Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Jason Zhu and Version 2 by Jason Zhu.
Molecularly imprinted polymers (MIPs) are synthetic receptors that mimic the specificity of biological antibody–antigen interactions. By using a “lock and key” process, MIPs selectively bind to target molecules that were used as templates during polymerization. While MIPs are typically prepared using conventional monomers, such as methacrylic acid and acrylamide, contemporary advancements have pivoted towards the functional potential of dopamine as a novel monomer. The overreaching goal of the proposed review is to fully unlock the potential of molecularly imprinted polydopamine (MIPda) within the realm of cutting-edge sensing applications.
- molecularly imprinted polymer
- polydopamine
1. Preparation Methods of MIPda
1.1. Self-Polymerization
The self-polymerization of dopamine is the most used technique for the synthesis of MIPda with a percentage of 65%. Dopamine, a small molecule, can undergo oxidative polymerization in the presence of a basic medium and room oxygen. The use of an oxygen-saturated solution [1] and purging air into the system was also applied [2]. The process begins with the oxidation of dopamine, which generates reactive intermediates, including quinones, leading to the formation of covalent bonds between dopamine molecules. As the polymerization progresses, template molecules are introduced into the reaction mixture, and they interact with the growing PDA network. This interaction results in the creation of specific binding sites within the polymer matrix, which can selectively recognize and bind to the target molecule. After the polymerization is complete, the template molecules are removed from the polymer matrix, leaving imprinted cavities that possess complementary shapes, sizes, and functional groups to the template molecule (Figure 1). The self-polymerization of dopamine offers advantages such as simplicity and versatility. The self-polymerization of PDA can be accelerated using chemical oxidants [3], UV irradiation [3], and microwave irradiation [4]. Ultrasound-assisted synthesis was successfully applied to accelerate the polymerization processes of conventional monomers such as methacrylic acid [5][6].
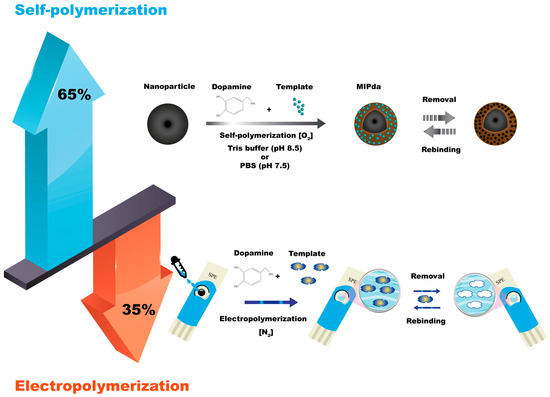
Figure 1. Self-polymerization and electropolymerization methods for synthesis of MIPda.
The self-polymerization method can be conducted in batches or by dipping solid support in a solution containing dopamine monomer [7]. Traditional self-polymerization usually requires several hours. The use of initiators to accelerate the self-polymerization of dopamine for the preparation of MIPda was introduced in 2021, achieving MIPda in only 1 h [8].
The self-polymerization of dopamine into PDA occurs using various solvents, such as sodium hydroxide, sodium bicarbonate, PBS, and Tris, resulting in PDA with distinct morphological and physicochemical characteristics associated with each solvent, as demonstrated by Patel et al. when they investigated their effects under similar pH = 8.5 [9]. Nevertheless, when it comes to the self-polymerization of dopamine for MIPda, both tris-buffer [8] and PBS [10] serve as frequently employed mediums. Tris-buffer is particularly well-suited for maintaining a pH of 8.5, while PBS is better suited for a pH of 7.5. These buffer solutions play a crucial role in facilitating the formation of PDA with desirable properties and functionalities.
Over the years, the use of the self-polymerization technique to prepare MIP was not applied only with dopamine monomer but also with other catecholamines such as norepinephrine [11][12]. In a recent development, the self-polymerization technique was successfully extended to include the 5,6-dihydroxy-1H-benzimidazole (DHBI) monomer [13]. Remarkably, DHBI exhibited comparable reaction pathways to dopamine, leading to the formation of a lightly cross-linked, p-conjugated poly(DHBI). Notably, the polymerization of DHBI demonstrated an accelerated rate compared to dopamine, and interestingly, it can be further enhanced under UV stimulation. This expansion of monomer options not only demonstrates the versatility of the self-polymerization approach but also enriches the diversity of properties of the resulting self-polymerized MIPs, opening new possibilities for adaptation to a wide range of templates.
1.2. Electropolymerization
The electrochemical synthesis of MIPda is a technique that utilizes an electrochemical process to fabricate MIPs. The process involves the electrooxidation of dopamine monomers on an electrode surface to initiate the polymerization and imprinting of target molecules. Typically, a conductive substrate, such as a glassy carbon electrode, is used as the working electrode. Dopamine monomers are dissolved in a suitable electrolyte solution, and the electrode is immersed in this solution. When an electrical potential is applied to the electrode, dopamine undergoes electrooxidation, leading to the formation of a PDA film on the electrode surface. During this electrochemical polymerization, template molecules are introduced into the system. The dopamine monomers polymerize around the template molecules, resulting in the formation of imprinted sites that possess a specific affinity for the target molecule (Figure 1).
The electrochemical synthesis of MIPda offers advantages such as precise control over the film thickness, improved accessibility of the imprinted sites, and the ability to integrate the MIPDA films onto various electrode surfaces for sensor and sensing applications.
