We present an overview of the current state of knowledge on the SARS-CoV-2 and COVID-19 pandemic. In addition to an overview of the epidemiological, clinical, and radiological features of SARS-CoV-2, we also summarize possible therapeutic options currently under investigation and the future outlook for the disease. Whereas the trials on SARS-CoV-2 genome-based specific vaccines and therapeutic antibodies are currently being tested, this solution is more long-term, as they require thorough testing of their safety. On the other hand, the repurposing of the existing therapeutic agents previously designed for other virus infections and pathologies happens to be the only practical approach as a rapid response measure to the emergent pandemic. The current pandemic emergency will be a trigger for more systematic drug repurposing design approaches based on big data analysis. Further on, regression analytical review is presented on the virological and evolutionary history of SARS-CoV viruses, indicating to the autoimmune pathogen.
- COVID-19
- SARS-CoV-2
- pneumonia
- ACE2
- clinical trials
1. General Clinical Features of COVID-19
The usual symptoms of COVID-19 include fever (83–98%), cough (59–82%), shortness of breath (19–55%), and muscle ache (11–44%), which are similar to those of SARS and MERS. [1] Some patients may have sore throat, rhinorrhea, headache and confusion a few days before the onset of fever, indicating that fever is a critical symptom, but not the only initial manifestation of infection. [1] The pattern of fever has not yet been fully understood. A small proportion of patients had hemoptysis [2][3], and a number of cases were found relatively asymptomatic. [4] COVID-19 patients may have normal or lower white blood cell counts, lymphopenia, or thrombocytopenia, with the increased C-reactive protein level. [1][2][3] People who have fever and upper respiratory tract symptoms with leukopenia or lymphopenia should be suspected for this disease, especially for patients with travel history to the endemic area or close exposure record.
However, the clinical course of COVID-19 pneumonia exhibits a broad spectrum of severity and progression patterns. In some patients, dyspnea develops within a median of 8 days after the onset of illness (range of 5–13 days), while in others, respiratory distress may be absent. [2] Around 3–29% patients may need the admission to the intensive care unit. Severely ill patients may have poor disease course of rapid progression to multiple organ dysfunction and even death [1][2], and those who have shortness of breath and hypoxemia can quickly progress into acute respiratory distress syndrome (ARDS), severe sepsis with shock, and even multiple organ dysfunction within one week. [3][5] ARDS was observed to develop in 17–29% of hospitalized patients approximately 8 days after symptoms onset, and the global mortality rate reached approximately 5.4% [2].
It is also worth noting that the gastrointestinal symptoms of COVID-19 may be caused by the direct viral damage to the intestine rather than the immunopathogenic response to the lung infection of the host. Since angiotensin-converting enzyme 2 (ACE2), the main cellular receptor of SARS-CoV-2 is expressed in the human gastrointestinal epithelial cells, it is believed that the viral shedding at the gastrointestinal tract and fecal–oral transmission is highly plausible. [6] Indeed, it was reported that the rectal swabs showed positive results even after the nasopharyngeal tests were constitutively negative [7]. Besides, the live virus was also detected in stool samples of diseased patients. This evidence strongly indicate that stool can be contagious for a long time after the discharge of patients based on two negative nasopharyngeal swabs. Thus, adding rectal swabs to the discharge criteria should be considered for the prevention of both nosocomial and community spread of COVID-19.
Aside from the gastrointestinal symptoms, a retrospective study of 214 patients in China reported that 5.6 % of patients experienced hypogeusia and 5.1 % experienced hyposmia [8]. Though the loss of olfaction during SARS-CoV-2 infection could be explained by the swelling of the nasal mucosa, a larger population of patients should be included to determine whether hypogeusia and hyposmia could be a common neurological manifestation of COVID-19. Nevertheless, hyposmia and hypogeusia are now being recommended as the early warning signs and an indication for early self-isolation.
