system for the conversion of methane to methanol has attracted researchers’ interest.
For this purpose, scientists have tried to take advantage of zeolite-based catalysts, metal-organic frameworks (MOFs), and graphene, which inherently have a large number of active sites as well as being perfect hosts for the incorporation of active sites, specifically those existing in nano-catalysts, resembling those found in the monooxygenase enzymes [20,21,22][20][21][22]. Such materials have gained interest for the catalytic conversion of methane to methanol in the last few years. One of the underlying reasons for the incorporation of nano-catalysts into porous media is to overcome a significant obstacle regarding these nano-catalysts high surface energy, which causes their aggregation and instability during the catalytic reaction and consequently their poor catalytic performance at short-medium times.
2. Conversion of Methane to Methanol Routes
2.1. Direct and Indirect Routes
As previously commented, the conversion of methane into value-added chemicals such as methanol, olefins, aromatics, and oxygenated compounds can be achieved through two different routes, as summarized in Figure 1. On one hand, the indirect route for methane to methanol conversion is a two-step process: (1) Partial oxidation or steam reforming of methane to syngas (CO + H2), and (2) catalytic conversion of syngas to methanol. It is known that the steam reforming step is an endothermic reaction (∆H0298K = +206.2 kJ mol−1) with an operating temperature between 800 and 1000 °C. Therefore, the process is extremely energy-demanding. Hence, scientists have attempted to circumvent the intermediate syngas production step and directly convert methane at low temperatures. Partial oxidation of methane (POM) to methanol and acetic acid, conversion of methane to olefins and aromatics through a non-oxidative route (NOCM), and oxidative coupling of methane (OCM) are among the well-known direct routes for methane conversion reactions.
Partial oxidation of methane is an interesting energy-saving process that converts methane to profitable oxygenates such as methanol, formic acid, formaldehyde, and methanol precursors. This route, using oxygen as an oxidant, is thermodynamically more convenient to carry out (Equation (1)). NO and H2O2 can also be exploited as oxidants in POM [13,14,15,16][13][14][15][16]. However, thermal catalytic conversion of methane to methanol encounters major challenges, such as the activation of C-H in methane, which occurs at extremely high temperatures.
2CH4 + O2 → 2CH3OH ΔG0 298K = −223 kJ mol−1
In addition to this, different works have been published regarding the use of zeolite-based catalysts that can contribute to the formation of methanol and acetic acid at low temperatures by activating methane and oxygen [23], although the reaction needs to be carried out at low methane conversion to preserve the target products from overoxidation.
Moreover, other studies have been aimed at addressing the challenges of the partial oxidation of methane to methanol. Some examples to overcome this phenomenon use different approaches such as the activation of methane in a liquid phase using H2O2 as an oxidant for the conversion of methane to methanol over copper-promoted Fe-ZSM-5 [24], a stepwise process for the conversion of CH4 over Cu-containing zeolite using H2O as oxidant [25], a new modified Au-Pd/zeolite catalyst for enhanced methanol productivity by in-situ generated hydrogen peroxide at low temperature (70 °C) [26], a hybrid system combining metal oxide (MOx)-coated glass beads as an alternative to thermal catalysis for the production of liquid oxygenates at atmospheric pressure and room temperature [27], a selective formation of methanol as unique oxygenate in a CO-assisted direct catalytic reaction over Cu-CHA zeolite catalyst [28], and the use of water for the mild oxidation of methane to methanol with high methanol selectivity over a gold single atom on phosphorous nanosheets under light irradiation [29].
On the other hand, NOCM (non-oxidative coupling of methane) is a promising route for the direct transformation of methane to hydrogen and ethane, despite the thermodynamically unfavorable nature of the reaction (Equation (2)):
2CH4 → C2H6 + H2 ΔG0 298K = 68.6 kJ mol−1
As mentioned, OCM (oxidative coupling of methane) is another direct route for methane conversion. During this route through Equations (3) and (4), the methane is primarily converted to C2H4 and C2H6 in the presence of an oxidant (Equation (3)):
4CH4 + O2→ 2C2H6 + 2H2O DG0 298K = −320.8 kJ mol−1
2C2H6 + O2→ 2C2H4 + 2H2O DG0 298K = −254.9 kJ mol−1
As observed, the change in Gibbs free energy is negative, and this route is thermodynamically favorable. Regarding OCM and NOCM, many studies have been presented in the literature [30].