Table 1 provides a summary of the various electrochemical methods used for the synthesis of imprinted PDA and their corresponding details. The table presents different preparation methods, core materials, templates, electrochemical conditions, sensors used, and additional comments for each method. The Comments column serves as a valuable addition to help provide context and insights that complement the data presented in the other columns. The first entry describes the use of an Au electrode and immunoglobulin G as the template, with specific electrochemical conditions applied in a PBS buffer solution [14].
Table 1. Electrochemical methods for the synthesis of imprinted PDA.
| Core Materials | Templates | Electrochemical Conditions | Sensors | Comments |
|---|---|---|---|---|
| Au electrode | Immunoglobulin G [14] | −0.45 and +0.55 Vat a scan rate of 50 mV s−1 in PBS buffer Solution |
QCM sensor | The igg was immobilized on the electrode surface before the synthesis of MIPda |
| Au electrode | Sulfamethoxazole [15] | Between−0.6 and 0.6 V at a rate of 20 mv s−1 for 60 cycle | Amperometric detection | No significant imprinting and no considerable difference between MIP and NIP |
| GCE modified with aunps@fullerene | An amino-aptamer for 2,4,6-trinitrotoluene [16] | −0.5 to 0.5 V At 20 mV s−1 (13 cycles) |
Impedimetric detection | The preparation method is not easy |
| Nickel nanoparticles Wrapped with carbon |
Uric acid [17] | −0.6 to 0.6 V for 10 cycles, scan rate 50 mv/s, and the electrolyte was 0.01 M Phosphate buffer solution (pH 7.4) |
DPV | The uric acid can be oxidized during electro-polymerization in the potential range of −0.6 to 0.6 V |
| AuNPs/CNTs/GCE | Urea–aptamer complex [18] | −0.5 to 0.5 V vs. Ag/Agcl at a scan rate of 20 mV s−1 (13 cycles) |
Impedance Spectroscopy | The urea was immobilized on the surface SH-AuNPs/CNTs/GCE |
| Cds/Zn/Ti substrate | L-phenylalanine [19] | +1.5 V to −1.5 V at 50 mV/s, 20 cycles | Photoelectrochemical | The template can be oxidized during polymerization |
| Au electrode | Carboxylic-acid-based Structural analogs (’dummy’ templates) for nitro-explosives (2,4,6-trinitrotoluene, TNT) And (1,3,5-trinitroperhydro-1,3,5-triazine [20]. |
−0.5 V and +0.5 V 0.02 V s−1 for 15 cycles | -- | Dopamine was identified in silico, based on DFT (density functional theory) calculations |
| Aunp-coated screen-printed carbon electrode | Ovalbumin Ige-binding epitope [21] |
−0.5 to +1.0 V at a scan rate of 50 mV/s for 10 cycles |
DPV | The pH of solution, concentration of template, and number of cycles of electropolymerization were optimized |
Before the synthesis of MIPda, the immobilization of IgG on the electrode surface takes place. Subsequent entries highlight diverse approaches, including the use of sulfamethoxazole [15], an amino-aptamer for 2,4,6-trinitrotoluene [16], uric acid [17], urea–aptamer complex [18], L-phenylalanine [19], carboxylic-acid-based structural analogs [20], and the ovalbumin IgE-binding epitope [21] as templates. Various core materials, such as Au nanoparticles, GCE modified with AuNP@fullerene, and nickel nanoparticles wrapped with carbon, are utilized in the electrochemical synthesis. The sensors employed for detection purposes range from QCM sensors to amperometric and impedance spectroscopy-based techniques. The comments provided in Table 1 shed light on aspects such as imprinting efficiency, the ease of the preparation method, and the optimization of the electrochemical parameters.
The number of electropolymerization cycles has a significant effect on the imprinting process. The development and sensing efficacy of the imprinted polymeric membrane hinged on the number of scan cycles [22]. A low cycle count resulted in a delicate and easily damaged MIP film during template removal. Moreover, an insufficient number of recognition sites formed [23]. Conversely, an excessive electropolymerization yielded a thick PDA layer that hindered template removal. Additionally, excessively coating the non-conductive PDA compromised electron transfer and sensitivity [24].
On the other hand, the pH of the electropolymerization solution plays an important role in MIPda-based electrochemical sensors. The pH of the MIP solution has a significant impact on the electrode sensing performance. At high pH levels, the phenolic group in PDA becomes deprotonated while, at low pH levels, the amino group becomes protonated. This property can influence PDA synthesis, necessitating pH optimization of the MIP solution. The findings revealed that the highest current values were observed at pH 7.5 and 8.0. While Yang et al. achieved the thickest PDA growth at pH 8.5, the resulting MIP PDA lacked satisfactory sensitivity [25]. Recent studies indicated that a pH of 8.5 or higher could trigger premature, uncontrolled DA polymerization before surface synthesis [25]. Table 1 provides information about the electrochemical methods used for the synthesis of MIPda, including the core materials used, templates, electrochemical conditions, sensors employed, and additional comments.
Self-polymerization may interfere with electropolymerization, particularly when electropolymerization is conducted in the basic medium at a low rate. However, the researchers avoid this interference by bubbling N2 to remove oxygen from the electropolymerization solutions [26][27].
As shown in Figure 1, the self-polymerization (65%) of dopamine offers versatility in preparing MIPda composites, including core-shell structures and nanocomposites. This allows integration into various type of sensors including optical and electrochemical sensors and SPE methods. In contrast, electropolymerization (35%) mainly focuses on electrochemical sensors by depositing PDA films on electrodes. While it provides control over film thickness and site accessibility, it is mainly used for electrode preparation.
