Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Jesús Ruiz-Ramos.
Despite the demonstrated effectiveness of antimicrobial stewardship programs in antibiotic consumption and minimizing multi-drug-resistant bacterium development, the characteristics of emergency departments pose a challenge to their implementation. The inclusion of rapid diagnostic tests, tracking microbiological results upon discharge, conducting audits with feedback, and implementing multimodal educational interventions have proven to be effective tools for optimizing antibiotic use in these units.
- antimicrobial stewardship
- drug resistance
- emergency care
1. Introduction
The progressive increase in antibiotic resistance over the past decades has had a significant impact on healthcare systems worldwide [1,2,3][1][2][3]. It is known that excessive and inappropriate uses of antimicrobials have contributed to the generation, acceleration, and perpetuation of these multi-drug-resistant strains [4]. To minimize the development of antimicrobial resistance, successful antimicrobial stewardship programs (ASPs) have been developed in recent decades, demonstrating optimization and reduction in antimicrobial use while minimizing the generation and spread of multi-drug-resistant infections [5,6][5][6]. Consequently, the implementation of these programs has been recognized as a priority by healthcare authorities and scientific societies [7,8][7][8].
Most of the described experiences of such programs to date have focused on hospitalized patients, particularly critical patients, as well as, more recently, in the outpatient setting [9,10][9][10]. Hospital emergency departments (EDs) are particularly relevant for the implementation of ASPs. These units are where the first doses of antibiotics are prescribed in the hospital, both for incoming patients and those returning to primary care, as well as for a significant number of patients discharged directly to their homes or other healthcare facilities. Moreover, several studies have highlighted a significant increase in the number of infections caused by multi-drug-resistant bacteria in these units [11,12][11][12]. Despite guidelines recognizing EDs as key sites for ASP implementation, multidisciplinary team participation in such units remains limited [13]. Additionally, there is a lack of uniformity in ASP activities carried out in EDs, as well as in the way clinical and antimicrobial use outcomes are monitored [14,15][14][15].
2. Multi-Drug-Resistant Bacteria in Emergency Departments
The widespread increase in antimicrobial resistance complicates the choice of appropriate empirical treatment, with a direct impact on the morbidity and mortality of patients, especially in cases of sepsis [16]. EDs present specific challenges for monitoring bacterial resistance and adopting measures to prevent its spread due to its inherent characteristics: the need for rapid care, short stays, and, in most cases, discharge of patients to their homes with empirical treatment for unidentified pathogens [17]. Moreover, up to 80% of hospitalized patients enter through EDs, underscoring the crucial role of this service in introducing multi-drug-resistant microorganisms to the hospital. On the other hand, empirical treatment initiated in these units often continues in hospital wards, significantly impacting antimicrobial consumption [17]. Several epidemiologic surveillance studies have shown an increase in the prevalence of infections caused by multi-drug-resistant strains. A longitudinal surveillance study conducted in several US hospitals from 2012 to 2017 showed a decrease in the incidence of infections caused by methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus spp., carbapenem-resistant Acinetobacter spp., and multi-drug-resistant Pseudomonas aeruginosa. The incidence of infections caused by carbapenem-resistant Enterobacterales did not change, but the incidence of infections caused by extended-spectrum β-lactamase (ESBL)-producing strains increased by 53.3%, especially due to a rise in community-acquired infections [18]. In the European Centre for Disease Prevention and Control (ECDC) surveillance 2021 report, an increase was observed in the percentage of carbapenem-resistant E. coli and K. pneumoniae strains, vancomycin-resistant Enterococcus faecium, and carbapenem-resistant Acinetobacter spp., as well as an increase in strains of Streptococcus pneumoniae with reduced susceptibility to penicillin (14% in 2017 to 16% in 2021) [19]. Furthermore, there has been a dramatic increase in the prevalence of Gram-negative bacteria producing narrow spectrum β-lactamase CTX-M in recent decades, replacing TEM and SHV variants as the most frequent type of ESBL. The blaCTX-M genes have migrated to highly transmissible plasmids associated with the community circulation of ESBLs [20]. In