Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Wendy Huang and Version 1 by Bingteng Xie.
ADP-ribosylation is a post-translational modification of proteins that plays a key role in various cellular processes, including DNA repair. ADP-ribosylation can regulate the recruitment and activity of DNA repair proteins by facilitating protein–protein interactions and regulating protein conformations. Moreover, ADP-ribosylation can influence additional post-translational modifications (PTMs) of proteins involved in DNA repair, such as ubiquitination, methylation, acetylation, phosphorylation, and SUMOylation. The interaction between ADP-ribosylation and these additional PTMs can fine-tune the activity of DNA repair proteins and ensure the proper execution of the DNA repair process.
- post-translational modification
- ADP-ribosylation
- crosstalk
- ubiquitination
- methylation
- acetylation
- phosphorylation
- SUMOylation
1. Introduction
Protein adenosine diphosphate (ADP)-ribosylation was first proposed in the early 1960s [1]. It involves the transfer of ADP-ribose (ADPr) from nicotinamide adenine nucleotide (NAD) to the target protein and the release of nicotinamide (NAM). This modification includes both mono-ADP-ribosylation (MAR) and poly-ADP-ribosylation (PAR). ADP-ribosylation plays a critical role in numerous biological processes such as DNA damage repair, gene regulation, and energy metabolism [2,3,4,5,6,7,8,9][2][3][4][5][6][7][8][9]. ADP-ribosylation is primarily catalyzed by ADP-ribosyltransferases (ARTs) [10], which is ubiquitous in cells and is associated with a growing number of biological processes, including DNA repair, replication, transcriptional regulation, intracellular and extracellular signaling, viral infection, cell death, and progression of mitosis.
The most well-known function of ADP-ribosylation is in DNA damage repair, where ADP-ribosylation works in a variety of ways. On the one hand, ADP-ribosylation can recruit proteins involved in DNA damage repair by interacting with the BRCT domains of these proteins. Previous studies have demonstrated that ADP-ribosylation occurs following the interaction of poly(ADP)-ribosylase 1 (PARP1) with the DSB terminus, leading to the recruitment and involvement of the DNA ligase XRCC1 and additional related repair proteins at the DNA damage site for efficient DNA repair [11]. On the other hand, ADP-ribosylation can also affect DNA repair through crosstalk with different post-translational modifications, including ubiquitination, methylation, acetylation, phosphorylation, and SUMOylation. These protein post-translational modifications interact with ADP-ribosylation in distinct ways that affect the DNA damage repair pathway.
Post-translational modification is central to regulating protein activity, stability, subcellular localization, and partner interaction. They considerably extend the functionality and diversity of the proteome and have become key players in the regulation of numerous cellular and physiological processes. As research has deepened, in addition to a single regulatory PTM, numerous proteins have been modified by multiple different types of PTM in a coordinated fashion to regulate biological outcomes. A pathway can be affected by multiple PTMs. The interaction between two PTMs involves two cases. One is positive crosstalk, where the first PTM promotes the formation or function of the second PTM. The other is negative crosstalk, where the first PTM hinders the formation or function of the second PTM. ADP-ribosylation can be in crosstalk with other modifications, such as ubiquitination, methylation, acetylation, phosphorylation, and SUMOylation (Figure 1).