2. Radiological Features of COVID-19
The radiological examinations, including chest X-ray (CXR) and chest computed tomography (CT) scan, are important for early detection and treatment of COVID-19 [9]. The imaging findings of COVID-19 pneumonia mimic influenza, SARS-CoV, and MERS-CoV pneumonia [10][11][12][13][14]. The primary Wuhan study revealed that upon diagnosis, 74 [75%] patients showed bilateral pneumonia, and the remaining 25 [25%] patients showed unilateral pneumonia. [1] In addition, 14 [14%] patients showed multiple mottling and ground-glass opacities [1]. In the subsequent study, it was reported that the predominant pattern of abnormality observed was peripheral (44 [54%]), ill-defined (66 [81%]), and mainly involved the right lower lobes (225 [27%] of 849 affected segments) [1]. Bilateral multiple consolidation usually occurs in more severe cases [9].
Chest CT is more efficient in detecting pneumonia at the early stages of COVID-19. However, the imaging findings of COVID-19 pneumonia on chest CT are variable and nonspecific [15][16][17]. The most common patterns of COVID-19 on chest CT scans include multiple GGO lesions (56.4%), and bilateral patchy shadowing (51.8%), and the other patterns consist of local patchy shadowing (28.1%), and interstitial abnormalities (4.4%). Severe cases tend to yield more prominent radiologic findings on chest CT scan, such as more bilateral patchy shadowing (82%), more multiple GGO lesions (60%), and more local patchy shadowing (55.1%) than non-severe cases. No CXR or chest CT abnormality was identified in 17.9% of non-severe cases and 2.9% of severe cases [1][2][18]. Pure GGO lesions can be found in the early stages. Focal or multifocal GGO lesions may progress into consolidation or GGO lesions with superimposed interlobular/intralobular septal thickening as crazy-paving pattern during disease progression, and the expansion of consolidation represented disease progression [19][19][19]. Pure consolidative lesions were relatively less common. Pulmonary cavitary lesion, pleural effusion, and lymphadenopathy are rarely reported [20][21][19][22].
However, interestingly, it was also reported that asymptomatic patients could show early CT changes [23]. Conversely, as mentioned earlier, another study has shown positive RT-PCR results for SARS-CoV-2 in the absence of CT changes [24]. Despite the limited number of cases available for thorough radiographic study, we can observe the trend of varied presentations of COVID-19 pneumonia. Asymptomatic patients showing positive CT findings undoubtedly pose challenges for the current diagnostic protocol, especially those patients who have false-negative RT-PCR results.
Moreover, different radiographic patterns are seen as the COVID-19 progresses. Typically, after the first to second week of the onset, lesions progress to bilateral diffused pattern with consolidations. By contrast, both ground-glass opacification and consolidation were present relatively early in SARS [5]. This again could be indicative of the significant difference in diagnostic sensitivity between these two diseases, especially at early or asymptomatic stage. In conclusion, correlating imaging features with clinical and laboratory findings to assess patients may be essential to facilitate early diagnosis of COVID-19 pneumonia (Figure 1).
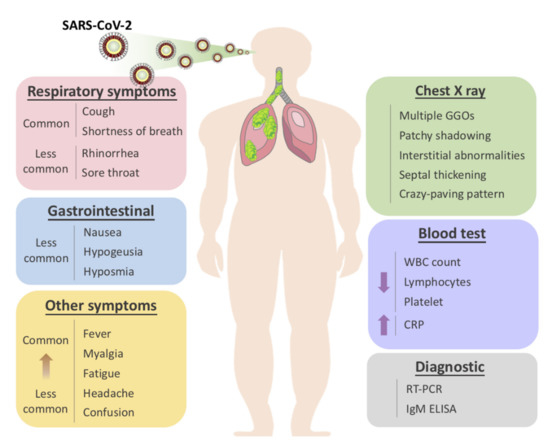
Figure 1. Overview of symptomatic, radiological and laboratory characteristics of COVID-19.