healthcare units (HUHs), despite having a limited number of records, a significant increase in infections caused by multidrug-resistant strains has been observed. A multicenter study conducted in HUHs in the United States between 2018 and 2019 found a prevalence of ESBL-producing strains in urinary tract infection (UTI) patient samples of 17%, ranging from 5% to 45%, depending on the region studied. Resistance rates to other antibiotics were 32.3% for fluoroquinolones, 13.7% for gentamicin, 1.3% for amikacin, and 0.3% for meropenem [21]. Another retrospective study of all patients presenting with febrile UTIs at HUHs in 21 different centers in the US between 2017 and 2019 found that of the 4107 included patients, 530 (12.9%) had infections caused by Escherichia coli, Klebsiella pneumoniae, or Proteus mirabilis that were resistant to third-generation cephalosporins. In this patient group, empirical antibiotic treatment was discordant in 63% of cases, compared to 7% of controls without resistance (OR 21.0; 95% CI 16.9 to 26.0). They also had a longer hospital stay (adjusted mean difference of 29.7 h; 95% CI 19.0 to 40.4) and higher 90-day mortality (12% in patients with resistance versus 8% in controls, adjusted OR 1.56; 95% CI 1.07 to 2.28) [22]. Regarding ED, there are limited published studies regarding variations in the resistance profile, specifically in these units, which will be a key element for improvement in the coming years. However, some studies have shown a progressive increase in infections by ESBL-producing Enterobacteriaceae in the case of urinary infections and bacteremia [23[23][24],24], which will be the main challenge for ASP in the coming years. A multinational survey of non-hospitalized patients with infections caused by ESBL-producing Enterobacterales identified risk factors of recent antibiotic use; these included residing in a long-term care facility, recent hospitalization, age of 65 years or older, and male gender [25]. Another study identified hospitalization, long-term care, antibiotic exposure in the previous 90 days, and isolation of a fluoroquinolone- or ceftriaxone-resistant strain in the previous year as risk factors [26]. Although several studies have attempted to develop clinical tools to predict the risk of infection by multidrug-resistant Enterobacterales [25[25][26][27],26,27], the results obtained lack specificity, as some community-acquired UTI patients caused by ESBL-producing Enterobacterales do not exhibit risk factors that imply the selection of appropriate empirical treatment [22]. In fact, in Ben-Ami et al.’s multinational study, 34% of ESBL-producing strain isolates (115 out of 336 strains) had no identified contact with the healthcare system [20]. Given all of the above, the appropriateness of empirical antibiotic treatment in EDs is becoming increasingly complex in an environment with constantly rising resistance rates. Physicians working in these units are aware to varying degrees of this issue, and they complain of a lack of feedback on antibiotic prescriptions and knowledge about local resistance rates. In this sense, artificial intelligence models should play a key role in EDs in the coming years, helping to predict the risk of multidrug resistance in patients treated in EDs [28].3. Antimicrobial Prescription in Emergency Departments
The EDs are an important setting for evaluating inappropriate antibiotic prescribing practices, given that they exist at the intersection of the community and the hospital. The EDs also present unique challenges for appropriate antibiotic prescribing, as clinicians often encounter diagnostic uncertainty and time constraints. Various studies have assessed the percentage of inappropriate antibiotic prescriptions in the ED, yielding divergent results ranging from 25% to 50% [29,30,31][29][30][31]. The variability in the definitions used to delineate this wide range can account for much of this inconsistency. Nevertheless, all studies indicate that there is substantial room for improvement in antimicrobial prescription practices in the ED, although their impact on the development of resistance and clinical outcomes remains uncertain.4. Antimicrobial Stewardship Indicators in Emergency Departments
