Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Koichiro Sumi.
Yogurt is a traditional fermented food that is accepted worldwide for its high palatability and various health values. The milk protein contained in yogurt exhibits different physical and biological properties from those of non-fermented milk protein due to the fermentation and manufacturing processes.
- yogurt
- milk protein
- digestion
- absorption
- skeletal muscle
1. Introduction
1.1. What Is Yogurt?
Yogurt is consumed in many cultures worldwide as a dessert and culinary ingredient because of its high palatability and nutritional value. Yogurt is produced mainly by fermenting cow milk via lactic acid bacteria and is defined in the International Food Standard (CODEX) as “milk or dairy products obtained by lactic acid fermentation by the action of Streptococcus thermophilus and Lactobacillus delbrueckii subsp. Bulgaricus” [1,2][1][2]. The production of yogurt involves various processes, such as homogenization, heat treatment (pasteurization), fermentation, and smoothing. Depending on the combination of these processes, various types of yogurts with completely different physical properties can be produced. For example, there is set yogurt, which is produced by coagulating the casein micelle by lowering the pH due to lactic acid fermentation after it is placed in a container; stirred yogurt, which is produced with a smooth texture by stirring after fermentation; drinking yogurt, which is transformed into a fluid by shearing after fermentation and stabilized with stabilizers; and concentrated yogurt, typically named “Greek style”, manufactured by straining after fermentation. In addition, even within the same type of yogurt, the physical and/or chemical properties, such as viscosity and hardness, can vary by changing the manufacturing conditions [3].
1.2. Benefits of Yogurt for Human Health
Yogurt has also been recognized as a functional health food in many biological aspects. As one of the most representative probiotics, yogurt has been reported to influence the composition of the intestinal flora by reducing pathogenic bacteria [3]. Other diverse benefits for human health have been reported for yogurt, such as intestinal regulation, immunomodulatory action, diabetes prevention, and cardiac disease prevention [4]. Furthermore, its nutritional value should also be noted. Yogurt is a source of various vitamins and minerals. In particular, yogurt is a good source of calcium because its absorption is facilitated by galacto-oligosaccharides and casein phosphopeptides in dairy products [5]. Yogurt, like milk, also contains high levels of short-chain fatty acids [6] and various milk protein-derived peptides, and it has been suggested that these components of yogurt help reduce intestinal inflammation and protect the immune system [7]. In addition, a large cohort study from 21 countries reported that consumption of full-fat dairy products, including yogurt, lowers the risks of metabolic syndrome, hypertension, and diabetes [8].
1.3. Physical/Biological Properties and Digestion/Absorption of Milk Protein
Yogurt is also important in that it is rich in protein. The major protein sources with higher bioavailability include milk, meat, fish, eggs, and soybeans, among others. Milk protein is rich in essential amino acids for humans and has a very good ratio at which it is absorbed and taken up into the body. To reflect this, the digestible indispensable amino acid score of milk protein is particularly superior among these major protein sources [9,10,11][9][10][11]. Milk protein is broadly divided into casein and whey proteins. Traditionally, when cheese is produced from milk using rennet, the protein in the solidified part of the cheese body is casein, and the protein in the residual water, called whey, is whey protein [12]. Total milk protein is composed of casein and whey proteins at an approximate 8:2 ratio. Casein proteins consist of alpha-, beta-, and kappa-casein, and these caseins are present in a uniform colloidal dispersion in milk by forming micelles together with calcium phosphate [13]. Whey protein is composed of approximately 50% β-lactoglobulin (β-Lg), 20–25% α-lactalbumin (α-La), and 25–30% other proteins, including serum albumin, immunoglobulin, and lactoferrin; these whey proteins are water soluble [14]. As mentioned above, whey is a by-product of cheese production, but whey proteins have a high content of essential amino acids, such as branched-chain amino acids (BCAAs), giving whey its high nutritional value. Therefore, these proteins are refined as whey protein concentrate or whey protein isolate, which are used as protein sources. β-Lg is rich in BCAAs, among other proteins, but is potentially the cause of bovine milk allergy because it is not present in human breast milk [15].
When milk proteins ingested from the mouth reach the stomach, casein is broken down by pepsin digestion, but by gastric juice, which has a very low pH, resulting in agglomerates and precipitates as large casein particles, and these particles remain in the stomach for a long time. On the other hand, whey protein is nearly unaffected by very low pH, and it is gastrically excreted to the small intestine over a short time [16]. In particular, β-Lg is highly resistant to pepsin and passes almost undigested through the stomach [14]. However, all of the proteins, including the pepsin-undigested whey proteins, are neutralized in the small intestine by pancreatic juice and then digested by enzymes in the pancreatic juice, including trypsin, chymotrypsin, and pancreatic elastase [17], into oligopeptides or amino acids [18]. Residual oligopeptides are degraded into amino acids by endoaminopeptidases expressed in small intestinal epithelial cells [17]. Amino acids are absorbed into the portal vein from the small intestine via various amino acid transporters for neutral amino acids, cationic amino acids, specific amino acids, and others present in the small intestine [19]. Moreover, di- and tripeptides are absorbed via peptide transporters [19].
