Circular RNA (CircRNA), a single-stranded closed-loop RNA that lacks terminal 5′ caps and 3′ poly (A) tails, is more stable than linear RNA. CircRNA was first discovered in plant-infected viroids and was later observed in eukaryotes, although it was not produced via the back-splicing mechanism.
1. Characteristics
Circular RNA (CircRNA), a single-stranded closed-loop RNA that lacks terminal 5′ caps and 3′ poly (A) tails, is more stable than linear RNA [1][8]. CircRNA was first discovered in plant-infected viroids [2][9] and was later observed in eukaryotes, although it was not produced via the back-splicing mechanism [3][10]. Increasing numbers of differentially expressed circRNAs have been identified as a result of improvements in high-throughput sequencing techniques and bioinformatics. Over the years, a variety of the biological functions of circRNAs have been disclosed. An increasing number of studies have suggested that circRNAs are involved in a variety of diseases and may serve as biomarkers for cancer diagnostic and therapeutic targets [4][11].
Through in-depth studies of circRNAs, scientists have obtained a fundamental understanding of the characteristics of circRNAs, including their diversity, expression, stability, and conservation.
-
Diversity: from fruit flies to humans, circRNAs have been discovered in a diverse range of metazoans, cell types, and species. Additionally, circRNAs have been detected in plants and other organisms;
-
Highly abundant expression: circRNAs are more abundantly distributed in human cells than their equivalent linear mRNAs, according to Salzaman
[5][12];
-
Stability: due to their distinctive topology, circRNAs, lacking 5′ caps and 3′ tails in comparison with other non-coding RNAs, are more stable than linear RNAs
[6][13]. In addition, owing to their resistance to RNA exonucleases or RNase R
[7][14], circRNAs exhibit a half-life over 48 h in most species
[6][13];
-
Conservation: the majority of circRNAs appear to be highly conservative as a result of splicing. Most circRNAs are highly conservative in various species, with only a few exceptions being unconservative
[8][15].
2. Biogenesis
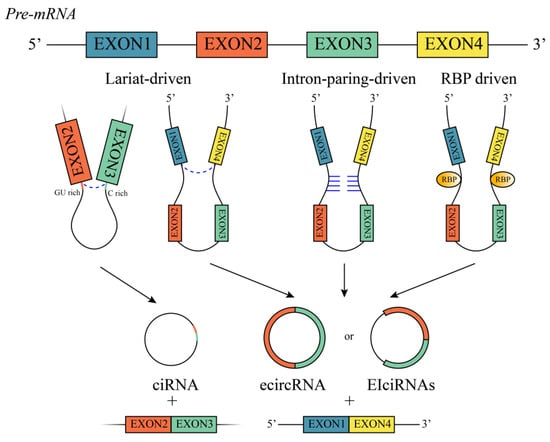
Both circRNAs and long non-coding RNAs (lncRNAs) are derived from primary mRNA; however, circRNA splicing is unique from that of other lncRNAs as they are mainly produced via back-splicing, which involves ligating an upstream 3′ splice site with a downstream 5′ splice site, generating a closed-loop structure with a specific junction point
[9][16]. CircRNAs are classified into three types according to their origins: exonic circle RNAs (ecircRNAs), circular intronic RNAs (ciRNAs), and exon–intron circular RNAs (EIciRNAs), with ecircRNAs accounting for roughly 80% of all the circRNAs that have been discovered
[10][17] (
Figure 1). Additionally, circRNAs undergo one of three commonly recognized back-splicing methods: lariat-driven, intron-pair-driven, and RNA-binding protein (RBP)-driven circularization (
Figure 1). In lariat-driven circularization, the splicing donor covalently binds to the splicing acceptor through exon skipping, thus forming an exon-containing lariat and eventually forming ecircRNAs or EIciRNAs
[6][13]. Furthermore, intron lariats with both a 7 nt GU-rich element and an 11 nt C-rich element can generate ciRNAs and resist debranching
[11][18]. With intron-paring-driven circularization, complementary base pairs between introns bring the two exons together, and the spliceosome chops away the exons and introns to form ecircRNAs or EIciRNAs
[12][19]. RBP-driven circularization involves the complementary base pairing of inverted repeats in the introns surrounding circRNA-forming exons
[13][20]. The type of circRNA produced is mostly determined by the constituents in the three possible models and by the splicing pattern.