Despite various scientific societies positioning EDs as preferred points for the implementation of ASPs [32[32][33],33], there still exists a lack of uniformity in the types of indicators used to monitor ASP activities in these units. Differences in ED operations, the diversity of treated pathologies and patient profiles, limitations of information systems, and loss of patient follow-up after discharge hinder their implementation. Over the past few years, studies describing ASP experiences in EDs have utilized diverse indicators. A recent systematic review of 26 studies categorized the ASP indicators used in EDs into four categories: antimicrobial consumption indicators, microbiological indicators, process indicators, and outcome indicators. It concluded that the high heterogeneity of indicators used in EDs makes monitoring these programs challenging, emphasizing the need to standardize indicators for optimizing antimicrobial use in these units [34]. Recently, Ruiz et al. [35], using a modified Delphi methodology, attempted to standardize the prioritized indicators for ASP implementation in these units, establishing different levels of priority for their adoption. Based on these reviews, ASP activities in EDs should primarily focus on reducing antimicrobial consumption and improving health outcomes, similar to hospital-based ASPs. However, the impact of ASP activities in EDs on microbiological indicators and resistance reduction remains uncertain. Difficulties exist that hinder indicator standardization. Firstly, not all hospitals have the necessary informatics tools to measure antimicrobial consumption in these units, making it challenging to assess its magnitude in the ED and, more importantly, to determine if a direct reduction is achieved. Another question is what is the most suitable unit of measurement for antimicrobial consumption in EDs. Since these services have high patient turnover and do not generate hospital stays, the percentage of patients receiving antibiotics in the ED, or the defined daily dose (DDD) per 1000 patients, has been the most commonly employed indicator [36]. Another unit of measurement that could be considered is the number of prescriptions per 1000 inhabitants for those antibiotics and infections that do not require admission and are managed on an outpatient basis. Another aspect to consider is which group of antibiotics would be a priority for monitoring their consumption. Due to their impact on resistance generation, relevant indicators would focus on carbapenems and fluoroquinolones due to their high impact on cross-resistance to other antibiotics; aminoglycosides due to their high nephrotoxicity and ototoxicity; and finally, coverage of anti-MRSA agents. It is also advisable to monitor antimicrobial consumption from the perspective of the most prevalent infectious syndromes. Some relevant indicators could include the percentage of patients diagnosed with pneumonia treated with quinolones, the percentage of patients treated with antibiotics for exacerbation of chronic obstructive pulmonary disease (COPD), the percentage of multi-resistant-organism-caused UTIs, or the percentage of patients with skin and soft tissue infections receiving anti-MRSA coverage [34]. The World Health Organization (WHO) created a new classification in 2017 in which antibacterial medicines were stratified into three groups, Access, Watch, and Reserve (AWaRe), based on their spectrum, anticipated risk of resistance development, risk of toxicity, and clinical utility; this was reviewed in 2021 [37]. The monitoring of antibiotics in the emergency department should consider the risk of antibiotic resistance described in this document. A recent systematic review found that more restricted utilization of “Watch” and “Reserve” agents such as carbapenems, third-generation cephalosporins, or quinolones might be greatly beneficial [38]. Most common ED infections can be either treated with no or Access antibiotics. It should be noted that all antibiotics, irrespective of AWaRe category, could be associated with antibiotic-resistant strain selection, indicating the need to enhance focus on symptomatic care for minor infections with no routine antibiotic treatment. On the other hand, to achieve better health outcomes, the most relevant interventions would be those that demonstrate a decrease in the number of return visits to the ED or infection-attributed mortality. An interesting measure is the review of cultures from discharged patients to verify if the microorganism is susceptible to the prescribed antibiotic.5. Antimicrobial Stewardship Interventions in the Emergency Department
Implementing ASP activities in EDs poses a significant challenge. The unique characteristics of this unit, including high patient and staff turnover, make it difficult to carry out many of the ASP activities typically applied in hospitalized patients, such as de-escalation of antibiotic treatment or optimization of duration based on patient evolution. It should be noted that each intervention requires a personalized approach, evaluating potential barriers and facilitators before execution. However, there are several activities that have proven highly effective in optimizing antimicrobial use in these units [14]. The main threats for ASP implementations in EDs and the most popular interventions described in these units are reflected in Figure 1 and Figure 2.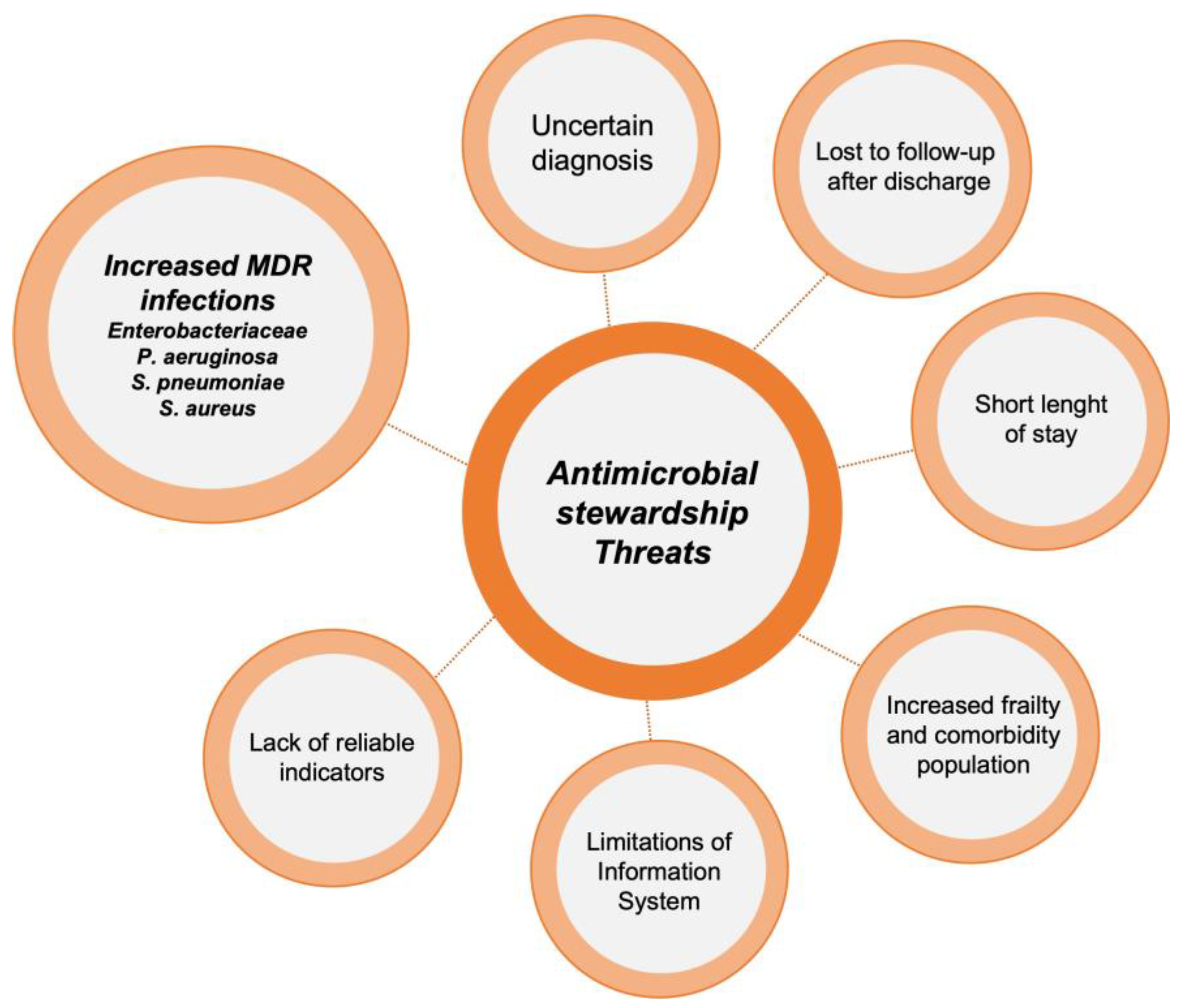
Figure 1.