Because protein digestion and absorption proceed rapidly in the small intestine after gastric emptying, gastric emptying dynamics are considered a major factor determining the rates of digestion and absorption of milk proteins [20]. Differences in the rate of digestion/absorption of proteins are reflected by blood amino acid dynamics, such as the timing and degree of the increase, peak, maintenance, and decrease in blood amino acid concentrations [21]. As mentioned above, casein has a longer residence time in the stomach, while whey protein’s residence time is shorter. Reflecting at least this property, it has been shown in humans and animals that blood amino acid concentrations rise faster during whey protein ingestion than during casein ingestion [22,23][22][23]. However, even when compared with whey protein, the rate of increase in blood amino acid concentrations is faster when enzymatically pre-degraded whey peptides are ingested [24]. These findings suggest that the digestibility of the protein itself and prior partial digestion may also affect the dynamics of blood amino acids.
1.4. Milk Protein and Skeletal Muscle Protein Synthesis
Skeletal muscle quantity and quality are important for healthy living in all people, as skeletal muscle is associated with not only improved performance in athletes [25,26][25][26] but also reduced risk of various diseases [27], maintenance of locomotor function in the elderly [28] and prolonged life span [29]. Skeletal muscle mass is specified by the net balance between muscle protein synthesis (MPS) and muscle protein breakdown (MPB) [30]. In other words, if the total MPS exceeds the total MPB within a time period, skeletal muscle mass will increase, although MPS and MPB are constantly fluctuating. One of the greatest factors affecting MPS/MPB is dietary (protein) intake, with low MPS and high MPB before meals, but high MPS and low MPB after meals, and this diurnal variation is repeated daily [31]. The increase in MPS and decrease in MPB after protein ingestion are due mainly to the increase in blood insulin and amino acid concentrations after a meal [32]. Insulin is a hormone that promotes glucose intracellular uptake from blood [33], but it also contributes to the increase in MPS and decreases in MPB. When insulin acts on insulin receptors in skeletal muscle cells, it activates the intracellular PI3K/Akt pathway, involving translocation of the glucose transporter Glut4 from inside the cell to the plasma membrane, resulting in increased glucose uptake into skeletal muscle and decreased blood glucose levels.
Akt also affects mTORC1, a kinase complex acting as a master regulator of protein synthesis [34], and FOXOs, transcription factors that control proteolysis via the ubiquitin–proteasome system [35]. Akt leads to the activation of mTORC1, inducing protein translation initiation and elongation via phosphorylation of the mTORC1 target proteins p70 S6K and 4E-BP1, and results in the elevation of MPS [36]. In addition, Akt directly phosphorylates FOXOs, thereby inhibiting their nuclear translocation [35]. FOXOs are transcription factors for Atrogin1 [37] and MuRF1 [38], which are ubiquitin ligases necessary for proteolysis, and Akt decreases MPB by suppressing their transcription. Insulin is secreted from the pancreas in response to the blood glucose level [33] and protein intake level [39]. Insulin elevation after protein intake is due to the secretion of gastrointestinal hormones, such as GLP-1 and GIP [40], and to the elevation of blood amino acids [41,42][41][42]. Therefore, insulin elevation is a mechanism of increasing MPS and decreasing MPB after protein intake.
The other major mechanism is due to specific amino acids supplied from food proteins that act as direct stimuli to activate MPS. The most well-studied is the BCAA leucine, which can activate mTORC1 in a PI3K/Akt-independent manner via the Sestrin2/GATOR2- and leucyl/tRNA synthase-mediated pathways [43], although it also affects PI3K/Akt mediated mTORC1 activation [44]. It has also become clear in recent years that other amino acids have a role in enhancing MPS [45]. Of course, since amino acids are the building blocks of nascent proteins, an adequate supply of all amino acids is necessary to increase skeletal muscle mass.
Besides diet, exercise has a major influence on muscle protein metabolism [46]. Resistance exercise (RE), commonly referred to as muscle training, especially increases both MPS and MPB [47]. During RE, if adequate protein supplementation is provided, MPS increases additively, and the skeletal muscle protein net balance tilts significantly toward positive [48]. It is also important to note that the effect of RE lasts longer than traditionally assumed [49], with additional increasing postprandial MPS for 1–2 days after RE [50]. Therefore, with habitual RE, MPS exceeds MPB, and muscle hypertrophy occurs as an adaptation to muscle training. Thus, considering the optimal quantity and quality of protein from food to promote MPS should be an effective strategy for maintaining and improving muscle mass [51]. In addition, elderly and chronically ill patients exhibit anabolic resistance, which is desensitivity to anabolic stimuli, such as exercise and nutrition, and the causes of skeletal muscle loss, such as primary/secondary sarcopenia [52]. In other words, to achieve the same gains as younger people, they would have to consume larger amounts of protein than younger people. However, this is not a practical solution, and therefore, a protein source that can elevate MPS more effectively is needed.