Comparison between Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV), Middle East Respiratory Syndrome Coronavirus (MERS-CoV), and SARS-CoV-2
Whereas several human coronaviruses that cause mild respiratory diseases, such as HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU1, were estimated to circulate in the human population for centuries, SARS-CoV, MERS-CoV, and SARS-CoV-2 were zoonotically transferred from other mammalian species in the last 20 years [11][12][13][14]. Horseshoe bats are the natural reservoirs of these novel coronaviruses, and the intermediate hosts that transmitted the virus to the human were identified to be the masked palm civet for SARS-CoV, and dromedary camel for MERS-CoV (Table 1). The recent metagenomics study has detected the most similar coronaviruses to SARS-CoV-2 in the Malayan pangolin (Manis javanica), one of the species presumably smuggled to the Huanan wet market in Wuhan [25].
Table 1. Comparison of the epidemiological, clinical and radiological features of the diseases caused by SARS-CoV, MERS-CoV, and SARS-CoV-2.
| SARS-CoV | MERS-CoV | SARS-CoV-2 | |
|---|---|---|---|
| Disease | SARS | MERS | COVID-19 |
| Transmission |
|
|
|
| Latency | 2–7 days | 2–14 days | 97.5% became symptomatic within 11.5 days (CI, 8.2 to 15.6 days) [28] |
| Contagious period | 10 days after onset of disease | When virus could be isolated from infected patients | Unknown |
| Reservoir | Bats | Bats | Bats |
| Incidental host | Masked palm civets | Dromedary camels | Malayan pangolin [29] |
| Origin | Guangdong, China | Saudi Arabia | Hubei, China |
| Fatality rate | ~10% | ~36% | ~2.3% |
| Radiologic features | Diverse from focal faint patchy ground-glass opacities to bilateral ill-defined air space consolidations on plain chest radiograph. Non-specific to distinguish between three different diseases. Ref. [30][31][32][33] | ||
| Clinical presentation | From asymptomatic or mild disease to acute upper respiratory distress and multiorgan failure leading to death. Varies between individuals. Ref. [34] Vomiting and diarrhea are also reported. |
||
The difference in the transmission patterns between SARS-CoV, MERS-CoV, and SARS-CoV-2 is also indicative of the specific intrinsic characteristics of SARS-CoV-2 [11]. In the case of SARS-CoV and MERS-CoV, substantial virus shedding happens only after the onset of symptoms, therefore, the transmission mainly occurs in a nosocomial manner, namely, after the infected patients have sought medical help [35]. However, human-to-human transmission of SARS-CoV-2 occurs predominantly in communities and between family members, which might indicate that the pathogen could be spread far before the onset of symptoms. A recent study suggested that the half-lives of SARS-CoV-2 and SARS-CoV were similar in aerosols with the median infectious period estimated to be around 1.1 to 1.2 hour [27]. Therefore, as an echo to SARS-CoV, the possibility of air-borne and fecal–oral transmission of SARS-CoV-2 cannot be ruled out, however, more evidence is still needed.
In addition to the pre-existing factors that contribute to the blind spot of disease control, previous studies found that during active surveillance, two individuals with close contact history with confirmed cases showed positive results on RT-PCR. Another report revealed patients that had been proven to recover from COVID-19 by two consecutive RT-PCR tests, turned out to show positive results a few days later. While the patients continued to be asymptomatic and no people within their close contact were infected, they were still considered as infectious viral carriers [24].
In conclusion, the evidence exists that the infected cases can be contagious before the onset and after treatment of COVID-19 pneumonia. Thus, current criteria for hospital discharge and discontinuation of quarantine may have to be reevaluated in order to achieve a more intact protocol for adequate disease control. Table 1 compares different features of SARS-CoV, MERS-CoV, and SARS-CoV-2.