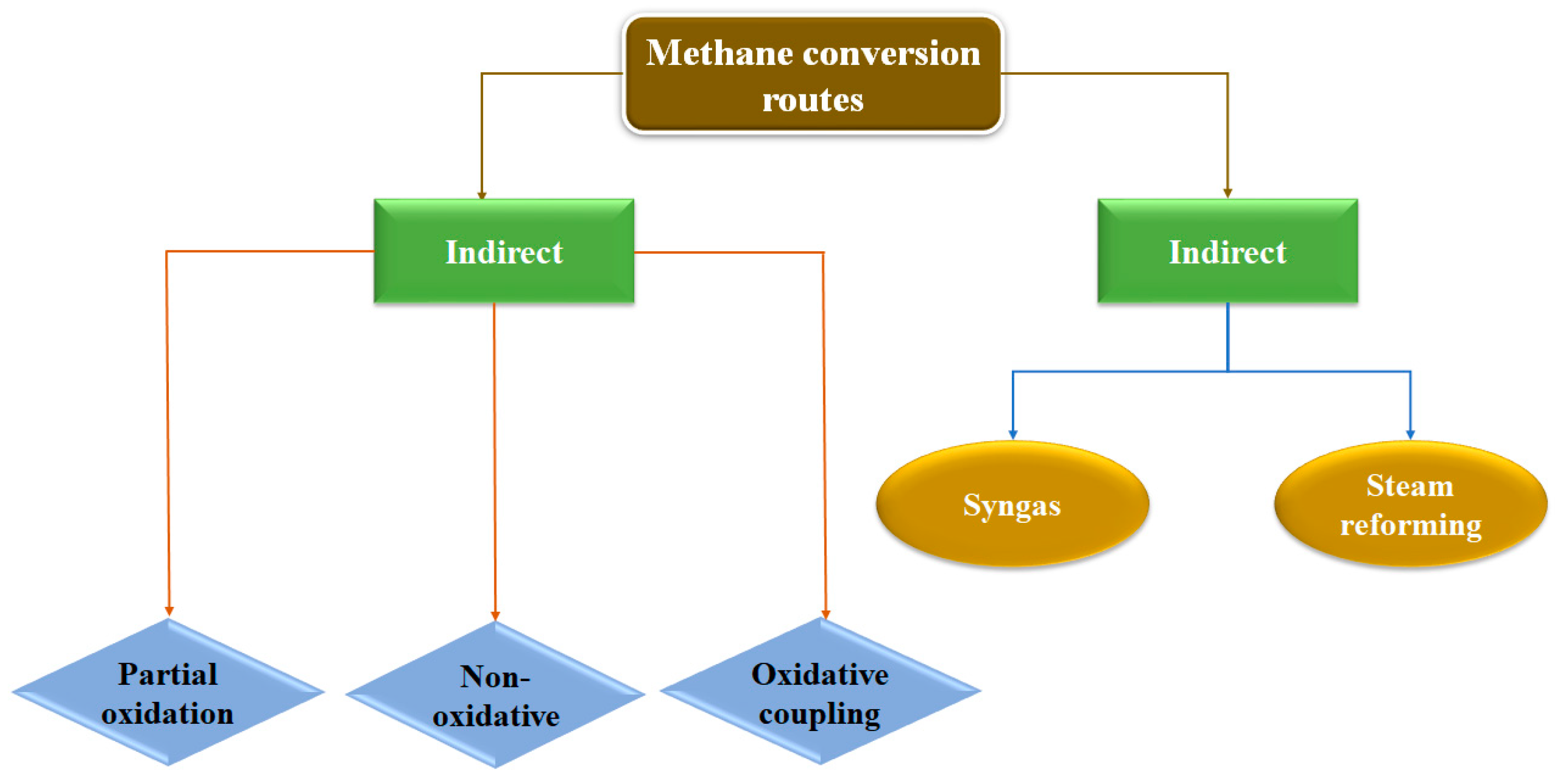
Figure 1. Routes for methane conversion.
2.2. Challenging Parameters in Methane to Methanol Catalysis
As previously commented, many catalysts have been developed and used for the direct partial oxidation of methane to methanol. However, there are several challenges regarding this catalytic process, such as the activation of the C-H bonds of methane, the need for catalyst activation, and the conditions of temperature and pressure necessary for acceptable methanol productivity and selectivity. In other words, developing a selective and efficient catalyst encounters a major challenge in the simultaneous control of the kinetics of methane transport, activation, hydroxylation, and the desorption and removal of methanol. Such challenges are summarized in Figure 2.