2. Characteristics of MIPda
2.1. Structure of PDA
The structure of PDA remains highly intricate and, as of yet, a definitive and comprehensive understanding of its formation and complete structure has not been conclusively established. Despite numerous proposed structures and mechanistic pathways, as presented in the literature [28], a clear mechanism for its formation still eludes researchers. Researchers have put forth various theories about the composition and structure of PDA. Some studies suggest that PDA is composed of covalently linked dihydroxy indole, indoledione, and dopamine units [29]. However, an alternative structural model has been proposed recently, suggesting that the dihydroxyindoline, indoline-dione, and dopamine units are not covalently linked but instead held together through hydrogen bonding between oxygen atoms or π stacking interactions [28]. Furthermore, Liebschera et al. developed another structural model, proposing mixtures of different oligomers in PDA, where indole units with varying degrees of (un)saturation and open-chain dopamine units coexist [30].
2.2. Morphology
Surface roughness plays a critical role in characterizing the properties of MIPda. To investigate surface morphologies, atomic force microscopy (AFM) was utilized to analyze MIPda-coated and NIP-coated quartz crystal microbalance (QCM) crystals [31] and SiO2 NPs [32]. The root means squared surface roughness (Rq) measurements were employed to monitor morphological changes on the surface of materials. Notably, the MIPda-QCM and MIPda-SiO2 exhibited a higher Rq value, suggesting a rougher surface compared to the NIP-QCM crystal and NIP-SiO2. Moreover, the surface of MIPda-SiO2 and NIP-SiO2 are rougher than the SiO2 surface, confirming the well decoration of PDA on the SiO2 [32]. These findings emphasize the influence of surface functionalization with the MIPda film on the overall roughness, highlighting the importance of surface roughness in characterizing MIPDA-based materials. The MIPda adopts various core/support shapes, including spherical [1], film [33], nanosheet [34], and nanotube [35] shapes.
The Fe3O4-MIPda image appeared smoother in comparison to the image of Fe3O4 NPs. The QCM crystals with the coating exhibited a slight blurriness when contrasted with the bare QCM crystal. This effect is likely attributed to charging artifacts arising from the deposition of non-conductive MIPda films on the surface. Upon applying the MIPda layer coating, there was a notable alteration in the surface morphology, transforming the previously gully-like surface into a granular texture.
MIPda’s properties are intricately linked to the thickness of its layers. Typically, MIPda exhibits an ultra-thin layer, with thicknesses measuring less than 10 nm [32][35][36], making it highly promising for precise drug delivery and molecular recognition applications. Surprisingly, researchers have also observed instances of relatively thicker MIPda layers [2][37], which further expands its potential for versatile use. The adaptability of MIPda in different shapes and layer thicknesses opens up exciting opportunities for advancing drug delivery systems and molecular sensing technologies.
2.3. Wettability
During the polymerization process of dopamine to form PDA, various functional groups, such as amine and hydroxyl, are incorporated into the material’s structure, leading to its hydrophilic nature. These functional groups facilitate hydrogen bonding and other interactions with water molecules. These interactions create a conducive environment for the diffusion of target analytes through the MIPda material, enhancing the accessibility of the imprinted cavities to the target molecules. This improved accessibility is essential for the efficient binding and recognition of the target analytes. Water contact angle measurements serve as a valuable tool for characterizing the wettability of MIPda. These measurements play a crucial role in validating the successful modification of substrate materials with MIPda films. Following surface functionalization, the contact angles of MIPda-based materials exhibit a notable decrease compared to bare materials, highlighting the enhanced wettability conferred by MIPda. This decrease in contact angles indicates an increase in the hydrophilicity of the substrate surface. The improved hydrophilicity can be attributed to the presence of hydroxyl groups within the PDA structure [31], along with the contribution of hydrophilic quinone/hydroquinone moieties present in the MIPda material [38]. These combined factors work synergistically to create a surface that strongly interacts with water molecules, holding significant implications for various applications where controlled wettability and specific molecular interactions are essential.
3. Applications of MIP-PDA in Sensors
MIPda has emerged as a groundbreaking material that significantly improves molecular-recognition-based sensing approaches. MIPda is a synthetic polymer with exceptional affinity and selectivity for specific target molecules, making it an ideal candidate for sensor applications. The unique properties of MIPda, such as its robustness, biocompatibility, and ease of synthesis, have fueled its integration into various sensing platforms, including optical, electrochemical, and SPE coupled to sensors. This introductory paragraph delves into the diverse applications of MIPda in these sensor technologies, showcasing its remarkable versatility and potential to revolutionize a wide array of fields, ranging from healthcare diagnostics and environmental monitoring to food safety and beyond.
3.1. MIPda-Based Optical Sensors
MIPda-based optical sensors have found diverse and impactful applications thanks to their unique combination of molecular imprinting and optical sensing. For instance, a microfiber interference sensor based on MIPda was developed by Liu et al. for the specific detection of C-reactive protein [39]. Unlike traditional imprinting methods, this approach induced the rearrangement of the template molecule during dopamine self-polymerization, creating complementary hydrophobic/hydrophilic and charge distributions in addition to shape and size within the imprinting cavity. By combining this approach with optical fiber interferometry, the optical sensor demonstrated a significantly low LOD of 5.8 × 10−10 ng/mL, outperforming commercial ELISA kits by eight orders of magnitude. The CMIP-PDA microfiber sensor exhibited strong repeatability, high selectivity, and the potential for ultrasensitive, label-free CRP diagnosis. This innovative technique opens doors to quantitatively monitoring biomarkers with extremely low concentrations and has broader applications for detecting various biomolecules.