2. Digestion/Absorption Properties of Protein in Yogurt
2.1. Digestion/Absorption Effectors of Yogurt Protein
Yogurt contains whey and casein in roughly the same proportions as those in original raw milk, although some of the proteins are partially broken down by fermentation [57][53]. Approximately 90–99% of the whey protein in raw milk is denatured by heat treatment prior to fermentation (generally 5–10 min at 90–95 °C) [58][54], and denatured whey proteins, such as β-Lg, are combined with casein micelle [59][55]. In addition, the decrease in pH due to lactic acid fermentation causes the casein to approach its isoelectric point (approximately pH 4.5–4.2), resulting in a decreased electrical repulsive force among casein micelles (Figure 1). Because the pH-decreasing process in fermentation is gradual and slow, the casein micelles in yogurt do not coagulate and precipitate as hard as in the case of abrupt contact between raw milk and gastric acids in the stomach. In the yogurt fermentation process, the casein micelle particles are hydrated and form a homogeneous mesh-like structure, which becomes soft white tissue [13] (Figure 1). Because whey proteins that do not combine with casein micelles are soluble in this gradually decreasing pH range, they remain dissolved and present in trapped water in the above casein micellar network or in the released free water from the yogurt structure. Therefore, the physical properties of milk proteins in yogurt differ from those in raw milk, based mainly on the organization of the casein micelle during the production process.
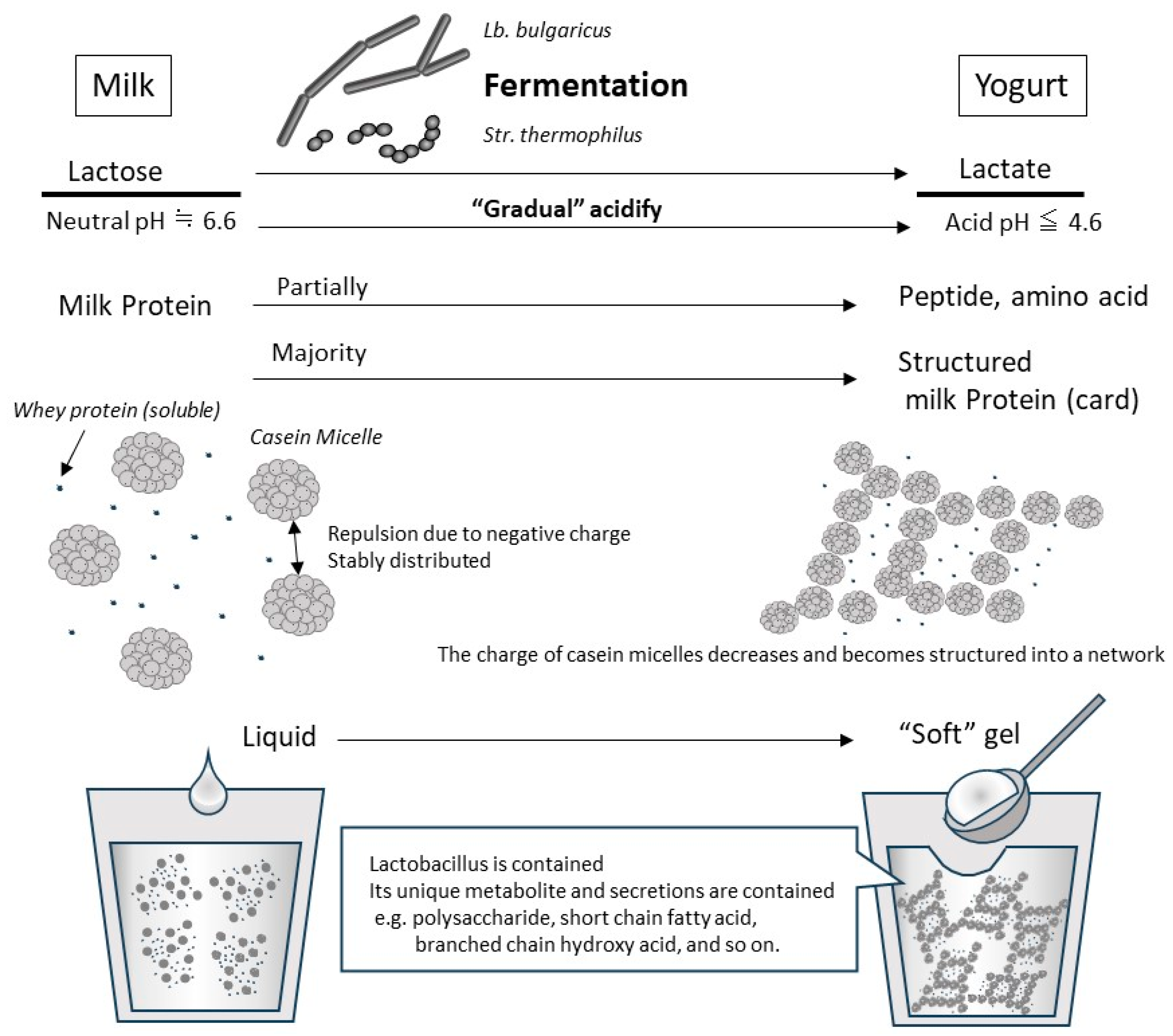
Figure 1. Typical changes due to lactobacillus fermentation at yogurt production from milk. An illustration of the changes during the production of “set yogurt”, which is produced by the most basic method of static fermentation. When the original milk is inoculated with Lb. bulgaricus and Str. thermophilus, they grow using lactose as a carbon source. In the process, lactose is converted to lactic acid, which decreases pH gradually. In fresh milk whose pH is neutral, casein micelles are negatively charged and are stably dispersed in the liquid due to their repulsion. As the fermentation progresses and the pH drops below 5.2, the casein micelles lose their charge and coagulate with each other. However, because this acidification and aggregation occurs slowly, casein micelles become a network structure that includes whey protein and water, so they are set as a soft gel (called card). By adding steps such as shearing and draining during or after fermentation, it is possible to create yogurt with different physical properties.