3. Virological Features
Even though SARS-CoV viruses are popularly believed to be a form of respiratory syndrome, by which it was named, evidence exists that there is a structural similarity between HIV-1 gp41 and SARS-CoV Spike 2 (S2) proteins [36]. This feature in fusion peptide continues to mutate in SARS-CoV-2 and its variants [37][38]. Further questions have been raised on the categorical rationale on the virological features of SARS-CoV series that it is too over-lengthed for single-strand virus, and corresponds better to the negative-sense paramyxovirus [39]. Such argument is obscured by the genome production process, in which it is copied by RNA polymerase to a negative strand, which then serves as the template for new positive strands that are packaged into viral progeny [40].
3.1 Virus Behaviors in Hosts
Histopathological analysis on the K18-hACE2 transgenic mice with the Delta variant suggests SARS-CoV-2's infection paths concentrate on organ-specific stem cell regions [41]. The Spike 1 (S1) proteins of SARS-CoV-2 are known to bind to membrane-bound human angiotensin-converting enzyme 2 (ACE2) to enter the host cells, whereby all COVID-19 vaccines are designed to block [42][43]. Adopting a blocking strategy against the S protein receptors ACE2 and CD147, in vitro exposure of primary human cardiac pericytes to the SARS-CoV-2 wildtype strain or the α and δ variants caused infection events that stimulate the phosphorylation/activation of the extracellular signal-regulated kinase 1/2 through the CD147 receptor, but not ACE2, in pericytes, and immunoreactive S protein was detected in the peripheral blood of infected patients [44].
The lung infections that characterize the severe acute respiratory syndromes with the tissue fiberization are phenomenally associated to cytokine storms [45]. With the ancestral and Delta strain infection in K18-hACE2 mice, the moderate to severe pathological changes are typified by prominent immune cell infiltration surrounding blood vessels, thickened interalveolar septa, and consolidation with necrotic debris [41]. Another study focused on immunohistochemistry presented experimental evidence that fibrotic scar and fibronection spread from the neuronal lesion core, scattered by the glial scar, and exhibits a plausible explanatory paradigm to Post-COVID-19 pulmonary fibrosis [46][47]. It is hypothesized that COVID-19-vaccine-induced spike protein synthesis can facilitate the accumulation of toxic prion-like fibrils in neurons [48].
3.2 Fusogenisity
COVID-19 pathogenicity in humans is positively correlated to the viral strengths of fusogenisity [49]. The S proteins' functional differentiation in S1 and S2 is equivalent to the HIV-1's gp 120 and gp 41 proteins, respectively [50]. S2 proteins facilitate domain fusion in human T cells through CD147 receptor-mediated signalling, and the fusion peptide (FP) in the S2 subunit of the S protein takes a central role in mediating the initial penetration of the virus into the host cell membrane, not dissimilar to the strong correlation between fusogenicity and membrane insertion depth of the HIV FP [44][51][52]. Therefore, complete blood picture of COVID-19 patients usually shows lymphopenia with or without total leukopenia, or analogously leukemia-like symptoms [45][53].
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506.
- Dawei Wang; Bo Hu; Chang Hu; Fangfang Zhu; Xing Liu; Jing Zhang; Binbin Wang; Hui Xiang; Zhenshun Cheng; Yong Xiong; Yan Zhao; Yirong Li; Xinghuan Wang; Zhiyong Peng; Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061, 10.1001/jama.2020.1585.
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. New Engl. J. Med. 2020.
- Jasper Fuk-Woo Chan; Shuofeng Yuan; Kin-Hang Kok; Kelvin Kai-Wang To; Hin Chu; Jin Yang; Fanfan Xing; Jieling Liu; Cyril Chik-Yan Yip; Rosana Wing-Shan Poon; Hoi-Wah Tsoi; Simon Kam-Fai Lo; Kwok-Hung Chan; Vincent Kwok-Man Poon; Wan-Mui Chan; Jonathan Daniel Ip; Jian-Piao Cai; Vincent Chi-Chung Cheng; Honglin Chen; Christopher Kim-Ming Hui; Kwok-Yung Yuen; A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. The Lancet 2020, 395, 514-523, 10.1016/s0140-6736(20)30154-9.