Lu et al. developed a unique approach using MIPda in a sandwich structure, combined with dual signal amplification using MnO2 nanosheets and MoS2 nanoflowers, to construct a fluorescence sensor for the ultrasensitive detection of trace amounts of carcinoembryonic antigen (CEA) [34]. The sensors utilize a combination of MIPda, MnO2 nanosheets, and MoS2 nanoflowers to achieve high sensitivity and specificity. The sensors can detect CEA concentrations in a linear range of 0.01 ng/mL to 10 μg/mL, with a remarkably low LOD of 3.5 pg/mL.
Another interesting approach was introduced by Wang et al. for the detection of antibiotics using an optical sensor based on SPR [40]. This aimed to create a localized surface plasmon resonance (LSPR) biosensor for swift, sensitive, and specific enrofloxacin (ENRO) detection, using MIPda as the recognition element. PDA-MIP film was formed on an LSPR sensor chip’s surface through dopamine and ENRO polymerization. The sensor selectively captured ENRO post-blocking and removal steps. To enhance detection signals, protein-conjugate competitors amplified LSPR signals. Detection took only 20 min, with a range of 25–1000 ng/mL. MIPda film showed higher ENRO binding than its non-imprinted counterparts, distinguishing ENRO from analogs. With high sensitivity, specificity, reusability, and stability, the developed LSPR/MIPda sensor holds the potential for rapid in-field ENRO residue detection.
Table 2 provides an overview of the key research works on MIPda-based optical sensors. It highlights target analytes, detection methods, and significant features of each study. MIPda-based optical sensors show great promise in enhancing detection precision and sensitivity. These sensors use tailored molecular imprinting to bind specifically with target molecules, allowing accurate measurement. PDA integration adds versatility, forming recognition sites through polymerization. Successful in various fields, like environmental monitoring and medical diagnostics, these sensors utilize light–MIP interactions for easily measurable optical changes. Real-time, label-free detection makes them invaluable for rapid and precise analyses. Continual technological progress ensures their potential for diverse applications in complex analytical scenarios.
Table 2. Overview of research works on MIPda-based optical sensors.
| Target Analyte | Detection Method | LOD | Notable Features |
|---|---|---|---|
| C-reactive protein (CRP) [39] | Microfiber interference sensor | 5.8 × 10−10 ng/mL | Low LOD, label-free diagnosis of CRP |
| Carcinoembryonic antigen (CEA) [34] | Fluorescence sensor | 3.5 pg/mL | Sandwich structure, dual signal amplification |
| Enrofloxacin (ENRO) [40] | Localized SPR biosensor | 25–1000 ng/mL | LSPR-based sensor for ENRO residue detection |
3.2. MIPda-Based Electrochemical Sensors
MIPda is emerging as a promising functional material for designing highly selective electrochemical sensors [20][21][27][35][41][42][43]. The unique properties of MIPda, including facile preparation, good stability, biocompatibility, and abundant functional groups, make them suitable for molecular imprinting/recognition and electrochemical sensing applications. MIPda can be electrodeposited on conductive surfaces modified electrodes by the one-step self-polymerization of dopamine in the presence of a template molecule. This creates selective recognition cavities in the polymer matrix complementary to the template in shape, size, and functional groups. Recently, MIPda has found many applications in the field of electrochemical sensing. Indeed, some of the key applications of the MIPda include the use of these sensitive materials for the electrochemical sensing of nitro-explosives, the detection of small molecule food contaminants, and the diagnosis of infectious diseases. In this context, Li et al. have created an electrochemical sensor to detect illicit stimulants MDA and MDMA (ecstasy) [27]. They used a MIPda film on a gold electrode, prepared via electrochemical polymerization. The film had specific binding sites for MDA and MDMA, leading to strong detection. The sensor showed excellent performances with low detection limits (37 nM for MDA, 54 nM for MDMA) using differential pulse voltammetry. It proved selective, stable, reproducible, and effective with real urine samples. This sensor could be a rapid diagnostic tool for detecting MDA and MDMA abuse in drug investigations.
Another interesting application of MIPda in electrochemical sensing has been introduced by Yin et al. for sunset yellow sensing [32]. They utilized self-polymerized PDA in water to form an MIP on multi-walled carbon nanotubes (MWCNTs), using sunset yellow as a template. The resulting nanocomposites were examined for their electrochemical response to sunset yellow (Figure 2). The modified electrode displayed precise and sensitive detection of sunset yellow due to well-matched cavities on MWCNTs and the blocking effect of non-imprinted PDA. The sensor showed a linear relationship from 2.2 nM to 4.64 μM, with a low LOD of 1.4 nM. It proved to have selectivity, stability, reproducibility, and successful real-sample detection. This technique holds promise for other PDA-based MIP sensors and practical uses.
Recently, the detection of nitro-explosives has been explored using the MIPda-based electrochemical sensor. In this context, Leibl et al. developed a sensitive electrochemical sensor to detect nitro-explosives in water using thin MIPda films [20]. The MIP films were electropolymerized on gold electrodes through cyclic voltammetry, with dummy templates to mimic the nitro-explosive molecules TNT and 1,3,5-trinitroperhydro-1,3,5-triazine. These imprinted films enhanced sensitivity 105-fold compared to unmodified gold electrodes. The MIP films concentrated the target molecules near the transduction element, resulting in improved sensitivity. The MIP films showed reproducible binding in a phosphate buffer, with a dynamic detection range of 0.1 nM to 10 nM for both TNT and 1,3,5-trinitroperhydro-1,3,5-triazine. They also exhibited increased selectivity over similar related compounds.