Figure 1. Biogenesis of circRNAs. Schematic showing three commonly recognized circRNA formations: lariat-driven circularization, intron-pair-driven circularization, and RBP-driven circularization; and three types of circRNAs, including ciRNA, ecircRNA and EIciRNA.
3. Functions
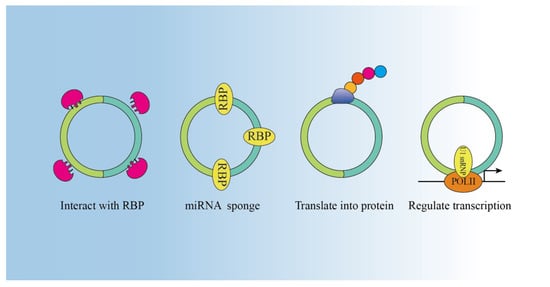
With advances in circRNA research, the biological functions of circRNAs have been revealed. The main biological functions include acting as miRNA sponges, interacting with RBPs, encoding proteins, and regulating transcription (Figure 2).
Figure 2. Functions of circRNAs: circRNAs function as miRNA sponges, thus regulating the expression of downstream genes. CircRNAs can bind with RBP. CircRNA can regulate transcription. Some circRNAs are able to be translated into protein.
3.1. miRNAs Sponge
miRNAs are endogenous RNAs that can pair with the 3′ untranslated regions of mRNAs
[14][21]. CircRNAs can interact with miRNAs and function as competing RNAs to eliminate the suppression of the miRNAs’ target gene of miRNA. CircRNAs play a crucial role in different cancers by acting as miRNA sponges. For instance, circPDSS1 promotes GC progression by acting as an miR-186-5p sponge, thus increasing NEK2 expression in gastric carcinoma
[15][22]. CircMOT1 binds with miR-9, inhibiting the progression in hepatocellular carcinoma (HCC)
[16][23]. Circ_0011946 can act as an miR-216a-5p sponge, promoting the expression of BCL2L2, whereas circ_0011946 knockdown constrains the tumorigenic phenotypes of OSCC cells
[17][24]. These results collectively indicate that the sequestering of miRNAs is a common function for circRNAs. Many circRNAs contain many different types of miRNA response elements, regulating various biological processes. For example, the knockdown of circPVT1, first reported to bind with miR-125b, suppresses OSCC cell proliferation. Later, it was found to bind with miR-106a-5p, thereby influencing important biological processes
[18][25].
3.2. Interaction with RNA Binding Proteins (RBPs)
CircRNAs directly bind with proteins to regulate cell mechanisms, participating in the progression of physiology and pathology. RBPs, a general term for proteins that can recognize RNA, play a critical role in gene transcription and translation. The absence or dysfunction of RBPs may induce various diseases. Recent data have revealed that circRNAs may interact with and alter the function of RBPs. For example, human antigen (HuR) is upregulated in various human cancers, playing a crucial role in cancer progression
[19][26]. Circ_0000745 is upregulated in OSCC cells and tissues and interacts with the HuR protein, thereby regulating the expression of CCND1
[20][27]. To summarize, circRNAs can combine with specific RBPs to facilitate or inhibit their functions.
3.3. Encoded Proteins
CircRNAs were previously recognized as endogenous RNAs unable to be translated into protein. However, some circRNAs from the cytoplasm can be translated into proteins
[21][28]. For instance, circZNF609 can act as a translation template for splicing-dependent and cap-independent translation. The product encoded by circZNF609 plays an important role in myogenesis
[22][29]. CircSHPRH is ORF-driven by the internal ribosome entry sites (IRESs) and the protein it encodes is highly expressed in the brain
[23][30]. In addition, Yi et al. found that circAXIN1 encodes a novel protein named AXIN1-295aa. AXIN1-295aa can activate the Wnt-signaling pathway, promoting GC tumorigenesis and progression by acting as an oncogenic protein
[24][31]. However, less research has been conducted on the translational function of circRNAs in OSCC. CircRNAs have the potential to encode proteins; however, bioinformatics and other methods are required to determine their mechanisms.
3.4. Transcription Regulation
CircRNAs regulate gene expression through various mechanisms, including transcription. CircRNAs can bind with U1 small nuclear ribonucleoprotein (U1 snRNP) to regulate the transcription of target genes
[25][32]. For example, ci-ankrd52 is able to interact with the RNA polymerase complex to promote parental gene transcription
[11][18].