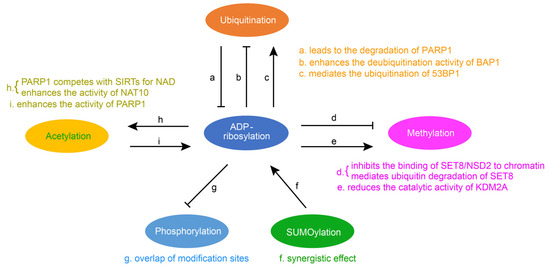
2. The Crosstalk between Ubiquitination and ADP-Ribosylation
Protein ubiquitination is one of the most significant post-translational modifications, and it leads to the degradation of proteins through proteasome or lysosome. The ubiquitin–proteasome system consists of a ubiquitin-activating enzyme (E1), a ubiquitin-coupled enzyme (E2), and a ubiquitin ligase (E3). E1 activates ubiquitin and transfers it to E2, and E3 specifically recruits ubiquitin protein substrates. The crosstalk between ubiquitin and ADP-ribosylation is mainly through E3. It has been reported that PARP1 is the substrate of ubiquitin ligase and that ADP-ribosylation is primarily affected by ubiquitin through the interaction of PARP1 with ubiquitin ligase E3. For example, as a ubiquitin ligase, WWP2 can mediate the polyubiquitination of PARP1 and lead to the degradation of PARP1 [45][12]. BAG3 is capable of binding to the BRCT domain of PARP1 and enhancing the activity of the E3 ubiquitin ligase WWP2, thereby promoting ubiquitination and subsequent degradation of PARP1 [46][13]. TRIP12, another ubiquitin ligase E3, binds PARP1 via the central PAR binding WWE domain and catalyzes PARP1 polyubiquitination by its carboxy-terminal HECT domain, triggering proteasome degradation and preventing PARP1 accumulation [47][14]. ADP-ribosylation can also affect deubiquitination. BAP1, a ubiquitin C-terminal hydrolase domain, can promote the repair of DNA damage induced by ultraviolet light (UV) through deubiquitination activity. PARP1 is able to recruit BAP1 to the site of damage and enhance the deubiquitination activity of BAP1 through PARylation [48][15]. ADP-ribosylated 53BP1 mediates the ubiquitination and degradation of 53BP1 in response to DNA damage. Recent studies have found that a hydrolase, NUDT16, can remove ADP-ribosylation from 53BP1 and inhibit the ubiquitin degradation of 53BP1, which helps to stabilize the 53BP1 protein and enables it to recruit to the histone methylation site for function [49][16].3. Crosstalk between Methylation and ADP-Ribosylation
Histone methylation is an important epigenetic modification that does not alter the charge. The crosstalk between histone methylation and ribosylation significantly impacts cell activity. The presence of ADP-ribosylation could reduce the catalytic activity of methyltransferase or demethyltransferase. SET8 is the only histone methyltransferase that manipulates histone H4K20, and it has been reported that PARP1 can interact with SET8, thereby inhibiting SET8′s binding to DNA and nucleosomes and affecting the monomethylation of histone H4K20. In addition, the binding of SET8 to PARP1 promotes PARylation of SET8 and thus mediates the ubiquitin degradation of SET8 [50][17]. NSD2 is a histone methyltransferase that functions similarly to SET8. Under oxidative stress, PARP1 can regulate NSD2 through PARylation, which inhibits the binding of NSD2 to chromatin and reduces its recruitment to target genes. This results in the decreased catalytic activity of the NSD2 histone methyltransferase [51][18]. The crosstalk between methylation and ADP-ribosylation is also reflected in the demethylase KDM2A that can be mono-ADP-ribosylated by the ADP-ribosyltransferase activity of SIRT6, resulting in increased H3K36me2 at the site of DNA damage. Finally, the initiation of transcription is inhibited, and the efficiency of nonhomologous end joining is improved [52][19].4. Crosstalk between Acetylation and ADP-Ribosylation
Acetylation in proteins occurs mainly on lysine residues. Protein acetylation can regulate a variety of protein properties, such as DNA–protein interactions, subcellular localization, transcriptional activity, and protein stability. The crosstalk between acetylation and ADP-ribosylation plays an essential role in various diseases. Sirtuins (SIRTs) are NAD-dependent deacetylases, which play their main functions through deacetylation. PARP1 indirectly regulates the activity of deacetylation and affects related physiological functions. PARP1 competes with SIRTs for NAD, resulting in aging muscle showing clear signs of mitochondrial