MIPda has found application in protein detection, especially for Ovalbumin protein. Indeed, in a recent study reported by Khumsap et al., a precise electrochemical sensor was developed by imprinting PDA with the OVA IgE-binding epitope to detect ovalbumin [21]. They optimized various factors in the process and used differential pulse voltammetry with K3Fe(CN)6 and KCl electrolyte to detect OVA. The sensor demonstrated remarkable sensitivity with a detection limit of 10.76 nM, a linear range from 23.25 to 232.50 nM, and strong selectivity against other proteins. Successful detection in wine samples showed potential for broader use in identifying allergenic proteins in the food chain.
Table 3 summarizes the notable research endeavors involving MIPda-based electrochemical sensors. The table outlines the target analytes, electrode modification methods, detection techniques, and key characteristics of each investigation. Its easy preparation, tunable selectivity, and sensitivity make MIPda a promising interface for the electroanalysis of various chemicals and biomolecules. The imprinting approach can be further explored to improve the analytical performance of PDA-based electrochemical sensors. These applications demonstrate the versatility and potential of MIPda in electrochemical sensors for various analytical purposes.
Table 3.
Overview of research works on MIPda-based electrochemical sensors.
. The authors synthesized a composite material comprising MIPda coated onto magnetic nanoparticles (Fe3O4@PDA@MIP) through an environmentally friendly process. This composite facilitated the magnetic dispersive solid-phase extraction (MDSPE) of ERT-B, ensuring rapid extraction, reusability, and effective pre-concentration. Notably, Fe3O4@PDA@MIP exhibited a strong imprinting factor (3.0 ± 0.05), showcasing remarkable selectivity against interfering substances such as patent blue and other food matrix components. The MDSPE approach was paired with a smartphone-based colorimetric detection method, yielding performance akin to UV–visible spectroscopy detection. The achieved reliable ERT-B quantification within the range of 0.5–10 mg/L, with a detection limit of 0.04 mg/L. The developed method was successfully applied to determine ERT-B content in various food samples, including juice, candy, and candied cherries, yielding satisfactory recovery values (82–97%).
Table 4 offers a concise compilation of the research efforts related to MIPda-based SPE. It details the target analytes, extraction methods, detection strategies, and noteworthy aspects of each study. The integration of MIPda into SPE has demonstrated remarkable potential and versatility in enhancing the selectivity, efficiency, and sensitivity of analyte extraction from complex matrices. The tailored molecular recognition properties of MIPda materials provide a unique advantage, allowing for the specific binding and efficient retention of target molecules. This synergy between MIPda and SPE addresses challenges in various fields, ranging from environmental monitoring to pharmaceutical analysis. The resultant MIPda-based SPE methods offer improved accuracy, reduced interference, and enhanced preconcentration capabilities, making them invaluable tools for sample preparation and analysis.
Table 4. Overview of research works on MIPda-based SPE.
| Target Analyte | Extraction Method | Detection Method | LOD | Notable Features |
|---|---|---|---|---|
| Cinnamic acid, ferulic acid, caffeic acid [45] | Magnetic imprinted polymers | HPLC analysis | -- | Selective extraction from complex matrix |
| Human serum albumin (HSA) [44] | Magnetic imprinted polymers | HPLC analysis | -- | Protein capture from urine samples |
| Erythrosine B (ERT-B) [46] | Magnetic dispersive SPE | Smartphone-based colorimetric detection | 0.04 mg/L | Selective extraction and colorimetric detection |
| Target Analyte | Electrode Modification Method | Detection Method | LOD | Notable Features |
|---|---|---|---|---|
| MDA and MDMA [27] | Electrochemical polymerization | Differential Pulse Voltammetry | 37 nM for MDA, 54 nM for MDMA | Rapid detection of illicit stimulants |
| Sunset Yellow [32] | Self-polymerized PDA on MWCNTs | Electrochemical response | 1.4 nM | Precise SY detection, MWCNT cavities |
| Nitro-explosives [20] | Electropolymerized MIP films | Cyclic Voltammetry | 0.1 nM–10 nM | Enhanced sensitivity |
| Ovalbumin protein [21] | Imprinted PDA electrode | Differential Pulse Voltammetry | 10.76 nM | Sensitivity for allergenic protein detection |
3.3. Solid-Phase Extraction Coupled to Sensors
MIPda combined with SPE forms a synergistic approach that enhances the selectivity and efficiency of analyte extraction and detection [44][45]. MIPda provides specific binding sites tailored for the target molecule, improving the extraction process’ selectivity. When integrated with SPE, the MIPda acts as a specialized sorbent, selectively capturing the target analyte from a complex sample matrix. This combination enhances pre-concentration, reduces interference, and improves overall sensitivity in analytical methods. A result is a powerful tool for the extraction and detection of specific compounds in diverse fields, such as environmental monitoring, pharmaceutical analysis, and food safety, where accuracy and precision are essential [45].