Threats to the implementation of antimicrobial stewardship programs in emergency departments.
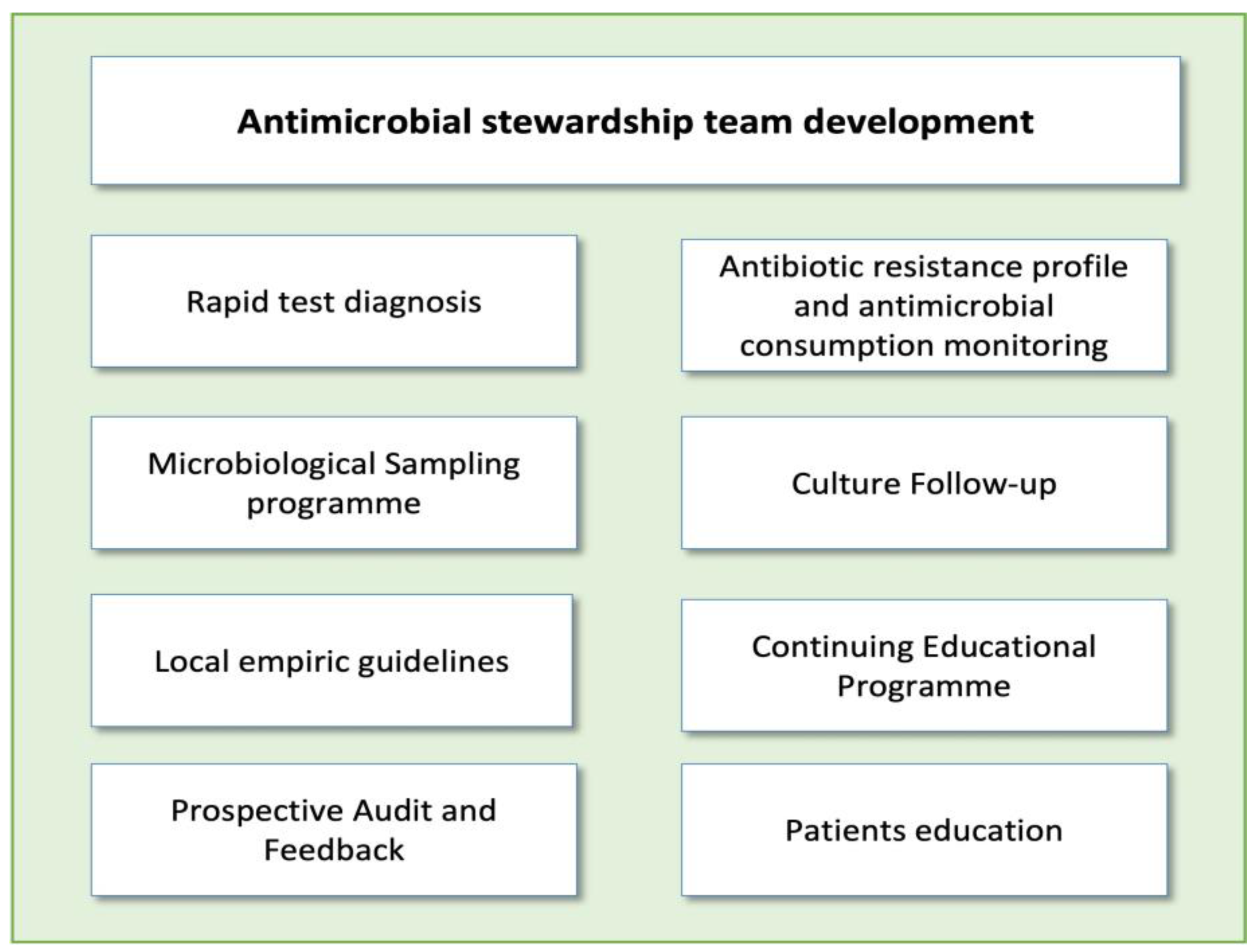
Figure 2.