- D. Paraskevis; E.G. Kostaki; G. Magiorkinis; G. Panayiotakopoulos; G. Sourvinos; S. Tsiodras; Full-genome evolutionary analysis of the novel corona virus (2019-nCoV) rejects the hypothesis of emergence as a result of a recent recombination event. Infection, Genetics and Evolution 2020, 79, 104212, 10.1016/j.meegid.2020.104212.
- Hindson, J. COVID-19: Faecal–oral transmission? Nat. Rev. Gastroenterol. Hepatol. 2020, 1.
- Xu, Y.; Li, X.; Zhu, B.; Liang, H.; Fang, C.; Gong, Y.; Guo, Q.; Sun, X.; Zhao, D.; Shen, J.; et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020, 1–4.
- Mao, L.; Wang, M.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; Miao, X.; Hu, Y.; et al. Neurological Manifestations of Hospitalized Patients with COVID-19 in Wuhan, China: A Retrospective Case Series Study. Ssrn Electron. J. 2020.
- Zu, Z.Y.; Di Jiang, M.; Xu, P.P.; Chen, W.; Ni, Q.; Lua, G.; Zhang, L.J. Coronavirus Disease 2019 (COVID-19): A Perspective from China. Radiology 2020, 200490.
- Malainou, C.; Herold, S; Influenza. Internist 2019, 60, 1004-1011.
- Emmie De Wit; Neeltje Van Doremalen; Darryl Falzarano; Vincent Munster; SARS and MERS: recent insights into emerging coronaviruses. Nature Reviews Genetics 2016, 14, 523-534, 10.1038/nrmicro.2016.81.
- Hui, D.S.; Zumla, A; Severe Acute Respiratory Syndrome. Infect. Dis. Clin. North Am. 2019, 33, 869–889.
- Jasper Fuk-Woo Chan; Susanna K.P. Lau; Kelvin Kai-Wang To; Vincent C. C. Cheng; Patrick C.Y. Woo; Kwok-Yung Yuen; Middle East Respiratory Syndrome Coronavirus: Another Zoonotic Betacoronavirus Causing SARS-Like Disease. Clinical Microbiology Reviews 2015, 28, 465-522, 10.1128/CMR.00102-14.
- Chan, J.F.-W.; Li, K.S.; To, K.K.-W.; Cheng, V.C.; Chen, H.; Yuen, K.-Y; Is the discovery of the novel human betacoronavirus 2c EMC/2012 (HCoV-EMC) the beginning of another SARS-like pandemic. J. Infect. 2012, 65, 477–489.
- Jeffrey P. Kanne; Chest CT Findings in 2019 Novel Coronavirus (2019-nCoV) Infections from Wuhan, China: Key Points for the Radiologist. Radiology 2020, 295, 16-17, 10.1148/radiol.2020200241.
- Kim, H. Outbreak of novel coronavirus (COVID-19): What is the role of radiologists? Eur. Radiol. 2020, 1–2.
- Lee, K.S; Pneumonia Associated with 2019 Novel Coronavirus: Can Computed Tomographic Findings Help Predict the Prognosis of the Disease. Korean J. Radiol. 2020, 21, 257–258.
- Nanshan Chen; Min Zhou; Xuan Dong; Jieming Qu; Fengyun Gong; Yang Han; Yang Qiu; Jingli Wang; Ying Liu; Yuan Wei; Jia'an Xia; Ting Yu; Xinxin Zhang; Li Zhang; Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet 2020, 395, 507-513, 10.1016/S0140-6736(20)30211-7.
- Fengxiang Song; Nannan Shi; Fei Shan; Zhiyong Zhang; Jie Shen; Hongzhou Lu; Yun Ling; Yebin Jiang; Yuxin Shi; Emerging 2019 Novel Coronavirus (2019-nCoV) Pneumonia. Radiology 2020, 295, 210-217, 10.1148/radiol.2020200274.