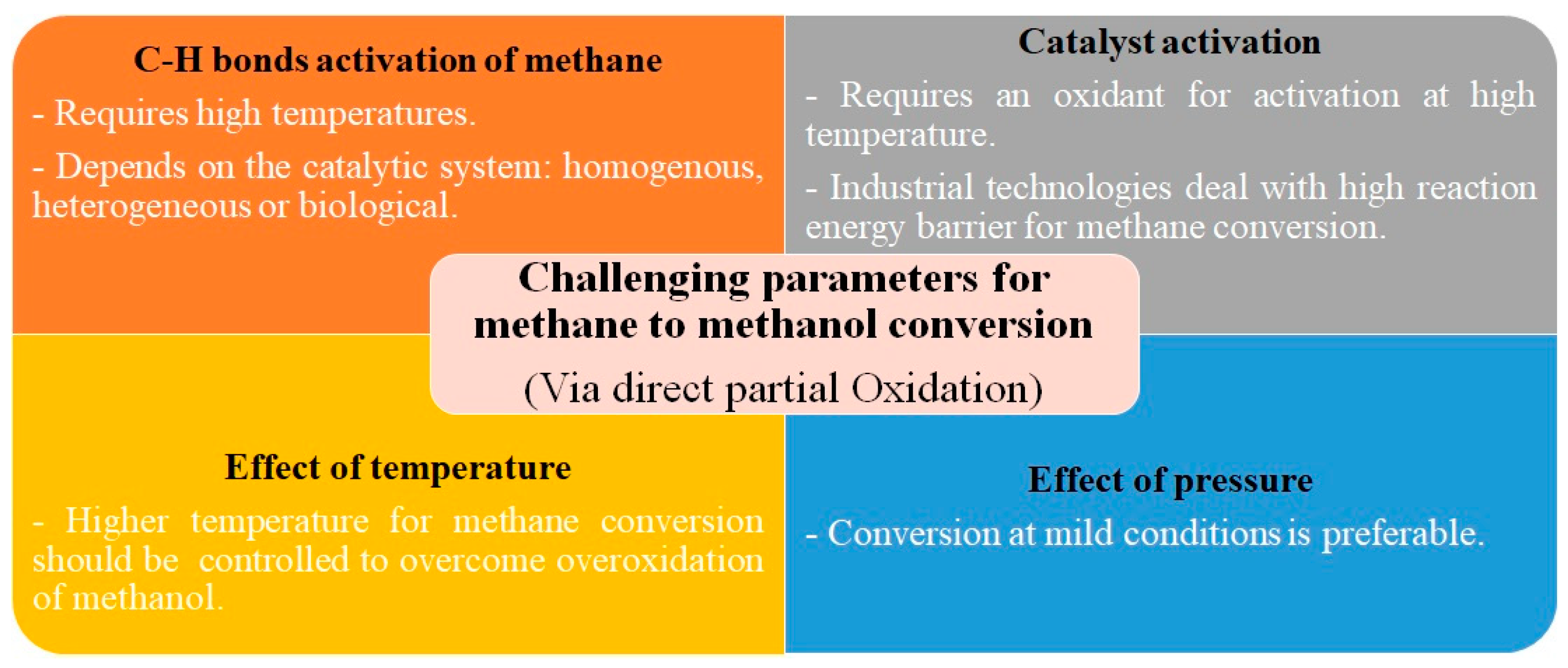
Figure 2. Challenges facing the methane activation and its conversion to methanol.
2.2.1. Activation of C-H Bonds and Its Connection to Selectivity
The activation of C-H bonds in methane requires high temperatures in traditional catalytic systems. However, under these conditions, the produced methanol can be overoxidized to produce thermodynamically more favorable products. In addition, the polar structure of methanol compared to the non-polar methane molecule contributes to the easier oxidation of methanol than methane since methanol molecules are more readily absorbed on the surface of the catalysts and activated for oxidation. Therefore, an ideal catalyst would be one that can facilitate methane activation and, at the same time, hamper methanol oxidation [17,18][17][18]. In this regard, a large number of strategies have been proposed in biological, homogenous, and heterogeneous catalytic systems. In nature, methane monooxygenase enzymes are present in aerobic methanotrophic bacteria that directly convert methane to methanol under ambient conditions due to their ability to control the transport of oxygen, methane, and protons to the active centers. Hydrophobic cavities linked together in the methane monooxygenase open the access gate to the oxygen and methane into the active center via the hydrophobic passage. Then, the activation of the oxygen in the metal center of the monooxygenase proteins leads to the formation of an oxidative intermediate that is able to perform the cleavage of the strong C-H bonds of methane [19]. When the enzymes rearrange their conformation, cavities dissociate from each other, resulting in the blockage of the hydrophobic passage and consequently restricting back diffusion and overoxidation of methanol while simultaneously opening separated hydrophilic pores for methanol to be removed. This biological system leads to an exceptionally high selectivity for methanol and can be an example of the control of mass transfer to and from the active sites. Therefore, it can be concluded that the presence of a hydrophobic cavity in the proximity of catalytic sites might lead to a higher affinity for methane than methanol [31]. However, such interesting ideas cannot be simply translated into simple homogenous catalysts [32]. In a homogenous catalytic system, the approach adopted is to functionalize methane in the form of a methyl ester that is more stable in this reaction environment. Afterward, this methyl ester is easily hydrolyzed for the recovery of methanol [33]. Regarding heterogeneous catalysis, the published studies have been focused on the investigation of materials that have a reactivity and a morphology resembling those found in methane monooxygenases. The exploitation of zeolite-based catalysts and the incorporation of different types of MOFs and graphene supports are among these attempts, and they will be discussed later.
2.2.2. Activation of Catalyst
One of the principal challenges in methane conversion to methanol is that the reaction has a stoichiometry of 1:1 [34]. This has originated the so-called “stepped conversion” process. In this procedure, the catalysts are first activated with an oxidant at a high temperature and then exposed to methane to form methanol at a lower temperature. Finally, methanol is extracted using steam flow. In this way, methanol selectivity is higher since the catalyst is exposed to the oxidant and methane separately. However, there are inevitably considerable obstacles, such as the fact that industrial technologies need a high reaction energy barrier for methane conversion; therefore, there are energy-intensive processes when it comes to practical and industrial terms [35].