Main antimicrobial stewardship interventions in emergency departments.
6. Outcomes of ED Antimicrobial Stewardship Programs
To date, there are few high-quality studies on the effectiveness of ASPs in EDs, with most of the described experiences being single-center descriptive studies with limited analysis of clinical evolution and resistance generation [14,40][14][39].
The implementation and adaptation of clinical guidelines and protocols associated with ASPs have been mainly described for respiratory infections and UTIs, yielding mixed results regarding antibiotic reduction. Angoulvant et al. [90][40] demonstrated a significant change in antibiotic prescription trends in seven pediatric EDs following the implementation of national empirical treatment guidelines for respiratory infections. On the other hand, Akenroye et al. [91][41], comparing outcomes before and after implementing a protocol for bronchiolitis, observed improvement in diagnostic test ordering without a significant reduction in antibiotic use.
The effectiveness of interventions seems to be related to the intensity of the intervention, primarily of an educational nature. Buising et al. [92][42] compared three different strategies over three different time periods: a first episode of delivering treatment guidelines to prescribers, a second episode incorporating educational activities, and a third episode introducing an electronic prescription support system with hospital guidelines. The risk of receiving guideline-concordant treatment significantly increased in the second (OR: 2.79 (1.88–4.14)) and third (OR: 1.99 (1.07–3.69)) periods compared to the first period.
Marrie et al. [93][43], in a clinical trial comparing conventional management versus an intervention protocol for pneumonia based on clinical guideline recommendations, showed a decrease in length of stay, hospitalization rates, and duration of intravenous antibiotic therapy. Metlay et al. [94][44] observed a 10% reduction in antibiotic prescriptions for acute respiratory infections in the intervention group in a clinical trial across 16 hospitals, including audit and feedback interventions and educational material delivery to clinicians and patients, which included posters, educational brochures, and a waiting room video. Borde et al. [95][45] implemented an ASP aimed at reducing cephalosporin and fluoroquinolone prescriptions in the ED. They reviewed and updated local guidelines for the most common infections, conducted an educational activity on antimicrobial use, and promoted consultation with infectious disease units, resulting in a reduction in daily antibiotic doses, especially for cephalosporins.
In the case of ASPs focused on UTIs, the main objectives have been centered on reducing antibiotic treatment for conditions with positive urine isolation without infection, including asymptomatic bacteriuria, facilitating appropriate diagnosis and selection of appropriate antimicrobial therapy, specifically with a reduction in quinolones for uncomplicated cystitis. Studies have focused on including educational interventions for prescribing physicians, with specific recommendations for microbiological sample collection and local guidelines based on institutional antibiograms. In a study that included the development of specific UTI guidelines based on institutional resistance patterns, recommendations for sample collection, and feedback to physicians, Hecker et al. [96][46] observed improved adherence to UTI guidelines with a 30% decrease in fluoroquinolone use for uncomplicated cystitis. Percival et al. [97][47] showed a 40% increase in adherence to UTI guidelines, including a specific educational program within the institution and educational material delivered via email, along with a retrospective audit and feedback to prescribers.
References
- Serra-Burriel, M.; Keys, M.; Campillo-Artero, C.; Agodi, A.; Barchitta, M.; Gikas, A.; Palos, C.; López-Casasnovas, G. Impact of multi-drug resistant bacteria on economic and clinical outcomes of healthcare-associated infections in adults: Systematic review and meta-analysis. PLoS ONE 2020, 15, e0227139.
- Eliopoulos, G.M.; Cosgrove, S.E.; Carmeli, Y. The Impact of Antimicrobial Resistance on Health and Economic Outcomes. Clin. Infect. Dis. 2003, 36, 1433–1437.
- Friedman, N.D.; Temkin, E.; Carmeli, Y. The negative impact of antibiotic resistance. Clin. Microbiol. Infect. 2016, 22, 416–422.
- Maortua, H.; Canut, A.; Ibáñez, B.; Martínez, D.; de Domingo, M.J.; Labora, A. Relationship between in-hospital bacterial resistance and antimicrobial use over a 13-year period. Enferm. Infect. Microbiol. Clin. 2009, 27, 441–448.