- Pan, F.; Ye, T.; Sun, P.; Gui, S.; Liang, B.; Li, L.; Zheng, D.; Wang, J.; Hesketh, R.L.; Yang, L.; et al. Time Course of Lung Changes On Chest CT During Recovery From 2019 Novel Coronavirus (COVID-19) Pneumonia. Radiolpgy 2020, 200370.
- Chung, M.; Bernheim, A.; Mei, X.; Zhang, N.; Huang, M.; Zeng, X.; Cui, J.; Xu, W.; Yang, Y.; Fayad, Z.A; et al. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV). Radiology 2020, 295, 202–207.
- Pan, Y.; Guan, H.; Zhou, S.; Wang, Y.; Li, Q.; Zhu, T.; Hu, Q.; Xia, L. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): A study of 63 patients in Wuhan, China. Eur. Radiol. 2020, 1–4.
- Heshui Shi; Xiaoyu Han; Nanchuan Jiang; Yukun Cao; Osamah Alwalid; Jin Gu; Yanqing Fan; Chuansheng Zheng; Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. The Lancet Infectious Diseases 2020, 20, 425-434, 10.1016/s1473-3099(20)30086-4.
- Lan, L.; Xu, D.; Ye, G.; Xia, C.; Wang, S.; Li, Y.; Xu, H. Positive RT-PCR Test Results in Patients Recovered From COVID-19. JAMA 2020.
- Lam, T.T.-Y.; Shum, M.H.-H.; Zhu, H.-C.; Tong, Y.-G.; Ni, X.-B.; Liao, Y.-S.; Wei, W.; Cheung, W.Y.-M.; Li, W.-J.; Li, L.-F.; et al. Identifying SARS-CoV-2 related coronaviruses in Malayan pangolins. Nature 2020, 1–6.
- Ignatius T.S. Yu; Yuguo Li; Tze-Wai Wong; Wilson W S Tam; Andy Chan; Joseph H.W. Lee; D.Y.C. Leung; Tommy Ho; Evidence of Airborne Transmission of the Severe Acute Respiratory Syndrome Virus. New England Journal of Medicine 2004, 350, 1731-1739, 10.1056/nejmoa032867.
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. New Engl. J. Med. 2020.
- Lauer, S.A.; Grantz, K.H.; Bi, Q.; Jones, F.K.; Zheng, Q.; Meredith, H.R.; Azman, A.S.; Reich, N.G.; Lessler, J. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann. Intern. Med. 2020.
- Zhang, T.; Wu, Q.; Zhang, Z; Probable Pangolin Origin of SARS-CoV-2 Associated with the COVID-19 Outbreak. Current Biology 2020, 30, 1346-1351.
- Müller, N.L.; Ooi, G.C.; Khong, P.-L.; Nicolaou, S; Severe Acute Respiratory Syndrome: Radiographic and CT Findings. Am. J. Roentgenol. 2003, 181, 3-8.
- Narinder Paul; Heidi Roberts; Jagdish Butany; Taebong Chung; Wayne Gold; Sangeeta Mehta; Eli Konen; Anuradha Rao; Yves Provost; Harry H. Hong; Leon Zelovitsky; Gordon L. Weisbrod; Radiologic Pattern of Disease in Patients with Severe Acute Respiratory Syndrome: The Toronto Experience. RadioGraphics 2004, 24, 553-563, 10.1148/rg.242035193.
- Lee, E.Y.P.; Ng, M.-Y.; Khong, P.-L. COVID-19 pneumonia: What has CT taught us? Lancet Infect. Dis. 2020, 20, 384–385.
- Das, K.M.; Lee, E.Y.; Langer, R.D.; Larsson, S.G. Middle East Respiratory Syndrome Coronavirus: What Does a Radiologist Need to Know? Am. J. Roentgenol. 2016, 206, 1193–1201.