2.2.3. Temperature and Pressure
Temperature and pressure are crucial parameters for methane oxidation to methanol in terms of the activation of catalysts and the cleavage of the methane C-H bond. In addition to the cost of having high temperatures, the issue of overoxidation of methanol at high temperatures is also noteworthy. A solution to achieve methanol formation at mild temperatures is the use of photocatalysts. As mentioned, the dissociation of the first C-H bond in methane is the rate-limiting step for methane activation. Under pure thermal conditions, extremely high temperatures will be required for the cleavage of the C-H bond [36]. Photocatalysis can be a potential solution to such a barrier. In photocatalysis, through the excitation of photons with high energies, active intermediates can be easily produced. Such active intermediates are capable of triggering the cleavage of the C-H bond at mild temperatures. This way, the sintering and agglomeration of active sites that usually happen due to the harsh reaction temperatures would be alleviated as well. Hence, by selecting the suitable photocatalyst, the high-temperature conversion of methane to methanol via the partial oxidation route would be feasible at low temperatures using solar energy. Photocatalytic partial oxidation of methane to methanol or formic acid can be carried out over oxide photocatalysts such as MoO3, VOX/SiO2 [14]. The active O species photogenerated on the surface of photocatalysts play a significant role in methane activation via cleaving the hydrogen from methane. Corma et al. [37] demonstrated that photoirradiation caused the dissociation of surface O-H bonds on the silica-zeolite. This resulted in the formation of Siloxyl radicals, which were capable of generating methyl radicals from methane. As far as the photocatalysis of methane is concerned, the oxidant agent is of importance. For instance, Anpo et al. [13] revealed that using nitric oxide (NO) instead of molecular oxygen as the oxidant and upon UV light irradiation over vanadium oxide immobolized on MCM-41 at ambient temperature, methanol can be formed with much higher selectivity, whereas overoxidation of methanol occurs in the presence of oxygen as the oxidant. Xie et al. [16] observed that when an appropriate amount of H2O2 was added, FeOx/TiO2 presented an outstanding performance as a photocatalyst for the methane oxidation to methanol at room temperature. FeOx/TiO2 catalyst showed a high methanol yield of 1056 mmol g−1 after 3 h of 300 W Xe lamp irradiation in a batch reactor purged with 70 mmol methane, with roughly 90% selectivity for methanol and 15% methane conversion. Yet, the challenge regarding the low selectivity of oxygenated products in photocatalytic partial oxidation of methane needs to be addressed. This low selectivity is largely due to the fact that the C-H dissociation energy of the oxygenates is lower than that of methane, inevitably resulting in the overoxidation of oxygenates such as methanol to CO2. As reported in the literature, various catalysts, including zeolites, MOFs, and graphene, together with nanomaterials immobilized in these supports, are the most commonly used systems to achieve this goal. These catalysts and their working conditions are presented in Table 1 and Table 2. It can be observed that many novel catalysts use relatively low temperatures. However, maintaining high catalytic activity and methanol selectivity under these mild conditions is a field of present research, and new findings are regularly published.
Table 1. Catalytic conditions of methanol yields and selectivity for various traditional zeolites used as catalysts for the conversion of methane to methanol.
| Catalyst | Reaction Time (min) |
Temp. (°C) |
Pressure (bar) |
Oxidant | Methanol Yield (µmol/gcat) |
Selectivity (%) |
Side Products |
Refs. |
|---|---|---|---|---|---|---|---|---|
| ZSM-5 | 60 | 600-700 | 0.01 | O2 | - | 10 | CH2O CO2 O2 |
[38] |
| FeHZSM-5 | 2.5 s (Contact time) |
630 | atmosphere | O2 | - | 16.51 | CO2 HCHO |
[39] |
| FeNaZSM-5 | 0.5 s (Contact time) |
390 | atmosphere | O2 | - | 74.37 | CO2 HCHO |
[39] |
| FeZSM-5 | 8–165 | 160 | 0.1 | N2O | 160 34 |
76 95 |
C2H5OH C2H4O |
[40] |
| Fe-ZSM-5 (84) | 30 | 50 | 30.5 | H2O2 | 74.4 | 10 | HCOOH CH3OOH |
[44][41] |
| ZSM-5 (86) | 30 | 50 | 30.5 | H2O2 | 5.55 | 72 | HCOOH CH3OOH |
[44][41] |
| Fe-silicalite-1 (86) | 30 | 50 | 30.5 | H2O2 | 65.18 | 19 | HCOOH CH3OOH |
[44][41] |
| Fe-Cu-ZSM-5 (30) | Steady state = 60 min | 50 | 20 | H2O2 | 81 (µmol gcat−1 h−1) |
92.2 | CO2 | [42] |
| Cu-SSZ-13 | 60 | 200 | 0.3 | N2O | 13.1 | 24 | CO2 HCHO |
[60][43] |
| Cu-MOR | 30 | 200 | 36 | O2 | 56 | 100 | - | [54][44] |
| Cu-MOR | 30 | 200 | 7 | H2O | 0.204 mol/molCu |
97 | H2O H2 |
[25] |
| Cu-ZSM-5-Cl | 30 | 50 | 30 | H2O2 H2O |
5866 | 79.93 | CH3OOH HOCH2OOH |
[61][45] |
| Cu-ZSM-5-N | 30 | 50 | 30 | H2O2 H2O |
3216 | 73.31 | CH3OOH HOCH2OOH |
[61][45] |
| Cu-ZSM-5-Ac | 30 | 50 | 30 | H2O2 H2O |
2851 | 74.78 | CH3OOH HOCH2OOH |
[61][45] |
| Cu-Fe(2/0.1)/ZSM-5 | 30 | 50 | 30 | H2O2 | 431 mol/molFe |
80 | HOCH2OOH CH3OOH CO2 |
[48][46] |
Table 2. Catalytic conditions and methanol yields and selectivity for metal organic frameworks (MOF) and zeolite used as supports and nanomaterials as active catalysts in the conversion of methane to methanol.