- Wagner, B.; Filice, G.A.; Drekonja, D.; Greer, N.; MacDonald, R.; Rutks, I.; Butler, M.; Wilt, T.J. Antimicrobial stewardship programs in inpatient hospital settings: A systematic review. Infect. Control Hosp. Epidemiol. 2014, 35, 1209–1228.
- Nathwani, D.; Varghese, D.; Stephens, J.; Ansari, W.; Martin, S.; Charbonneau, C. Value of hospital antimicrobial stewardship programs : A systematic review. Antimicrob. Resist. Infect. Control 2019, 8, 35.
- Society for Healthcare Epidemiology of America; Infectious Diseases Society of America; Pediatric Infectious Diseases Society. Policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS). Infect. Control Hosp. Epidemiol. 2012, 33, 322–327.
- Rodríguez-Baño, J.; Paño-Pardo, J.R.; Alvarez-Rocha, L.; Asensio, A.; Calbo, E.; Cercenado, E.; Cisneros, J.M.; Cobo, J.; Delgado, O.; Garnacho-Montero, J.; et al. Programs for optimizing the use of antibiotics (PROA) in Spanish hospitals: GEIH-SEIMC, SEFH and SEMPSPH consensus document. Enferm. Infect. Microbiol. Clin. 2012, 30, 22.e1–22.e23.
- Mas-Morey, P.; Valle, M. A systematic review of inpatient antimicrobial stewardship programmes involving clinical pharmacists in small-to-medium-sized hospitals. Eur. J. Hosp. Pharm. 2018, 25, e69–e73.
- Drekonja, D.M.; Filice, G.A.; Greer, N.; Olson, A.; MacDonald, R.; Rutks, I.; Wilt, T.J. Antimicrobial stewardship in outpatient settings: A systematic review. Infect. Control Hosp. Epidemiol. 2015, 36, 142–152.
- Frazee, B.W.; Trivedi, T.; Montgomery, M.; Petrovic, D.-F.; Yamaji, R.; Riley, L. Emergency Department Urinary Tract Infections Caused by Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae: Many Patients Have No Identifiable Risk Factor and Discordant Empiric Therapy Is Common. Ann. Emerg. Med. 2018, 72, 449–456.
- Talan, D.A.; Takhar, S.S.; Krishnadasan, A.; Abrahamian, F.M.; Mower, W.R.; Moran, G.J.; EMERGEncy ID Net Study Group. Fluoroquinolone-Resistant and Extended-Spectrum β-Lactamase-Producing Escherichia coli Infections in Patients with Pyelonephritis, United States. Emerg. Infect. Dis. 2016, 22, 1594.
- Losier, M.; Ramsey, T.D.; Wilby, K.J.; Black, E.K. Systematic Review of Antimicrobial Stewardship Interventions in the Emergency Department. Ann. Pharmacother. 2017, 51, 774–790.
- Pulia, M.; Redwood, R.; May, L. Antimicrobial Stewardship in the Emergency Department. Emerg. Med. Clin. North Am. 2018, 36, 853–872.
- Ruiz-Ramos, J.; Vallvé Alcón, E.; Moreno Ramos, F.; Santolaya-Perrín, R.; Guardiola Tey, J.M. Antimicrobial stewardship programs in emergency departments: How do we measure antimicrobial use? A systematic review. Rev. Esp. Quimioter. 2021, 34, 610–617.
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377.
- Pin, M.; Somasundaram, R.; Wrede, C.; Schwab, F.; Gastmeier, P.; Hansen, S. Antimicrobial resistance control in the emergency department: A need for concrete improvement. Antimicrob. Resist. Infect. Control 2022, 11, 94.
- Jernigan, J.A.; Hatfield, K.M.; Wolford, H.; Nelson, R.E.; Olubajo, B.; Reddy, S.C.; McCarthy, N.; Paul, P.; McDonald, L.C.; Kallen, A.; et al. Multidrug-Resistant Bacterial Infections in U.S. Hospitalized Patients, 2012–2017. N. Engl. J. Med. 2020, 382, 1309–1319.
- European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report 2021. Stockholm: ECDC. 2022. Available online: https://https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2021 (accessed on 14 July 2023).
- Ben-Ami, R.; Rodríguez-Baño, J.; Arslan, H.; Pitout, J.D.; Quentin, C.; Calbo, E.S.; Azap, O.K.; Arpin, C.; Pascual, A.; Livermore, D.M.; et al. A multinational survey of risk factors for infection with extended-spectrum beta-lactamase-producing enterobacteriaceae in nonhospitalized patients. Clin. Infect. Dis. 2009, 49, 682–690.
- Talan, D.A.; Takhar, S.S.; Krishnadasan, A.; Mower, W.R.; Pallin, D.J.; Garg, M.L.; Femling, J.; Rothman, R.E.; Moore, J.C.; Jones, A.E.; et al. Emergence of Extended-Spectrum β-Lactamase Urinary Tract Infections among Hospitalized Emergency Department Patients in the United States. Ann. Emerg. Med. 2021, 77, 32–43.