dysfunction, oxidative stress, and inflammation [53][20]. ADP-ribosylation can also directly affect the catalytic activity of acetyltransferase. NAT10 is a member of the GNAT family of lysine acetyltransferases, and PARP1 is able to catalyze NAT10 PARylation on three conserved lysines in its C-terminal nucleolus localization signal sequence. It has been shown that the PARylation of the acetyltransferase NAT10 is key to its effects [54][21]. Additionally, the function of PARP1 can be directly affected by deacetylase. Sirtuin 3 (SIRT3) is a type III histone deacetylase, which can inhibit cardiac hypertrophy. It has been shown that, by interacting with PARP1, SIRT3 inhibits the acetylation of PARP1, thereby reducing the activity of PARP1 to inhibit cardiac hypertrophy [55][22].5. Crosstalk between Phosphorylation and ADP-Ribosylation
Phosphorylation is the first known post-translational modification of a protein. The main players in phosphorylation are protein kinases and protein phosphatases, which phosphorylate and dephosphorylate specific amino acid residues of proteins to regulate the catalytic activity of proteins. It has been shown that the serine ADP-ribosylation and phosphorylation sites greatly overlap [56][23]. Thus the ADP-ribosylation and phosphorylation crosstalk is mostly negative crosstalk. The presence of ADP-ribosylation suppresses phosphorylation so that phosphorylation-related pathways are weakened or unable to function. It has been shown that PARP1 can perform the ADP-ribosylation of the histone H2B-Glu35 to inhibit the AMP kinase-mediated phosphorylation of the neighboring H2B-Ser36 [57][24]. ADP-ribosylation at E141 of the histone mutant H2XA inhibits phosphorylation at the adjacent site S139 [58][25]. PARP1 also inhibits the phosphorylation of STAT3, which can be combined with the promoter of PD-L1 (programmed death ligand 1) to regulate it [59][26].6. Crosstalk between SUMOylation and ADP-Ribosylation
The SUMOylation process is similar to ubiquitination: It consists of maturation, activation, coupling, and defibrination steps. The amino acid sequence of SUMO proteins is similar to that of ubiquitin. The entire process of SUMOylation also requires the involvement of three enzymes, namely the SUMO-activating E1 enzyme, the SUMO-conjugating E2 enzyme, and the SUMO E3 ligase. It has been shown that the SUMOylation of PARP1 abolishes P300-mediated PARP1 acetylation and has no effect on the ADP-ribosylation of PARP1. In addition, SUMOylation inhibits the transcriptional coactivator function of PARP1, resulting in decreased expression of PARP1-regulated genes [60][27]. TDP1 is tyrosine-DNA phosphodiesterase 1, and it has been shown that the ADP-ribosylation of TDP1 in cooperation with its SUMO can promote protein stabilization and promote its function in repairing the topoisomerase I (TOP1)-trapping cleavage complex [61][28]. PIASy is a small ubiquitin-associated modifier ligase that mediates the SUMO-2/3 coupling of PARP1 on mitotic chromosomes, and SUMO-2/3 heavily binds to PARP1 on mitotic chromosomes. The polyADP-ribosylase activity of SUMO PARP1 also did not alter the accumulation of PARP1 on mitotic chromosomes. However, SUMO PARP1 has the ability to modify additional chromosomal proteins [62][29].
Figure 1. Crosstalk of ADP-ribosylation with other protein post-translational modifications. Summary of interaction relationships for ADP-ribosylation and the remaining five modifications, i.e., ubiquitination, methylation, acetylation, phosphorylation, and SUMOylation. Examples are given to illustrate the relationship between them (a [52][19], b [53][20], c [54][21], d [55[22][23],56], e [57][24], f [62][29], g [61][28], h [58[25][26],59], i [60][27]).
References
- Chambon, P.; Weill, J.; Mandel, P. Nicotinamide mononucleotide activation of a new DNA-dependent polyadenylic acid synthesizing nuclear enzyme. Biochem. Biophys. Res. Commun. 1963, 11, 39–43.
- Ray, S.; Abugable, A.A.; Parker, J.; Liversidge, K.; Palminha, N.M.; Liao, C.; Acosta-Martin, A.E.; Souza, C.D.; Jurga, M.; Sudbery, I. A mechanism for oxidative damage repair at gene regulatory elements. Nature 2022, 609, 1038–1047.