| Catalyst | Reaction Time (min) |
Temp. (°C) |
Pressure (bar) |
Oxidant | Methanol Yield (µmol/gcat) |
Methanol Selectivity (%) |
Side Products |
Refs. |
|---|---|---|---|---|---|---|---|---|
| Rh-ZSM-5 | 60 | 150 | 30 | O2 | 1224 | 8.78 | CH3COOH HCOOH |
[67][47] |
| 1%Pd/HZS-5 (30) | 30 | 50 | 30.5 | H2O2 | 51.1 | 33.6 | CH3OOH HCOOH CO2 |
[69][48] |
| Au/H-MOR | 60 | 150 | 30 | O2 | 1300 | 75 | CH3OOH HCOOH CO2 |
[70][49] |
| MIL-53 (Fe, Al) | 60 | ≤60 | 30.5 | H2O2 | - | - | CH3OOH CH2O2 CO2 |
[120][50] |
| CuxOy@UiO-bpy | 180 | 200 | 1 | O2 | 24 | 88.1 | C2H5OH | [121][51] |
| Uio-67-Pt-Z | 120 | 60 | 50 | H2O2 | - | 12.4 | C2H5OH CH3COOH |
[122][52] |
| MOF derived IrO2/CuO | 180 | 150 | 3 | H2O | 872 | 95 | C2H5OH CH3COOH |
[123][53] |
| AuPd@ZIF-8 | 30 | 90 | 15 | H2O2/O2 | 10.85 | 21.9 | CH3OOH HCOOH |
[124][54] |
| Au@ZIF-8 | 30 | 90 | 15 | H2O2/O2 | 0.7 | - | CH3OOH HCOOH |
[124][54] |
| Pd@ZIF-8 | 30 | 90 | 15 | H2O2/O2 | 1.2 | - | CH3OOH HCOOH |
[124][54] |
| MOF-808-His-Cu | 60 | 150 | - | N2O | 31.7 | 100 | - | [9] |
| MOF-808-Iza-Cu | 60 | 150 | - | N2O | 61.8 | 100 | - | [9] |
| MOF-808-Bzz-Cu | 60 | 150 | - | N2O | 71.8 | 100 | - | [9] |
| CU-NU-1000 | 30-180 | 150-200 | 1-40 | O2 | 1.5–15.81 | 70–90 | C2H5OH CO2 |
[125][55] |
| CU-NU-1000 | 180 | 200 | 1 | O2 | 17.7 | ≤46 | C2H5OH CO2 |
[126][56] |
| MIL-100(Fe) | 120 | 200 | 0.015 | N2O | 0.2 | ≥98 | CO2 | [127][57] |
| Fe-ZSM-5@ZIF-8 | 300 | 150 | 1 | - | 0.12 | - | - | [128][58] |
| Pd/Pt core-shell | 30 | 50 | 30 | H2O2 | 83 mmol gcat−1 h−1 | 92.4 | CH3OOH HCOOH HOCH2OOH |
[140][59] |
| Rh/TiO2 | 60 | 150 | 31 | H2O2 | - | 92 | [141][60] |
References
- Karl, T.R.; Trenberth, K.E. Modern Global Climate Change. Science 2003, 302, 1719–1723.
- Vali, S.A.; Markeb, A.A.; Moral-Vico, J.; Font, X.; Sánchez, A. A Novel Cu-Based Catalyst Supported in Chitosan Nanoparticles for the Hydrogenation of Carbon Dioxide to Methanol: From the Optimization of the Catalyst Performance to the Reaction Mechanism. Catal. Commun. 2023, 182, 106747.
- Bradforf, M.C.J.; Vannice, M.A. CO2 Reforming of CH4. Catal. Rev. 1999, 41, 1–42.