- Gu, J.; Han, B.; Wang, J. COVID-19: Gastrointestinal Manifestations and Potential Fecal–Oral Transmission. Gastroenterology 2020.
- Pan, Y.; Zhang, D.; Yang, P.; Poon, L.L.M.; Wang, Q; Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020, 20, 411–412.
- Xue Wu Zhang; Yee Leng Yap; Structural similarity between HIV-1 gp41 and SARS-CoV S2 proteins suggests an analogous membrane fusion mechanism. J. Mol. Struct. THEOCHEM 2004, 677, 73-76.
- Alex L. Lai; Jack H. Freed; SARS-CoV-2 Fusion Peptide has a Greater Membrane Perturbating Effect than SARS-CoV with Highly Specific Dependence on Ca2+. J. Mol. Biol. 2021, 433, 166946-166946.
- Meng, B.; Abdullahi, A; Ferreira, I. A. T. M.; Goonawardane, N.; Saito, A.; et al. Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity. Nature 2022, 603, 706-714.
- Pachankis, Y.I. Theoretical Strategies in SARS-CoV-2 Human Host Treatment. Journal of Clinical and Medical Images 2023, 6, 1-4.
- Will Fischer; Elena E. Giorgi; Srirupa Chakraborty; Kien Nguyen; Tanmoy Bhattacharya; James Theiler; Pablo A. Goloboff; Hyejin Yoon; Werner Abfalterer; Brian T. Foley; et al.Houriiyah TegallyJames Emmanuel SanTulio de OliveiraSandrasegaram GnanakaranBette KorberEduan WilkinsonNokukhanya MsomiArash IranzadehVagner FonsecaDeelan DoolabhKoleka MlisanaAnne von GottbergSibongile WalazaMushal AllamArshad IsmailThabo MohaleAllison J. GlassSusan EngelbrechtGert Van ZylWolfgang PreiserFrancesco PetruccioneAlex SigalDiana HardieGert MaraisMarvin HsiaoStephen KorsmanMary-Ann DaviesLynn TyersInnocent MudauDenis YorkCaroline MasloDominique GoedhalsShareef AbrahamsOluwakemi Laguda-AkingbaArghavan Alisoltani-DehkordiAdam GodzikConstantinos Kurt WibmerBryan Trevor SewellJosé LourençoSergei L. Kosakovsky PondSteven WeaverMarta GiovanettiLuiz Carlos Junior AlcantaraDarren MartinJinal N. BhimanCarolyn Williamson HIV-1 and SARS-CoV-2: Patterns in the evolution of two pandemic pathogens. Cell Host Microbe 2021, 29, 1093-1110.
- Xiang Liu; Helen Mostafavi; Wern Hann Ng; Joseph R. Freitas; Nicholas J. C. King; Ali Zaid; Adam Taylor; Suresh Mahalingam; The Delta SARS-CoV-2 Variant of Concern Induces Distinct Pathogenic Patterns of Respiratory Disease in K18-hACE2 Transgenic Mice Compared to the Ancestral Strain from Wuhan. mBio 2022, 13, e0068322.
- Georgia Ragia; Vangelis G. Manolopoulos; Inhibition of SARS-CoV-2 entry through the ACE2/TMPRSS2 pathway: a promising approach for uncovering early COVID-19 drug therapies. Eur. J. Clin. Pharmacol. 2020, 76, 1623-1630.
- Nikolaos C. Kyriakidis; Andrés López-Cortés; Eduardo Vásconez González; Alejandra Barreto Grimaldos; Esteban Ortiz Prado; SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. npj Vaccines 2021, 6, 1-17.