- Mark, D.G.; Hung, Y.-Y.; Salim, Z.; Tarlton, N.J.; Torres, E.; Frazee, B.W. Third-Generation Cephalosporin Resistance and Associated Discordant Antibiotic Treatment in Emergency Department Febrile Urinary Tract Infections. Ann. Emerg. Med. 2021, 78, 357–369.
- Tamas, V.; Shah, S.; Hollenbach, K.A.; Kanegaye, J.T. Emergence of Extended-Spectrum β-Lactamase-Producing Pathogens in Community-Acquired Urinary Tract Infections Among Infants at a Pediatric Emergency Department. Pediatr. Emerg. Care 2022, 38, e1053–e1057.
- Clemenceau, M.; Ahmed-Elie, S.; Vilfaillot, A.; Chocron, R.; Compain, F.; Lebeaux, D.; Grohs, P. Appropriateness of empirical antibiotic prescription for bloodstream infections in an emergency department from 2006 to 2018: Impact of the spread of ESBL-producing Enterobacterales. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 71–77.
- Quaegebeur, A.; Brunard, L.; Javaudin, F.; Vibet, M.A.; Bemer, P.; Le Bastard, Q.; Batard, E.; Montassier, E.; EuroUTI 2010–2016 Study Group. Trends and prediction of antimicrobial susceptibility in urinary bacteria isolated in European emergency departments: The EuroUTI 2010–2016 Study. J. Antimicrob. Chemother. 2019, 74, 3069–3076.
- González Del Castillo, J.; Julián-Jiménez, A.; Gamazo-Del Rio, J.J.; García-Lamberechts, E.J.; Llopis-Roca, F.; Guardiola Tey, J.M.; Martínez-Ortiz de Zarate, M.; Navarro Bustos, C.; Piñera Salmerón, P.; Álvarez-Manzanares, J.; et al. A multidrug-resistant microorganism infection risk prediction model: Development and validation in an emergency medicine population. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 309–323.
- Göransson, J.; Sundqvist, M.; Ghaderi, E.; Lisby, J.G.; Molin, Y.; Eriksson, E.; Carlsson, S.; Cederlöf, A.; Ellis, L.; Melin, J. Performance of a System for Rapid Phenotypic Antimicrobial Susceptibility Testing of Gram-Negative Bacteria Directly from Positive Blood Culture Bottles. J. Clin. Microbiol. 2023, 61, e01525-22.
- Denny, K.J.; Gartside, J.G.; Alcorn, K.; Cross, J.W.; Maloney, S.; Keijzers, G. Appropriateness of antibiotic prescribing in the Emergency Department. J. Antimicrob. Chemother. 2019, 74, 515–520.
- Yunquera-Romero, L.; Márquez-Gómez, I.; Henares-López, A.; Morales-Lara, M.J.; Gallego Fernández, C.; Asensi-Díez, R. Appropriateness of antimicrobial prescriptions in the emergency department of a tertiary hospital. Rev. Esp. Quimioter. 2018, 31, 209–216.
- Koh, H.P.; Ambaras Khan, R.; Tay, S.L.; Gill, M.K.; Wong, J.Y.; Zainuddin, M.K. Appropriateness of antimicrobial prescribing in the high-burden emergency department of a tertiary hospital in Malaysia. Int. J. Clin. Pharm. 2021, 43, 1337–1344.
- Mora-Jiménez, I.; Tarancón-Rey, J.; Álvarez-Rodríguez, J.; Soguero-Ruiz, C. Artificial Intelligence to Get Insights of Multi-Drug Resistance Risk Factors during the First 48 Hours from ICU Admission. Antibiotics 2021, 10, 239.
- Cercenado, E.; Rodríguez-Baño, J.; Alfonso, J.L.; Calbo, E.; Escosa, L.; Fernández-Polo, A.; García-Rodríguez, J.; Garnacho, J.; Gil-Navarro, M.V.; Grau, S.; et al. Antimicrobial stewardship in hospitals: Expert recommendation guidance document for activities in specific populations, syndromes and other aspects (PROA-2) from SEIMC, SEFH, SEMPSPGS, SEMICYUC and SEIP. Enfermedades Infecc. Y Microbiol. Clínica 2023, 41, 238–242.
- Lipsky, B.A.; Dryden, M.; Gottrup, F.; Nathwani, D.; Seaton, R.A.; Stryja, J. Antimicrobial stewardship in wound care: A Position Paper from the British Society for Antimicrobial Chemotherapy and European Wound Management Association. J. Antimicrob. Chemother 2016, 71, 3026–3035.