- Bitter, J.H.; Seshan, K.; Lercher, J.A. Mono and Bifunctional Pathways of CO2/CH4 Reforming over Pt and Rh Based Catalysts. J. Catal. 1998, 176, 93–101.
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837.
- Arutyunov, V. Low-scale direct methane to methanol—Modern status and future prospects. Catal. Today 2013, 215, 243–250.
- Yang, J.; Guo, Y. Nanostructured Perovskite Oxides as Promising Substitutes of Noble Metals Catalysts for Catalytic Combustion of Methane. Chin. Chem. Lett. 2018, 29, 252–260.
- Cihlar, J.; Vrba, R.; Castkova, K.; Cihlar, J. Effect of Transition Metal on Stability and Activity of La-Ca-M-(Al)-O (M = Co, Cr, Fe and Mn) Perovskite Oxides during Partial Oxidation of Methane. Int. J. Hydrogen Energy 2017, 42, 19920–19934.
- Beckner, M.; Dailly, A. A Pilot Study of Activated Carbon and Metal-Organic Frameworks for Methane Storage. Appl. Energy 2016, 162, 506–514.
- Baek, J.; Rungtaweevoranit, B.; Pei, X.; Park, M.; Fakra, S.C.; Liu, Y.S.; Matheu, R.; Alshmimri, S.A.; Alshehri, S.; Trickett, C.A.; et al. Bioinspired Metal-Organic Framework Catalysts for Selective Methane Oxidation to Methanol. J. Am. Chem. Soc. 2018, 140, 18208–18216.
- Aseem, A.; Jeba, G.G.; Conato, M.T.; Rimer, J.D.; Harold, M.P. Oxidative Coupling of Methane over Mixed Metal Oxide Catalysts: Steady State Multiplicity and Catalyst Durability. Chem. Eng. J. 2018, 331, 132–143.
- Alizadeh, R.; Jamshidi, E.; Zhang, G. Transformation of Methane to Synthesis Gas over Metal Oxides without Using Catalyst. J. Nat. Gas. Chem. 2009, 18, 124–130.
- Hu, Y.; Higashimoto, S.; Takahashi, S.; Nagai, Y.; Anpo, M. Selective Photooxidation of Methane into Methanol by Nitric Oxide over V-MCM-41 Mesoporous Molecular Sieves. Catal. Lett. 2005, 100, 35–37.
- Kaliaguine, S.L.; Shelimov, B.N.; Kazansky, V.B. Reactions of Methane and Ethane with Hole Centers O−. J. Catal. 1978, 55, 384–393.
- Ward, M.D.; Brazdil, J.F.; Mehandru, S.P.; Anderson, A.B. Methane Photoactivation on Copper Molybdate: An Experimental and Theoretical Study. J. Phys. Chem. 1987, 91, 6515–6521.
- Xie, J.; Jin, R.; Li, A.; Bi, Y.; Ruan, Q.; Deng, Y.; Zhang, Y.; Yao, S.; Sankar, G.; Ma, D.; et al. Highly Selective Oxidation of Methane to Methanol at Ambient Conditions by Titanium Dioxide-Supported Iron Species. Nat. Catal. 2018, 1, 889–896.
- Ahlquist, M.; Nielsen, R.J.; Periana, R.A.; Goddard, W.A. Product Protection, the Key to Developing High Performance Methane Selective Oxidation Catalysts. J. Am. Chem. Soc. 2009, 131, 17110–17115.
- Otsuka, K.; Wang, Y. Direct conversion of methane into oxygenates. Appl. Catal. A-Gen. 2001, 222, 145–161.
- Sirajuddin, S.; Rosenzweig, A.C. Enzymatic Oxidation of Methane. Biochemistry 2015, 54, 2283–2294.
- White, R.J.; Luque, R.; Budarin, V.L.; Clark, J.H.; Macquarrie, D.J. Supported Metal Nanoparticles on Porous Materials. Methods and Applications. Chem. Soc. Rev. 2009, 38, 481–494.
- Goel, S.; Wu, Z.; Zones, S.I.; Iglesia, E. Synthesis and Catalytic Properties of Metal Clusters Encapsulated within Small-Pore (SOD, GIS, ANA) Zeolites. J. Am. Chem. Soc. 2012, 134, 17688–17695.
- Zhu, Q.L.; Xu, Q. Immobilization of Ultrafine Metal Nanoparticles to High-Surface-Area Materials and Their Catalytic Applications. Chem 2016, 1, 220–245.
- Tomkins, P.; Ranocchiari, M.; van Bokhoven, J.A. Direct Conversion of Methane to Methanol under Mild Conditions over Cu-Zeolites and Beyond. Acc. Chem. Res. 2017, 50, 418–425.
- Hammond, C.; Forde, M.M.; Ab Rahim, M.H.; Thetford, A.; He, Q.; Jenkins, R.L.; Dimitratos, N.; Lopez-Sanchez, J.A.; Dummer, N.F.; Murphy, D.M.; et al. Direct Catalytic Conversion of Methane to Methanol in an Aqueous Medium by Using Copper-Promoted Fe-ZSM-5. Angew. Chem.-Int. Ed. 2012, 51, 5129–5133.