Thus, the absorption rate of milk proteins may be higher in yogurt than in the original raw milk because milk proteins become frangible soft tissue at a pH below the isoelectric point of casein during the fermentation process. It was originally also considered that a portion of the milk protein is partially broken down during fermentation, which increases digestibility [60][56]. Thus, these properties have been expected to increase the protein absorption of yogurt. Indeed, Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus, which are used in yogurt production, have the ability to at least partially decompose and utilize casein [61][57], β-Lg, and α-La [62][58] as their amino acid sources. Reflecting this, it has been shown that peptides produced by artificial digestion are also different between yogurt and original raw milk [57][53]. However, in more recent artificial digestion studies using more physiological conditions, the digestibility of the protein in yogurt was more influenced by heating, such as during pasteurization, and by the milk protein composition of the raw milk [63][59] than by fermentation-based changes [64,65][60][61]. In macroscopic protein digestion, at least as measured by the increase in soluble protein and by the disappearance of major protein bands on SDS-PAGE, the influence of lactobacillus fermentation as the main factor is not clear.
2.2. Slower Rate of Digestion/Absorption of Yogurt Protein Compared with Milk Protein
Various factors are involved in the digestion and absorption kinetics of proteins in yogurt. Reflecting this, in vivo studies have shown mixed results regarding how the digestion and absorption kinetics of milk proteins in yogurt differ from those in raw milk. In a study in which 15N-labeled milk or yogurt was given to miniature pigs, their digestion and absorption kinetics were examined based on the intestinal residue after 1, 2, 4, 8, and 12 h. As a result, the absorption of protein was faster during the first 2 h but slower after 2 h in raw milk than in yogurt. However, proteins in each were rapidly absorbed after reaching the intestines [20]. In a study conducted by the same group in adult males and females, the jejunal contents were continuously sampled through a nasogastric tube from before to 4 h after ingestion of 15N-labeled milk or yogurt. Similarly, in an animal study, the fastest rate of excretion from the stomach to the intestines was 20 min or 60 min after ingestion of milk or yogurt, respectively; both were rapidly digested and absorbed after reaching the proximal jejunum [66][62]. In a study in which young men ingested a test food mixed with 13C-labeled sodium acetate and their exerted 13CO2 in the breath was measured, Caspian Sea yogurt (strictly, this is not yogurt because it is fermented with Lactobacillus acidophilus and Lactococcus cremoris) took longer to digest/absorb compared to milk (195 vs. 110–150 min) [67][63]. In summary, those studies show that gastric emptying had the most important effect on the rate of digestion and absorption, and these results might be explained by yogurt’s longer gastric emptying because of its higher viscosity compared with milk.
2.3. Faster Rate of Digestion/Absorption of Yogurt Protein Compared with Milk Protein
On the other hand, in a study examining changes in blood total amino acid (TAA) concentration in elderly people over a 5 h period following consumption of various dairy products, the peak blood TAA concentration was higher, and the time to reach peak TAA concentration was shorter for yogurt ingestion compared with raw milk or cheese ingestion [68][64]. Moreover, using rat portal blood TAA concentrations as an indicator of protein digestion/absorption, wresearchers have consistently found that yogurt is more digestible and absorbable compared with original raw milk. In brief, in yogurt and original low-fat milk, which were pasteurized and homogenized under identical conditions, the portal TAA collected by dissection at 30 and 60 min after oral yogurt consumption was significantly higher than that after original milk administration. The portal TAA concentrations in blood collected at 90, 120, and 240 min after administration of yogurt or original milk were almost the same [69][65]. Almost the same results were obtained in an experiment in which a portal vein catheter was introduced in rats to measure changes in portal TAA concentrations over time in the same individuals (unpublished data). In another study comparing original milk and yogurt produced by a different fermentation starter, the portal TAA concentration was significantly higher 30 min after yogurt than the original skim milk administration and was comparable after 60 and 90 min [70][66]. It has been shown that dairy beverages stabilized in an acidified state have better protein absorption compared with non-acidified dairy beverages [71][67]. In our study [69], sSimilar to fermented milk, unfermented milk acidified by adding lactic acid had higher TAA absorption compared with the original raw milk. These results suggest that the presence or absence of acid coagulation of milk proteins when mixed with gastric juice has a large impact on protein digestion/absorption. This is supported by the finding in [69][65] that when gastric juice was depleted using a proton pump inhibitor, amino acid absorption improved 15–30 min after raw milk ingestion. On the other hand, proton pump inhibitors do not affect amino acid absorption after ingestion of acidified milk but reduce it after ingestion of fermented milk [69][65]. Casein protein may be more highly digestible by pepsin in fermented milk than in raw milk in the stomach [70][66]. These results indicate that casein protein in fermented milk is partially digested by lactic acid bacterial fermentation, potentially contributing to its reduced resistance to pepsin.