- Doernberg, S.B.; Abbo, L.M.; Burdette, S.D.; Fishman, N.O.; Goodman, E.L.; Kravitz, G.R.; Leggett, J.E.; Moehring, R.W.; Newland, J.G.; Robinson, P.A.; et al. Essential Resources and Strategies for Antibiotic Stewardship Programs in the Acute Care Setting. Clin. Infect. Dis. 2018, 67, 1168–1174.
- Ruiz Ramos, J.; Santolaya Perrín, M.R.; González Del Castillo, J.; Candel, F.J.; Quirós, A.M.; López-Contreras González, J.; Jiménez, A.J.; Suárez-Lledó Grande, A.; PROA-Urgencias Group. Design of a panel of indicators for antibiotic stewardship programs in the Emergency Department. Farm. Hosp. 2023, 20, S1130-6343(23)00089-2.
- Schoffelen, T.; Schouten, J.; Hoogerwerf, J.; Martín Quirós, A.; May, L.; Ten Oever, J.; Hulscher, M. Quality indicators for appropriate antimicrobial therapy in the emergency department: A pragmatic Delphi procedure. Clin. Microbiol. Infect. 2021, 27, 210–214.
- Executive Summary. Executive Summary. The selection and use of essential medicines 2021. In Proceedings of the 23rd WHO Expert Committee on the Selection and Use of Essential Medicines, Virtual Meeting, 21 June–2 July 2021; World Health Organization (WHO): Geneva, Switzerland, 2021. Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2022.02 (accessed on 12 September 2023).
- Sulis, G.; Sayood, S.; Katukoori, S.; Bollam, N.; George, I.; Yaeger, L.H.; Chavez, M.A.; Tetteh, E.; Yarrabelli, S.; Pulcini, C.; et al. Exposure to World Health Organization’s AWaRe antibiotics and isolation of multidrug resistant bacteria: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 1193–1202.
- Kooda, K.; Canterbury, E.; Bellolio, F. Impact of Pharmacist-Led Antimicrobial Stewardship on Appropriate Antibiotic Prescribing in the Emergency Department: A Systematic Review and Meta-Analysis. Ann. Emerg. Med. 2020, 79, 374–387.
- Angoulvant, F.; Cohen, R.; Doit, C.; Elbez, A.; Werner, A.; Béchet, S.; Bonacorsi, S.; Varon, E.; Levy, C. Trends in antibiotic resistance of Streptococcus pneumoniae and Haemophilus influenzae isolated from nasopharyngeal flora in children with acute otitis media in France before and after 13 valent pneumococcal conjugate vaccine introduction. BMC Infect. Dis. 2015, 15, 236.
- Akenroye, A.T.; Baskin, M.N.; Samnaliev, M.; Stack, A.M. Impact of a bronchiolitis guideline on ED resource use and cost: A segmented time-series analysis. Pediatrics 2014, 133, e227–e234.
- Buising, K.L.; Thursky, K.A.; Black, J.F.; MacGregor, L.; Street, A.C.; Kennedy, M.P.; Brown, G.V. Improving antibiotic prescribing for adults with community acquired pneumonia: Does a computerised decision support system achieve more than academic detailing alone?—A time series analysis. BMC Med. Inform. Decis. Mak. 2008, 8, 35.
- Marrie, T.J.; Lau, C.Y.; Wheeler, S.L.; Wong, C.J.; Vandervoort, M.K.; Feagan, B.G. A controlled trial of a critical pathway for treatment of community-acquired pneumonia. CAPITAL Study Investigators. Community-Acquired Pneumonia Intervention Trial Assessing Levofloxacin. JAMA 2000, 283, 749–755.
- Metlay, J.P.; Camargo, C.A., Jr.; MacKenzie, T.; McCulloch, C.; Maselli, J.; Levin, S.K.; Kersey, A.; Gonzales, R.; IMPAACT Investigators. Cluster-randomized trial to improve antibiotic use for adults with acute respiratory infections treated in emergency departments. Ann. Emerg. Med. 2007, 50, 221–230.
- Borde, J.P.; Kern, W.V.; Hug, M.; Steib-Bauert, M.; de With, K.; Busch, H.J.; Kaier, K. Implementation of an intensified antibiotic stewardship programme targeting third-generation cephalosporin and fluoroquinolone use in an emergency medicine department. Emerg. Med. J. 2015, 32, 509–515.
- Hecker, M.T.; Son, A.H.; Murphy, N.N.; Sethi, A.K.; Wilson, B.M.; Watkins, R.R.; Donskey, C.J. Impact of syndrome-specific antimicrobial stewardship interventions on use of and resistance to fluoroquinolones: An interrupted time series analysis. Am. J. Infect. Control 2019, 47, 869–875.
- Percival, K.M.; Valenti, K.M.; Schmittling, S.E.; Strader, B.D.; Lopez, R.R.; Bergman, S.J. Impact of an antimicrobial stewardship intervention on urinary tract infection treatment in the ED. Am. J. Emerg. Med. 2015, 33, 1129–1133.
More