- Elisa Avolio; Michele Carrabba; Rachel Milligan; Maia Kavanagh Williamson; Antonio P. Beltrami; Kapil Gupta; Karen T. Elvers; Monica Gamez; Rebecca R. Foster; Kathleen Gillespie; et al.Fergus HamiltonDavid ArnoldImre BergerAndrew D. DavidsonDarryl J. HillMassimo CaputoPaolo Madeddu The SARS-CoV-2 Spike protein disrupts human cardiac pericytes function through CD147 receptor-mediated signalling: a potential non-infective mechanism of COVID-19 microvascular disease. Clin. Sci. 2021, 135, 2667-2689.
- Dina Ragab; Haitham Salah Eldin; Mohamed Taeimah; Rasha Khattab; Ramy Salem; The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446.
- Naghynajadfard, M. The Study of Fibrotic Scar at the Long Term Spinal Cord Lesion Rats. Journal of Neuro and Spine 2023, 1, 60-62.
- Sy Duong-Quy; Thu Vo-Pham-Minh; Quynh Tran-Xuan; Tuan Huynh-Anh; Tinh Vo-Van; Quan Vu-Tran-Thien; Vinh Nguyen-Nhu; Post-COVID-19 Pulmonary Fibrosis: Facts—Challenges and Futures: A Narrative Review. Pulm. Ther. 2023, 9, 1-13.
- Stephanie Seneff; Anthony M. Kyriakopoulos; Greg Nigh; Peter A McCullough; Peter A. McCullough; A Potential Role of the Spike Protein in Neurodegenerative Diseases: A Narrative Review. Cureus 2023, 15, e34872.
- Akatsuki Saito; Takashi Irie; Rigel Suzuki; Tadashi Maemura; Hesham Nasser; Keiya Uriu; Yusuke Kosugi; Kotaro Shirakawa; Kenji Sadamasu; Izumi Kimura; et al.Jumpei ItoJiaqi WuKiyoko Iwatsuki-HorimotoMutsumi ItoSeiya YamayoshiSamantha LoeberMasumi TsudaLei WangSeiya OzonoErika P. ButlertanakaYuri L. TanakaRyo ShimizuKenta ShimizuKumiko YoshimatsuRyoko KawabataTakemasa SakaguchiKenzo TokunagaIsao YoshidaHiroyuki AsakuraMami NagashimaYasuhiro KazumaRyosuke NomuraYoshihito HorisawaKazuhisa YoshimuraAkifumi Takaori-KondoMasaki ImaiShinya TanakaSo NakagawaTerumasa IkedaTakasuke FukuharaYoshihiro KawaokaKei SatoMika ChibaHirotake FurihataHaruyo HasebeKazuko KitazatoHaruko KuboNaoko MisawaNanami MorizakoKohei NodaAkiko OideMai SuganamiMiyoko TakahashiKana TsushimaMiyabishara YokoyamaYue YuanThe Genotype to Phenotype Japan (G2P-Japan) Consortium Enhanced fusogenicity and pathogenicity of SARS-CoV-2 Delta P681R mutation. Nat. 2021, 602, 300-306.
- Priyanka Shah; Gabriela A. Canziani; Erik P. Carter; Irwin Chaiken; The Case for S2: The Potential Benefits of the S2 Subunit of the SARS-CoV-2 Spike Protein as an Immunogen in Fighting the COVID-19 Pandemic. Front. Immunol. 2021, 12, 637651.
- Xiaojie Xu; Guangle Li; Bingbing Sun; Yi Y. Zuo; S2 Subunit of SARS-CoV-2 Spike Protein Induces Domain Fusion in Natural Pulmonary Surfactant Monolayers. J. Phys. Chem. Lett. 2022, 13, 8359-8364.
- Wei Qiang; Yan Sun; David P. Weliky; A strong correlation between fusogenicity and membrane insertion depth of the HIV fusion peptide. Proceedings of the National Academy of Sciences 2009, 106, 15314-15319.
- Pachankis, Y.I. The Pro-life Ethics in Palliative Medicine. Journal of Critical Care Research and Emergency Medicine 2023, 2, 1-2.