Thus, the apparent digestion and absorption rates of protein can be faster or slower in yogurt than in its unfermented origins, such as raw milk, depending on the manufacturing conditions. However, if fluidity is ensured by avoiding fermentation conditions that result in firmness or by adding a low-viscosity process after fermentation, fermentation should substantially improve the digestion and absorption of proteins. For example, in practical terms, fermented products with reduced viscosity, such as drinking yogurt, are presumed to be advantageous in terms of protein absorption. In addition, increasing the proportion of whey protein in raw material may also be effective in creating yogurt with a higher protein concentration and reduced viscosity [72][68]. However, processing such as heating, separation, desalting, drying, and storage conditions, such as temperature, humidity, and duration, can alter the state of milk proteins (crosslinking, saccharification, etc.) [73,74,75][69][70][71] and have unintended effects on its digestion and absorption properties. For example, Trommelen et al. tested the hypothesis that caseinate increases postprandial blood amino acid levels more than micellar casein or crosslinked caseinate because of its superior water solubility, but the obtained results were the opposite of their hypothesis [76][72]. Therefore, it will be necessary to assess the effects of lactic acid bacterial fermentation on the digestion and absorption of milk proteins in more detail via precise studies that not only control physical properties, such as viscosity but also clarify the processing history of the original dairy ingredients.
References
- CXS 243-2003; WHO Standard for Fermented Milks. Food and Agriculture Organization of the United Nations: Rome, Italy, 2018.
- FAO. Food and Agriculture Organization of the United Nations: Food Energy—Methods of Analysis and Conversion Factors; FAO Food and Nutrition Paper 77; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003.
- Aslam, H.; Marx, W.; Rocks, T.; Loughman, A.; Chandrasekaran, V.; Ruusunen, A.; Dawson, S.L.; West, M.; Mullarkey, E.; Pasco, J.A.; et al. The effects of dairy and dairy derivatives on the gut microbiota: A systematic literature review. Gut Microbes 2020, 12, 1799533.
- Hadjimbei, E.; Botsaris, G.; Chrysostomou, S. Beneficial Effects of Yoghurts and Probiotic Fermented Milks and Their Functional Food Potential. Foods 2022, 11, 2691.
- Ilesanmi-Oyelere, B.L.; Kruger, M.C. The Role of Milk Components, Pro-, Pre-, and Synbiotic Foods in Calcium Absorption and Bone Health Maintenance. Front. Nutr. 2020, 7, 578702.
- Khiaosa-Ard, R.; Kaltenegger, A.; Humer, E.; Zebeli, Q. Effect of inclusion of bakery by-products in the dairy cow’s diet on milk fatty acid composition. J. Dairy Res. 2022, 89, 236–242.
- Sigala-Robles, R.; Santiago-López, L.; Hernández-Mendoza, A.; Vallejo-Cordoba, B.; Mata-Haro, V.; Wall-Medrano, A.; González-Córdova, A.F. Peptides, Exopolysaccharides, and Short-Chain Fatty Acids from Fermented Milk and Perspectives on Inflammatory Bowel Diseases. Dig. Dis. Sci. 2022, 67, 4654–4665.
- Bhavadharini, B.; Dehghan, M.; Mente, A.; Rangarajan, S.; Sheridan, P.; Mohan, V.; Iqbal, R.; Gupta, R.; Lear, S.; Wentzel-Viljoen, E.; et al. Association of dairy consumption with metabolic syndrome, hypertension and diabetes in 147,812 individuals from 21 countries. BMJ Open Diabetes Res. Care 2020, 8, e000826.
- Herreman, L.; Nommensen, P.; Pennings, B.; Laus, M.C. Comprehensive overview of the quality of plant- And animal-sourced proteins based on the digestible indispensable amino acid score. Food Sci. Nutr. 2020, 8, 5379–5391.
- Kendler, S.; Thornes, F.W.; Jakobsen, A.N.; Lerfall, J. Nutritional profiling and contaminant levels of five underutilized fish species in Norway. Front. Nutr. 2023, 10, 1118094.
- Rutherfurd, S.M.; Fanning, A.C.; Miller, B.J.; Moughan, P.J. Protein digestibility-corrected amino acid scores and digestible indispensable amino acid scores differentially describe protein quality in growing male rats. J. Nutr. 2015, 145, 372–379.