- Hoch, N.C.; Polo, L.M. ADP-ribosylation: From molecular mechanisms to human disease. Genet. Mol. Biol. 2019, 43, e20190075.
- Zhu, H.; Zheng, C. When PARPs Meet Antiviral Innate Immunity. Trends Microbiol. 2021, 29, 776–778.
- Rodríguez, M.I.; Majuelos-Melguizo, J.; Martí Martín-Consuegra, J.M.; Ruiz de Almodóvar, M.; López-Rivas, A.; Javier Oliver, F. Deciphering the insights of poly(ADP-ribosylation) in tumor progression. Med. Res. Rev. 2015, 35, 678–697.
- Rodriguez, K.M.; Cohen, M.S. Chemical genetic methodologies for identifying protein substrates of PARPs. Trends Biochem. Sci. 2022, 47, 390–402.
- Rack, J.G.M.; Palazzo, L.; Ahel, I. (ADP-ribosyl)hydrolases: Structure, function, and biology. Genes Dev. 2020, 34, 263–284.
- Qi, H.; Price, B.D.; Day, T.A. Multiple Roles for Mono- and Poly(ADP-Ribose) in Regulating Stress Responses. Trends Genet. TIG 2019, 35, 159–172.
- Puvar, K.; Luo, Z.Q.; Das, C. Uncovering the Structural Basis of a New Twist in Protein Ubiquitination. Trends Biochem. Sci. 2019, 44, 467–477.
- Lüscher, B.; Ahel, I.; Altmeyer, M.; Ashworth, A.; Bai, P.; Chang, P.; Cohen, M.; Corda, D.; Dantzer, F.; Daugherty, M.D. ADP-ribosyltransferases, an update on function and nomenclature. FEBS J. 2022, 289, 7399–7410.
- Caldecott, K.W. XRCC1 protein; Form and function. DNA Repair 2019, 81, 102664.
- Lu, X.; Huang, X.; Xu, H.; Lu, S.; You, S.; Xu, J.; Zhan, Q.; Dong, C.; Zhang, N.; Zhang, Y. The role of E3 ubiquitin ligase WWP2 and the regulation of PARP1 by ubiquitinated degradation in acute lymphoblastic leukemia. Cell Death Discov. 2022, 8, 421.
- Zhang, N.; Zhang, Y.; Miao, W.; Shi, C.; Chen, Z.; Wu, B.; Zou, Y.; Ma, Q.; You, S.; Lu, S. An unexpected role for BAG3 in regulating PARP1 ubiquitination in oxidative stress-related endothelial damage. Redox Biol. 2022, 50, 102238.
- Gatti, M.; Imhof, R.; Huang, Q.; Baudis, M.; Altmeyer, M. The ubiquitin ligase TRIP12 limits PARP1 trapping and constrains PARP inhibitor efficiency. Cell Rep. 2020, 32, 107985.
- Lee, S.-A.; Lee, D.; Kang, M.; Kim, S.; Kwon, S.-J.; Lee, H.-S.; Seo, H.-R.; Kaushal, P.; Lee, N.S.; Kim, H. BAP1 promotes the repair of UV-induced DNA damage via PARP1-mediated recruitment to damage sites and control of activity and stability. Cell Death Differ. 2022, 29, 2381–2398.
- Zhang, F.; Lou, L.; Peng, B.; Song, X.; Reizes, O.; Almasan, A.; Gong, Z. Nudix hydrolase NUDT16 regulates 53BP1 protein by reversing 53BP1 ADP-ribosylation. Cancer Res. 2022, 80, 999–1010.
- Estève, P.-O.; Sen, S.; Vishnu, U.S.; Ruse, C.; Chin, H.G.; Pradhan, S. Poly ADP-ribosylation of SET8 leads to aberrant H4K20 methylation in mammalian nuclear genome. Commun. Biol. 2022, 5, 1292.