Yin et al. presented a new approach involving magnetic imprinted polymers coated with cinnamic acid polydopamine, designed for the simultaneous and selective extraction of cinnamic acid, ferulic acid, and caffeic acid from radix scrophulariae samples [45]. These innovative magnetic imprinted polymers were created through surface imprinting polymerization, utilizing magnetic MWCNTs as support material, cinnamic acid as the template, and dopamine as the functional building block. The analysis demonstrated excellent magnetic properties, a high adsorption capacity, selectivity, and rapid binding kinetics for cinnamic acid, ferulic acid, and caffeic acid. By coupling with high-performance liquid chromatography, researchers extensively investigated the magnetic imprinted polymers’ use as a magnetic SPE sorbent under varying extraction conditions. Successfully employed for the purification and enrichment of cinnamic acid, ferulic acid, and caffeic acid from radix scrophulariae extracts, the proposed imprinted magnetic SPE method achieved recoveries of 92.4–115.0% for cinnamic acid, 89.4–103.0% for ferulic acid, and 86.6–96.0% for caffeic acid.
Another interesting application of MIPda in SPE, as reported by Yin et al., is for protein capture [44]. Indeed, an innovative and cost-effective approach was developed to imprint proteins onto magnetic MMWNTs surfaces. Human serum albumin (HSA) was used as the template, while dopamine served as the functional monomer. The maximum adsorption capacity for HSA by the magnetic imprinted polymers was determined to be 66.23 mg g−1, and equilibrium adsorption was achieved within 20 min. The MIPda demonstrated remarkable selective adsorption capabilities, specifically for HSA. When combined with high-performance liquid chromatography (HPLC) analysis, the magnetic imprinted polymers were effectively employed for SPE and the detection of HSA in urine samples, yielding successful recoveries ranging from 91.95% to 97.8%.
Recently, Elfadil et al. have introduced a study for extracting and quantifying erythrosine B (ERT-B) in food samples [46]
References
- Wang, L.; Miao, L.; Yang, H.; Yu, J.; Xie, Y.; Xu, L.; Song, Y. A Novel Nanoenzyme Based on Fe3O4 Nanoparticles@thionine-Imprinted Polydopamine for Electrochemical Biosensing. Sens. Actuators B Chem. 2017, 253, 108–114.
- Chen, J.; Lei, S.; Xie, Y.; Wang, M.; Yang, J.; Ge, X. Fabrication of High-Performance Magnetic Lysozyme-Imprinted Microsphere and Its NIR-Responsive Controlled Release Property. ACS Appl. Mater. Interfaces 2015, 7, 28606–28615.
- Du, X.; Li, L.; Li, J.; Yang, C.; Frenkel, N.; Welle, A.; Heissler, S.; Nefedov, A.; Grunze, M.; Levkin, P.A. UV-Triggered Dopamine Polymerization: Control of Polymerization, Surface Coating, and Photopatterning. Adv. Mater. 2014, 26, 8029–8033.
- Lee, M.; Lee, S.-H.; Oh, I.-K.; Lee, H. Microwave-Accelerated Rapid, Chemical Oxidant-Free, Material-Independent Surface Chemistry of Poly(Dopamine). Small 2017, 13, 1600443.
- Lamaoui, A.; Lahcen, A.A.; García-Guzmán, J.J.; Palacios-Santander, J.M.; Cubillana-Aguilera, L.; Amine, A. Study of Solvent Effect on the Synthesis of Magnetic Molecularly Imprinted Polymers Based on Ultrasound Probe: Application for Sulfonamide Detection. Ultrason. Sonochem. 2019, 58, 104670.
- Lamaoui, A.; María Palacios-Santander, J.; Amine, A.; Cubillana-Aguilera, L. Computational Approach and Ultrasound Probe–Assisted Synthesis of Magnetic Molecularly Imprinted Polymer for the Electrochemical Detection of Bisphenol A. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2022, 277, 115568.
- Arabi, M.; Ostovan, A.; Wang, Y.; Mei, R.; Fu, L.; Li, J.; Wang, X.; Chen, L. Chiral Molecular Imprinting-Based SERS Detection Strategy for Absolute Enantiomeric Discrimination. Nat. Commun. 2022, 13, 5757.
- Lamaoui, A.; Palacios-Santander, J.M.; Amine, A.; Cubillana-Aguilera, L. Molecularly Imprinted Polymers Based on Polydopamine: Assessment of Non-Specific Adsorption. Microchem. J. 2021, 164, 106043.
- Patel, K.; Singh, N.; Yadav, J.; Nayak, J.M.; Sahoo, S.K.; Lata, J.; Chand, D.; Kumar, S.; Kumar, R. Polydopamine Films Change Their Physicochemical and Antimicrobial Properties with a Change in Reaction Conditions. Phys. Chem. Chem. Phys. 2018, 20, 5744–5755.
- Khumsap, T.; Bamrungsap, S.; Thu, V.T.; Nguyen, L.T. Development of Epitope-Imprinted Polydopamine Magnetic Nanoparticles for Selective Recognition of Allergenic Egg Ovalbumin. Chem. Pap. 2022, 76, 6129–6139.
- Chen, J.; Liang, R.-P.; Wang, X.-N.; Qiu, J.-D. A Norepinephrine Coated Magnetic Molecularly Imprinted Polymer for Simultaneous Multiple Chiral Recognition. J. Chromatogr. A 2015, 1409, 268–276.
- Baldoneschi, V.; Palladino, P.; Banchini, M.; Minunni, M.; Scarano, S. Norepinephrine as New Functional Monomer for Molecular Imprinting: An Applicative Study for the Optical Sensing of Cardiac Biomarkers. Biosens. Bioelectron. 2020, 157, 112161.