- Amaro-Hernández, J.C.; Olivas, G.I.; Acosta-Muñiz, C.H.; Gutiérrez-Méndez, N.; Sepulveda, D.R. Structure rearrangement during rennet coagulation of milk modifies curd density. J. Dairy Sci. 2020, 103, 3088–3094.
- Rasic, J.L.; Kurmann, J.A. Yoghurt. Scientific Grounds, Technology, Manufacture and Preparations; Technical Dairy Publishing House: Copenhagen, Denmark, 1978; 466p.
- Chatterton, D.E.; Nguyen, D.N.; Bering, S.B.; Sangild, P.T. Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. Int. J. Biochem. Cell Biol. 2013, 45, 1730–1747.
- Sélo, I.; Clément, G.; Bernard, H.; Chatel, J.; Créminon, C.; Peltre, G.; Wal, J. Allergy to bovine β-lactoglobulin: Specificity of human IgE to tryptic peptides. Clin. Exp. Allergy 1999, 29, 1055–1063.
- Ye, A.; Cui, J.; Dalgleish, D.; Singh, H. Formation of a structured clot during the gastric digestion of milk: Impact on the rate of protein hydrolysis. Food Hydrocoll. 2016, 52, 478–486.
- Fu, Z.; Akula, S.; Thorpe, M.; Hellman, L. Marked difference in efficiency of the digestive enzymes pepsin, trypsin, chymotrypsin, and pancreatic elastase to cleave tightly folded proteins. Biol. Chem. 2021, 402, 861–867.
- Abrahamse, E.; Thomassen, G.G.M.; Renes, I.B.; Wierenga, P.A.; Hettinga, K.A. Assessment of milk protein digestion kinetics: Effects of denaturation by heat and protein type used. Food Funct. 2022, 13, 5715–5729.
- Jochems, P.G.M.; Garssen, J.; van Keulen, A.M.; Masereeuw, R.; Jeurink, P.V. Evaluating Human Intestinal Cell Lines for Studying Dietary Protein Absorption. Nutrients 2018, 10, 322.
- Gaudichon, C.; Roos, N.; Mahé, S.; Sick, H.; Bouley, C.; Tomé, D. Gastric emptying regulates the kinetics of nitrogen absorption from 15N-labeled milk and 15N-labeled yogurt in miniature pigs. J. Nutr. 1994, 124, 1970–1977.
- Dangin, M.; Boirie, Y.; Garcia-Rodenas, C.; Gachon, P.; Fauquant, J.; Callier, P.; Ballèvre, O.; Beaufrère, B. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E340–E348.
- Gorissen, S.H.M.; Trommelen, J.; Kouw, I.W.K.; Holwerda, A.M.; Pennings, B.; Groen, B.B.L.; Wall, B.T.; Churchward-Venne, T.A.; Horstman, A.M.H.; Koopman, R.; et al. Protein Type, Protein Dose, and Age Modulate Dietary Protein Digestion and Phenylalanine Absorption Kinetics and Plasma Phenylalanine Availability in Humans. J. Nutr. 2020, 150, 2041–2050.
- Kanda, A.; Nakayama, K.; Sanbongi, C.; Nagata, M.; Ikegami, S.; Itoh, H. Effects of whey, caseinate, or milk protein ingestion on muscle protein synthesis after exercise. Nutrients 2016, 8, 339.
- Morifuji, M.; Ishizaka, M.; Baba, S.; Fukuda, K.; Matsumoto, H.; Koga, J.; Kanegae, M.; Higuchi, M. Comparison of different sources and degrees of hydrolysis of dietary protein: Effect on plasma amino acids, dipeptides, and insulin responses in human subjects. J. Agric. Food Chem. 2010, 58, 8788–8797.
- Abe, T.; Kawamoto, K.; Dankel, S.J.; Bell, Z.W.; Spitz, R.W.; Wong, V.; Loenneke, J.P. Longitudinal associations between changes in body composition and changes in sprint performance in elite female sprinters. Eur. J. Sport Sci. 2020, 20, 100–105.
- Ishida, A.; Travis, S.K.; Stone, M.H. Associations of Body Composition, Maximum Strength, Power Characteristics with Sprinting, Jumping, and Intermittent Endurance Performance in Male Intercollegiate Soccer Players. J. Funct. Morphol. Kinesiol. 2021, 6, 7.
- Wolfe, R.R. The underappreciated role of muscle in health and disease. Am. J. Clin. Nutr. 2006, 84, 475–482.
- Visser, M.; Goodpaster, B.H.; Kritchevsky, S.B.; Newman, A.B.; Nevitt, M.; Rubin, S.M.; Simonsick, E.M.; Harris, T.B. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2005, 60, 324–333.
- Srikanthan, P.; Karlamangla, A.S. Muscle mass index as a predictor of longevity in older adults. Am. J. Med. 2014, 127, 547–553.
- Phillips, S.M. Protein requirements and supplementation in strength sports. Nutrition 2004, 20, 689–695.
- Phillips, S.M.; Tang, J.E.; Moore, D.R. The role of milk-and soy-based protein in support of muscle protein synthesis and muscle protein accretion in young and elderly persons. J. Am. Coll. Nutr. 2009, 28, 343–354.