- Sushkevich, V.L.; Palagin, D.; Ranocchiari, M.; van Bokhoven, J.A. Selective anaerobic oxidation of methane enables direct synthesis of methanol. Science 2017, 356, 523–527.
- Jin, Z.; Wang, L.; Zuidema, E.; Mondal, K.; Zhang, M.; Zhang, J.; Wang, C.; Meng, X.; Yang, H.; Mesters, C.; et al. Hydrophobic zeolite modification for in situ peroxide formation in methane oxidation to methanol. Science 2020, 367, 193–197.
- Chawdhury, P.; Bhargavi, K.V.S.S.; Subrahmanyam, C. A Single-Stage Partial Oxidation of Methane to Methanol: A Step Forward in the Synthesis of Oxygenates. Sustain. Energy Fuels 2021, 5, 3351–3362.
- Sogukkanli, S.; Moteki, T.; Ogura, M. Selective Methanol FormationviaCO-Assisted Direct Partial Oxidation of Methane over Copper-Containing CHA-Type Zeolites Prepared by One-Pot Synthesis. Green. Chem. 2021, 23, 2148–2154.
- Luo, L.; Luo, J.; Li, H.; Ren, F.; Zhang, Y.; Liu, A.; Li, W.X.; Zeng, J. Water Enables Mild Oxidation of Methane to Methanol on Gold Single-Atom Catalysts. Nat. Commun. 2021, 12, 1218.
- Liu, Y.; Deng, D.; Bao, X. Catalysis for Selected C1 Chemistry. Chem 2020, 6, 2497–2514.
- Ikbal, S.A.; Colomban, C.; Zhang, D.; Delecluse, M.; Brotin, T.; Dufaud, V.; Dutasta, J.-P.; Sorokin, A.B.; Martinez, A. Bioinspired Oxidation of Methane in the Confined Spaces of Molecular Cages. Inorg. Chem. 2019, 58, 7220–7228.
- Dinh, K.T.; Sullivan, M.M.; Serna, P.; Meyer, R.J.; Dincǎ, M.; Román-Leshkov, Y. Viewpoint on the Partial Oxidation of Methane to Methanol Using Cu- and Fe-Exchanged Zeolites. ACS Catal. 2018, 8, 8306–8313.
- Gunsalus, N.J.; Koppaka, A.; Park, S.H.; Bischof, S.M.; Hashiguchi, B.G.; Periana, R.A. Homogeneous Functionalization of Methane. Chem. Rev. 2017, 117, 8521–8573.
- Marenich, A.v.; Jerome, S.v.; Cramer, C.J.; Truhlar, D.G. Charge Model 5: An Extension of Hirshfeld Population Analysis for the Accurate Description of Molecular Interactions in Gaseous and Condensed Phases. J. Chem. Theory Comput. 2012, 8, 527–541.
- Latimer, A.A.; Kakekhani, A.; Kulkarni, A.R.; Nørskov, J.K. Direct Methane to Methanol: The Selectivity-Conversion Limit and Design Strategies. ACS Catal. 2018, 8, 6894–6907.
- Kwon, Y.; Kim, T.Y.; Kwon, G.; Yi, J.; Lee, H. Selective Activation of Methane on Single-Atom Catalyst of Rhodium Dispersed on Zirconia for Direct Conversion. J. Am. Chem. Soc. 2017, 139, 17694–17699.
- Sastre, F.; Fornés, V.; Corma, A.; García, H. Selective, Room-Temperature Transformation of Methane to C1 Oxygenates by Deep UV Photolysis over Zeolites. J. Am. Chem. Soc. 2011, 133, 17257–17261.
- Kudo, H.; Ono, T. Partial oxidation of CH4 over ZSM-5 catalysts. Appl. Surf. Sci. 1997, 121, 413–416.
- Michalkiewicz, B. Partial Oxidation of Methane to Formaldehyde and Methanol Using Molecular Oxygen over Fe-ZSM-5. Appl. Catal. A Gen. 2004, 277, 147–153.
- Starokon, E.v.; Parfenov, M.v.; Arzumanov, S.S.; Pirutko, L.v.; Stepanov, A.G.; Panov, G.I. Oxidation of Methane to Methanol on the Surface of FeZSM-5 Zeolite. J. Catal. 2013, 300, 47–54.
- Hammond, C.; Dimitratos, N.; Lopez-Sanchez, J.A.; Jenkins, R.L.; Whiting, G.; Kondrat, S.A.; Ab Rahim, M.H.; Forde, M.M.; Thetford, A.; Hagen, H.; et al. Aqueous-Phase Methane Oxidation over Fe-MFI Zeolites; Promotion through Isomorphous Framework Substitution. ACS Catal. 2013, 3, 1835–1844.