- Fan, K.W.; Peterson, M.B.; Ellersdorfer, P.; Granville, A.M. Expanding the Aqueous-Based Redox-Facilitated Self-Polymerization Chemistry of Catecholamines to 5,6-Dihydroxy-1H-Benzimidazole and Its 2-Substituted Derivatives. RSC Adv. 2016, 6, 25203–25214.
- Tretjakov, A.; Syritski, V.; Reut, J.; Boroznjak, R.; Volobujeva, O.; Öpik, A. Surface Molecularly Imprinted Polydopamine Films for Recognition of Immunoglobulin G. Microchim. Acta 2013, 180, 1433–1442.
- Turco, A.; Corvaglia, S.; Mazzotta, E.; Pompa, P.P.; Malitesta, C. Preparation and Characterization of Molecularly Imprinted Mussel Inspired Film as Antifouling and Selective Layer for Electrochemical Detection of Sulfamethoxazole. Sens. Actuators B Chem. 2018, 255, 3374–3383.
- Shahdost-fard, F.; Roushani, M. Impedimetric Detection of Trinitrotoluene by Using a Glassy Carbon Electrode Modified with a Gold Nanoparticle@fullerene Composite and an Aptamer-Imprinted Polydopamine. Microchim. Acta 2017, 184, 3997–4006.
- Wang, Y.; Liu, X.; Lu, Z.; Liu, T.; Zhao, L.; Ding, F.; Zou, P.; Wang, X.; Zhao, Q.; Rao, H. Molecularly Imprinted Polydopamine Modified with Nickel Nanoparticles Wrapped with Carbon: Fabrication, Characterization and Electrochemical Detection of Uric Acid. Microchim. Acta 2019, 186, 414.
- Yarahmadi, S.; Azadbakht, A.; Derikvand, R.M. Hybrid Synthetic Receptor Composed of Molecularly Imprinted Polydopamine and Aptamers for Impedimetric Biosensing of Urea. Microchim. Acta 2019, 186, 71.
- Dashtian, K.; Hajati, S.; Ghaedi, M. L-Phenylalanine-Imprinted Polydopamine-Coated CdS/CdSe n-n Type II Heterojunction as an Ultrasensitive Photoelectrochemical Biosensor for the PKU Monitoring. Biosens. Bioelectron. 2020, 165, 112346.
- Leibl, N.; Duma, L.; Gonzato, C.; Haupt, K. Polydopamine-Based Molecularly Imprinted Thin Films for Electro-Chemical Sensing of Nitro-Explosives in Aqueous Solutions. Bioelectrochemistry 2020, 135, 107541.
- Khumsap, T.; Bamrungsap, S.; Thu, V.T.; Nguyen, L.T. Epitope-Imprinted Polydopamine Electrochemical Sensor for Ovalbumin Detection. Bioelectrochemistry 2021, 140, 107805.
- Lian, W.; Liu, S.; Yu, J.; Xing, X.; Li, J.; Cui, M.; Huang, J. Electrochemical Sensor Based on Gold Nanoparticles Fabricated Molecularly Imprinted Polymer Film at Chitosan-Platinum Nanoparticles/Graphene-Gold Nanoparticles Double Nanocomposites Modified Electrode for Detection of Erythromycin. Biosens. Bioelectron. 2012, 38, 163–169.
- Razavipanah, I.; Alipour, E.; Deiminiat, B.; Rounaghi, G.H. A Novel Electrochemical Imprinted Sensor for Ultrasensitive Detection of the New Psychoactive Substance “Mephedrone”. Biosens. Bioelectron. 2018, 119, 163–169.
- Jia, Z.; Li, H.; Zhao, Y.; Frazer, L.; Qian, B.; Borguet, E.; Ren, F.; Dikin, D.A. Electrical and Mechanical Properties of Poly(Dopamine)-Modified Copper/Reduced Graphene Oxide Composites. J. Mater. Sci. 2017, 52, 11620–11629.
- Yang, B.; Lv, S.; Chen, F.; Liu, C.; Cai, C.; Chen, C.; Chen, X. A Resonance Light Scattering Sensor Based on Bioinspired Molecularly Imprinted Polymers for Selective Detection of Papain at Trace Levels. Anal. Chim. Acta 2016, 912, 125–132.
- Yaman, Y.T.; Bolat, G.; Abaci, S.; Saygin, T.B. Peptide Nanotube Functionalized Molecularly Imprinted Polydopamine Based Single-Use Sensor for Impedimetric Detection of Malathion. Anal. Bioanal. Chem. 2022, 414, 1115–1128.
- Li, C.; Han, D.; Wu, Z.; Liang, Z.; Han, F.; Chen, K.; Fu, W.; Han, D.; Wang, Y.; Niu, L. Polydopamine-Based Molecularly Imprinted Electrochemical Sensor for the Highly Selective Determination of Ecstasy Components. Analyst 2022, 147, 3291–3297.
- Dreyer, D.R.; Miller, D.J.; Freeman, B.D.; Paul, D.R.; Bielawski, C.W. Elucidating the Structure of Poly(Dopamine). Langmuir 2012, 28, 6428–6435.
- Shalev, T.; Gopin, A.; Bauer, M.; Stark, R.W.; Rahimipour, S. Non-Leaching Antimicrobial Surfaces through Polydopamine Bio-Inspired Coating of Quaternary Ammonium Salts or an Ultrashort Antimicrobial Lipopeptide. J. Mater. Chem. 2012, 22, 2026–2032.