- Atherton, P.J.; Smith, K. Muscle protein synthesis in response to nutrition and exercise. J. Physiol. 2012, 590, 1049–1057.
- Linder, M.C. Nutrition and Metabolism of Carbohydrates. In Nutritional Biochemistry and Metabolism: With Clinical Applications; Elsevier: Amsterdam, The Netherlands, 1991; Chapter 2.
- Hay, N.; Sonenberg, N. Upstream and downstream of mTOR. Genes Dev. 2004, 18, 1926–1945.
- McKinnell, I.W.; Rudnicki, M.A. Molecular Mechanisms of Muscle Atrophy. Cell 2004, 119, 907–910.
- Drummond, M.J.; Dreyer, H.C.; Fry, C.S.; Glynn, E.L.; Rasmussen, B.B. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J. Appl. Physiol. 2009, 106, 1374–1384.
- Gomes, M.D.; Lecker, S.H.; Jagoe, R.T.; Navon, A.; Goldberg, A.L. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc. Natl. Acad. Sci. USA 2001, 98, 14440–14445.
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K.; et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001, 294, 1704–1708.
- Claessens, M.; Saris, W.H.M.; van Baak, M.A. Glucagon and insulin responses after ingestion of different amounts of intact and hydrolysed proteins. Br. J. Nutr. 2008, 100, 61–69.
- Amigo-Benavent, M.; Power-Grant, O.; FitzGerald, R.J.; Jakeman, P. The insulinotropic and incretin response to feeding a milk based protein matrix in healthy young women. J. Funct. Foods 2020, 72, 104056.
- Floyd, J.C.; Fajans, S.S.; Conn, J.W.; Knopf, R.F.; Rull, J. Stimulation of insulin secretion by amino acids. J. Clin. Investig. 1966, 45, 1487–1502.
- Kuhara, T.; Ikeda, S.; Ohneda, A.; Sasaki, Y. Effects of intravenous infusion of 17 amino acids on the secretion of GH, glucagon, and insulin in sheep. Am. J. Physiol. Endocrinol. Metab. 1991, 260, E21–E26.
- Suryawan, A.; Rudar, M.; Fiorotto, M.L.; Davis, T.A. Differential regulation of mTORC1 activation by leucine and β-hydroxy-β-methylbutyrate in skeletal muscle of neonatal pigs. J. Appl. Physiol. 2020, 128, 286–295.
- Anthony, J.C.; Lang, C.H.; Crozier, S.J.; Anthony, T.G.; MacLean, D.A.; Kimball, S.R.; Jefferson, L.S. Contribution of insulin to the translational control of protein synthesis in skeletal muscle by leucine. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E1092–E1101.
- Shimobayashi, M.; Hall, M.N. Multiple amino acid sensing inputs to mTORC1. Cell Res. 2016, 26, 7–20.
- Brook, M.S.; Wilkinson, D.J.; Phillips, B.E.; Perez-Schindler, J.; Philp, A.; Smith, K.; Atherton, P.J. Skeletal muscle homeostasis and plasticity in youth and ageing: Impact of nutrition and exercise. Acta Physiol. 2016, 216, 15–41.
- Ato, S.; Fujita, S. Regulation of muscle protein metabolism by nutrition and exercise. J. Phys. Fit. Sports Med. 2017, 6, 119–124.
- Joanisse, S.; McKendry, J.; Lim, C.; Nunes, E.A.; Stokes, T.; McLeod, J.C.; Phillips, S.M. Understanding the effects of nutrition and post-exercise nutrition on skeletal muscle protein turnover: Insights from stable isotope studies. Clin. Nutr. Open Sci. 2021, 36, 56–77.
- Aragon, A.A.; Schoenfeld, B.J. Nutrient timing revisited: Is there a post-exercise anabolic window? J. Int. Soc. Sports Nutr. 2013, 10, 5.
- Churchward-Venne, T.A.; Burd, N.A.; Phillips, S.M. Nutritional regulation of muscle protein synthesis with resistance exercise: Strategies to enhance anabolism. Nutr. Metab. 2012, 9, 40.
- Morton, R.W.; McGlory, C.; Phillips, S.M. Nutritional interventions to augment resistance training-induced skeletal muscle hypertrophy. Front. Physiol. 2015, 6, 245.
- Paulussen, K.J.M.; McKenna, C.F.; Beals, J.W.; Wilund, K.R.; Salvador, A.F.; Burd, N.A. Anabolic Resistance of Muscle Protein Turnover Comes in Various Shapes and Sizes. Front. Nutr. 2021, 8, 615849.
- Nguyen, H.T.H.; Gathercole, J.L.; Day, L.; Dalziel, J.E. Differences in peptide generation following in vitro gastrointestinal digestion of yogurt and milk from cow, sheep and goat. Food Chem. 2020, 317, 126419.
- Ichimura, T.; Osada, T.; Yonekura, K.; Horiuchi, H. A new method for producing superior set yogurt, focusing on heat treatment and homogenization. J. Dairy Sci. 2022, 105, 2978–2987.