- Huang, X.; LeDuc, R.D.; Fornelli, L.; Schunter, A.J.; Bennett, R.L.; Kelleher, N.L.; Licht, J.D. Defining the NSD2 interactome: PARP1 PARylation reduces NSD2 histone methyltransferase activity and impedes chromatin binding. J. Biol. Chem. 2019, 294, 12459–12471.
- Rezazadeh, S.; Yang, D.; Biashad, S.A.; Firsanov, D.; Takasugi, M.; Gilbert, M.; Tombline, G.; Bhanu, N.V.; Garcia, B.A.; Seluanov, A. SIRT6 mono-ADP ribosylates KDM2A to locally increase H3K36me2 at DNA damage sites to inhibit transcription and promote repair. Aging 2020, 12, 11165.
- Yeo, D.; Kang, C.; Ji, L.L. Aging alters acetylation status in skeletal and cardiac muscles. GeroScience 2020, 42, 963–976.
- Liu, H.-Y.; Liu, Y.-Y.; Zhang, Y.-L.; Ning, Y.; Zhang, F.-L.; Li, D.-Q. Poly (ADP-ribosyl) ation of acetyltransferase NAT10 by PARP1 is required for its nucleoplasmic translocation and function in response to DNA damage. Cell Commun. Signal. 2022, 20, 127.
- Feng, X.; Wang, Y.; Chen, W.; Xu, S.; Li, L.; Geng, Y.; Shen, A.; Gao, H.; Zhang, L.; Liu, S. SIRT3 inhibits cardiac hypertrophy by regulating PARP-1 activity. Aging 2020, 12, 4178.
- Larsen, S.C.; Hendriks, I.A.; Lyon, D.; Jensen, L.J.; Nielsen, M.L. Systems-wide analysis of serine ADP-ribosylation reveals widespread occurrence and site-specific overlap with phosphorylation. Cell Rep. 2018, 24, 2493–2505.e4.
- Huang, D.; Camacho, C.V.; Setlem, R.; Ryu, K.W.; Parameswaran, B.; Gupta, R.K.; Kraus, W.L. Functional interplay between histone H2B ADP-ribosylation and phosphorylation controls adipogenesis. Mol. Cell 2020, 79, 934–949.e14.
- Chen, Q.; Bian, C.; Wang, X.; Liu, X.; Ahmad Kassab, M.; Yu, Y.; Yu, X. ADP-ribosylation of histone variant H2AX promotes base excision repair. EMBO J. 2021, 40, e104542.
- Ding, L.; Chen, X.; Xu, X.; Qian, Y.; Liang, G.; Yao, F.; Yao, Z.; Wu, H.; Zhang, J.; He, Q. PARP1 Suppresses the Transcription of PD-L1 by Poly (ADP-Ribosyl) ating STAT3. Cancer Immunol. Res. 2019, 7, 136–149.
- Messner, S.; Schuermann, D.; Altmeyer, M.; Kassner, I.; Schmidt, D.; Schär, P.; Müller, S.; Hottiger, M.O. Sumoylation of poly (ADP-ribose) polymerase 1 inhibits its acetylation and restrains transcriptional coactivator function. FASEB J. 2009, 23, 3978–3989.
- Das, B.B.; Huang, S.-y.N.; Murai, J.; Rehman, I.; Ame, J.-C.; Sengupta, S.; Das, S.K.; Majumdar, P.; Zhang, H.; Biard, D. PARP1–TDP1 coupling for the repair of topoisomerase I–induced DNA damage. Nucleic Acids Res. 2014, 42, 4435–4449.
- Ryu, H.; Al-Ani, G.; Deckert, K.; Kirkpatrick, D.; Gygi, S.P.; Dasso, M.; Azuma, Y. PIASy mediates SUMO-2/3 conjugation of poly (ADP-ribose) polymerase 1 (PARP1) on mitotic chromosomes. J. Biol. Chem. 2010, 285, 14415–14423.
More