- Liebscher, J.; Mrówczyński, R.; Scheidt, H.A.; Filip, C.; Hădade, N.D.; Turcu, R.; Bende, A.; Beck, S. Structure of Polydopamine: A Never-Ending Story? Langmuir 2013, 29, 10539–10548.
- Lim, H.J.; Saha, T.; Tey, B.T.; Tan, W.S.; Hassan, S.S.; Ooi, C.W. Quartz Crystal Microbalance-Based Biosensing of Hepatitis B Antigen Using a Molecularly Imprinted Polydopamine Film. Talanta 2022, 249, 123659.
- Bonyadi, S.; Ghanbari, K. Development of Highly Sensitive and Selective Sensor Based on Molecular Imprinted Polydopamine-Coated Silica Nanoparticles for Electrochemical Determination of Sunset Yellow. Microchem. J. 2021, 167, 106322.
- Lim, H.J.; Saha, T.; Tey, B.T.; Lal, S.K.; Ooi, C.W. Quartz Crystal Microbalance-Based Biosensing of Proteins Using Molecularly Imprinted Polydopamine Sensing Films: Interplay between Protein Characteristics and Molecular Imprinting Effect. Surf. Interfaces 2023, 39, 102904.
- Lu, H.; Xu, S. Carbon Nanofibers Coated with Fe3O4 Nanoparticles and MnO2 Nanosheets Further Modified with Molecularly Imprinted Polydopamine for Fluorescence Sensing of Carcinoembryonic Antigen. ACS Appl. Nano Mater. 2022, 5, 2532–2540.
- Yin, Z.-Z.; Cheng, S.-W.; Xu, L.-B.; Liu, H.-Y.; Huang, K.; Li, L.; Zhai, Y.-Y.; Zeng, Y.-B.; Liu, H.-Q.; Shao, Y.; et al. Highly Sensitive and Selective Sensor for Sunset Yellow Based on Molecularly Imprinted Polydopamine-Coated Multi-Walled Carbon Nanotubes. Biosens. Bioelectron. 2018, 100, 565–570.
- Chen, W.; Fu, M.; Zhu, X.; Liu, Q. Protein Recognition by Polydopamine-Based Molecularly Imprinted Hollow Spheres. Biosens. Bioelectron. 2019, 142, 111492.
- Lan, F.; Ma, S.; Yang, Q.; Xie, L.; Wu, Y.; Gu, Z. Polydopamine-Based Superparamagnetic Molecularly Imprinted Polymer Nanospheres for Efficient Protein Recognition. Colloids Surf. B Biointerfaces 2014, 123, 213–218.
- Almeida, L.C.; Correia, J.P.; Viana, A.S. Electrochemical and Optical Characterization of Thin Polydopamine Films on Carbon Surfaces for Enzymatic Sensors. Electrochim. Acta 2018, 263, 480–489.
- Liu, X.; Lin, W.; Xiao, P.; Yang, M.; Sun, L.-P.; Zhang, Y.; Xue, W.; Guan, B.-O. Polydopamine-Based Molecular Imprinted Optic Microfiber Sensor Enhanced by Template-Mediated Molecular Rearrangement for Ultra-Sensitive C-Reactive Protein Detection. Chem. Eng. J. 2020, 387, 124074.
- Wang, W.; Wang, R.; Liao, M.; Kidd, M.T.; Li, Y. Rapid Detection of Enrofloxacin Using a Localized Surface Plasmon Resonance Sensor Based on Polydopamine Molecular Imprinted Recognition Polymer. Food Meas. 2021, 15, 3376–3386.
- Cheng, S.; Tang, D.; Zhang, Y.; Xu, L.; Liu, K.; Huang, K.; Yin, Z. Specific and Sensitive Detection of Tartrazine on the Electrochemical Interface of a Molecularly Imprinted Polydopamine-Coated PtCo Nanoalloy on Graphene Oxide. Biosensors 2022, 12, 326.
- Roushani, M.; Sarabaegi, M.; Rostamzad, A. Novel Electrochemical Sensor Based on Polydopamine Molecularly Imprinted Polymer for Sensitive and Selective Detection of Acinetobacter Baumannii. J. Iran Chem. Soc. 2020, 17, 2407–2413.
- Tlili, A.; Attia, G.; Khaoulani, S.; Mazouz, Z.; Zerrouki, C.; Yaakoubi, N.; Othmane, A.; Fourati, N. Contribution to the Understanding of the Interaction between a Polydopamine Molecular Imprint and a Protein Model: Ionic Strength and PH Effect Investigation. Sensors 2021, 21, 619.
- Yin, Y.; Yan, L.; Zhang, Z.; Wang, J. Magnetic Molecularly Imprinted Polydopamine Nanolayer on Multiwalled Carbon Nanotubes Surface for Protein Capture. Talanta 2015, 144, 671–679.
- Yin, Y.; Yan, L.; Zhang, Z.; Wang, J.; Luo, N. Polydopamine-Coated Magnetic Molecularly Imprinted Polymer for the Selective Solid-Phase Extraction of Cinnamic Acid, Ferulic Acid and Caffeic Acid from Radix Scrophulariae Sample: Liquid Chromatography. J. Sep. Sci. 2016, 39, 1480–1488.
- Elfadil, D.; Della Pelle, F.; Compagnone, D.; Amine, A. Green Synthesis of Molecularly Imprinted Polymers for Dispersive Magnetic Solid-Phase Extraction of Erythrosine B Associated with Smartphone Detection in Food Samples. Materials 2022, 15, 7653.
More