- Dannenberg, F.; Kessler, H.G. Effect of denaturation of beta-lactoglobulin on texture properties of set-style nonfat yoghurt. 2. Firmness and flow properties. Milchwiss. Milk Sci. Int. 1988, 43, 700–704.
- Breslaw, E.S.; Kleyn, D.H. In Vitro Digestibility of Protein in Yogurt at Various Stages of Processing. J. Food Sci. 1973, 38, 1016–1021.
- Savijoki, K.; Ingmer, H.; Varmanen, P. Proteolytic systems of lactic acid bacteria. Appl. Microbiol. Biotechnol. 2006, 71, 394–406.
- Tzvetkova, I.; Dalgalarrondo, M.; Danova, S.; Iliev, I.; Ivanova, I.; Chobert, J.-M.; Haertlé, T. Hydrolysis of Major Dairy Proteins by Lactic Acid Bacteria from Bulgarian Yogurts. J. Food Biochem. 2007, 31, 680–702.
- Rioux, L.-E.; Turgeon, S.L. The Ratio of Casein to Whey Protein Impacts Yogurt Digestion In Vitro. Food Dig. 2012, 3, 25–35.
- Dupont, D.; Mandalari, G.; Mollé, D.; Jardin, J.; Rolet-Répécaud, O.; Duboz, G.; Léonil, J.; Mills, C.E.N.; Mackie, A.R. Food processing increases casein resistance to simulated infant digestion. Mol. Nutr. Food Res. 2010, 54, 1677–1689.
- Rinaldi, L.; Gauthier, S.F.; Britten, M.; Turgeon, S.L. In vitro gastrointestinal digestion of liquid and semi-liquid dairy matrixes. LWT—Food Sci. Technol. 2014, 57, 99–105.
- Gaudichon, C.; Mahé, S.; Roos, N.; Benamouzig, R.; Luengo, C.; Huneau, J.F.; Sick, H.; Bouley, C.; Rautureau, J.; Tome, D. Exogenous and endogenous nitrogen flow rates and level of protein hydrolysis in the human jejunum after milk and yoghurt ingestion. Br. J. Nutr. 1995, 74, 251–260.
- Sanggaard, K.M.; Holst, J.J.; Rehfeld, J.F.; Sandström, B.; Raben, A.; Tholstrup, T. Different effects of whole milk and a fermented milk with the same fat and lactose content on gastric emptying and postprandial lipaemia, but not on glycaemic response and appetite. Br. J. Nutr. 2004, 92, 447–459.
- Horstman, A.M.H.; Ganzevles, R.A.; Kudla, U.; Kardinaal, A.F.M.; van den Borne, J.J.G.C.; Huppertz, T. Postprandial blood amino acid concentrations in older adults after consumption of dairy products: The role of the dairy matrix. Int. Dairy J. 2021, 113, 104890.
- Sumi, K.; Osada, K.; Ashida, K.; Nakazato, K. Lactobacillus-fermented milk enhances postprandial muscle protein synthesis in Sprague-Dawley rats. J. Funct. Foods 2020, 66, 103789.
- Sumi, K.; Osada, K.; Sakuda, M.; Ashida, K.; Nakazato, K. Fermented milk retains beneficial effects on skeletal muscle protein anabolism after processing by centrifugation and supernatant removal. J. Dairy Sci. 2021, 104, 1336–1350.
- Nakayama, K.; Kanda, A.; Tagawa, R.; Sanbongi, C.; Ikegami, S.; Itoh, H. Post-exercise muscle protein synthesis in rats after ingestion of acidified bovine milk compared with skim milk. Nutrients 2017, 9, 1071.
- Morell, P.; Fiszman, S.; Llorca, E.; Hernando, I. Designing added-protein yogurts: Relationship between in vitro digestion behavior and structure. Food Hydrocoll. 2017, 72, 27–34.
- Anema, S.G.; Pinder, D.N.; Hunter, R.J.; Hemar, Y. Effects of storage temperature on the solubility of milk protein concentrate (MPC85). Food Hydrocoll. 2006, 20, 386–393.
- Nyakayiru, J.; van Lieshout, G.A.A.; Trommelen, J.; van Kranenburg, J.; Verdijk, L.B.; Bragt, M.C.E.; van Loon, L.J.C. The glycation level of milk protein strongly modulates post-prandial lysine availability in humans. Br. J. Nutr. 2020, 123, 545–552.
- Van Lieshout, G.A.A.; Lambers, T.T.; Bragt, M.C.E.; Hettinga, K.A. How processing may affect milk protein digestion and overall physiological outcomes: A systematic review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2422–2445.
- Trommelen, J.; Weijzen, M.E.G.; van Kranenburg, J.; Ganzevles, R.A.; Beelen, M.; Verdijk, L.B.; van Loon, L.J.C. Casein Protein Processing Strongly Modulates Post-Prandial Plasma Amino Acid Responses In Vivo in Humans. Nutrients 2020, 12, 2299.
More