- Xu, J.; Armstrong, R.D.; Shaw, G.; Dummer, N.F.; Freakley, S.J.; Taylor, S.H.; Hutchings, G.J. Continuous Selective Oxidation of Methane to Methanol over Cu- and Fe-Modified ZSM-5 Catalysts in a Flow Reactor. Catal. Today 2016, 270, 93–100.
- Ipek, B.; Lobo, R.F. Catalytic Conversion of Methane to Methanol on Cu-SSZ-13 Using N2O as Oxidant. Chem. Commun. 2016, 52, 13401–13404.
- Tomkins, P.; Mansouri, A.; Bozbag, S.E.; Krumeich, F.; Park, M.B.; Alayon, E.M.C.; Ranocchiari, M.; Vanbokhoven, J.A. Isothermal Cyclic Conversion of Methane into Methanol over Copper-Exchanged Zeolite at Low Temperature. Angew. Chem.-Int. Ed. 2016, 55, 5467–5471.
- Fang, Z.; Huang, M.; Liu, B.; Jiang, F.; Xu, Y.; Liu, X. Identifying the Crucial Role of Water and Chloride for Efficient Mild Oxidation of Methane to Methanol over a 2+-ZSM-5 Catalyst. J. Catal. 2022, 405, 1–14.
- Yu, T.; Li, Z.; Lin, L.; Chu, S.; Su, Y.; Song, W.; Wang, A.; Weckhuysen, B.M.; Luo, W. Highly Selective Oxidation of Methane into Methanol over Cu-Promoted Monomeric Fe/ZSM-5. ACS Catal. 2021, 11, 6684–6691.
- Shan, J.; Li, M.; Allard, L.F.; Lee, S.; Flytzani-Stephanopoulos, M. Mild Oxidation of Methane to Methanol or Acetic Acid on Supported Isolated Rhodium Catalysts. Nature 2017, 551, 605–608.
- Lewis, R.J.; Bara-Estaun, A.; Agarwal, N.; Freakley, S.J.; Morgan, D.J.; Hutchings, G.J. The Direct Synthesis of H2O2 and Selective Oxidation of Methane to Methanol Using HZSM-5 Supported AuPd Catalysts. Catal. Lett. 2019, 149, 3066–3075.
- Wang, W.; Zhou, W.; Tang, Y.; Cao, W.; Docherty, S.R.; Wu, F.; Cheng, K.; Zhang, Q.; Copéret, C.; Wang, Y. Selective Oxidation of Methane to Methanol over Au/H-MOR. J. Am. Chem. Soc. 2023, 145, 12928–12934.
- Li, B.; Chrzanowski, M.; Zhang, Y.; Ma, S. Applications of Metal-Organic Frameworks Featuring Multi-Functional Sites. Coord. Chem. Rev. 2016, 307, 106–129.
- Huang, Y.B.; Liang, J.; Wang, X.S.; Cao, R. Multifunctional Metal-Organic Framework Catalysts: Synergistic Catalysis and Tandem Reactions. Chem. Soc. Rev. 2017, 46, 126–157.
- Herbst, A.; Janiak, C. MOF Catalysts in Biomass Upgrading towards Value-Added Fine Chemicals. CrystEngComm 2017, 19, 4092–4117.
- Trickett, C.A.; Helal, A.; Al-Maythalony, B.A.; Yamani, Z.H.; Cordova, K.E.; Yaghi, O.M. The chemistry of metal-organic frameworks for CO2 capture, regeneration and conversion. Nat. Rev. Mater. 2017, 2, 17045.
- Maina, J.W.; Pozo-Gonzalo, C.; Kong, L.; Schütz, J.; Hill, M.; Dumée, L.F. Metal Organic Framework Based Catalysts for CO2 Conversion. Mater. Horiz. 2017, 4, 345–361.
- Vali, S.A.; Moral-Vico, J.; Font, X.; Sánchez, A. Adsorptive Removal of Siloxanes from Biogas: Recent Advances in Catalyst Reusability and Water Content Effect. Biomass Convers. Biorefin. 2023, 1–15.
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444.
- Corma, A.; García, H.; Llabrés I Xamena, F.X. Engineering Metal Organic Frameworks for Heterogeneous Catalysis. Chem. Rev. 2010, 110, 4606–4655.
- Lee, J.; Farha, O.K.; Roberts, J.M.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal—Organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459.
- Chen, J.; Wang, S.; Peres, L.; Collière, V.; Philippot, K.; Lecante, P.; Chen, Y.; Yan, N. Oxidation of Methane to Methanol over Pd@Pt Nanoparticles under Mild Conditions in Water. Catal. Sci. Technol. 2021, 11, 3493–3500.
- Gu, F.; Qin, X.; Li, M.; Xu, Y.; Hong, S.; Ouyang, M.; Giannakakis, G.; Cao, S.; Peng, M.; Xie, J.; et al. Selective Catalytic Oxidation of Methane to Methanol in Aqueous Medium over Copper Cations Promoted by Atomically Dispersed Rhodium on TiO2. Angew. Chem. Int. Ed. 2022, 61, e202201540.
More
