The screening and early diagnosis of diseases are crucial for a patient’s treatment to be successful and to improve their survival rate, especially for cancer. The development of non-invasive analytical methods able to detect the biomarkers of pathologies is a critical point to define a successful treatment and a good outcome. A biopsy is a technique in which tissue samples are taken from the body and examined under a microscope to see if cancer (though the concept is applicable to many other diseases) or abnormal cells are present. Electrochemical methods, such as electrochemical impedance spectroscopy (EIS), differential pulse voltammetry (DPV), and cyclic voltammetry (CV), play a crucial role in both the development of biosensors and the evaluation of their performance. These methods are highly valuable approaches in the field of liquid biopsy.
1. Introduction
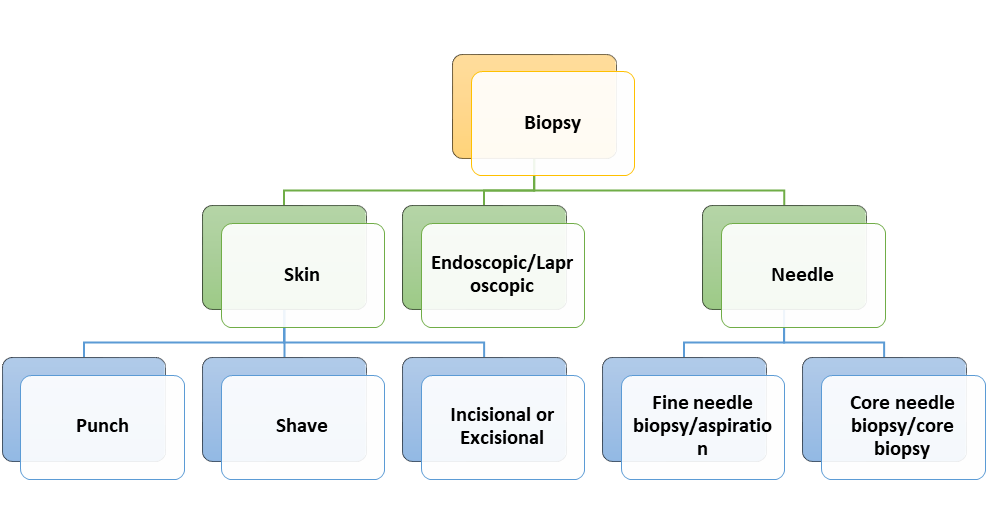
A biopsy is a technique in which tissue samples are taken from the body and examined under a microscope to see if cancer (though the concept is applicable to many other diseases) or abnormal cells are present. Biopsies can be classified into the following categories based on the sample being taken (Figure 1).
Figure 1.
Classification of biopsies: understanding the different categories.
In the last decades, the liquid biopsy, namely, the possibility to have a diagnosis from body fluids without resorting to a tissue biopsy, has been increasingly investigated. The possibility to detect and classify tumors or other diseases, even at a very early stage, in a minimally invasive and repeatable way could have a significant clinical impact, and significant progress has been made in the development of devices able to do this in a smarter manner compared to standard analytical methods. Despite the great advantages for patients’ compliance and the minimally invasive features, this approach has not yet attained the status of a conventional tool in the armory of clinical oncologists
[1].
Biosensors are considered to be promising tools for the quantitative or semi-quantitative detection of analytes
[2]. In this type of sensor, a biological molecule interacts with the analyte, previously immobilized on the biosensor, producing a physicochemical signal that is detected by the transducer. Biosensors can be divided into two categories: catalytic-based, which produce a substance starting from substrate’s compounds, and affinity-based, which directly bind the analyte. According to the type of signal being transmitted, biosensors can be classified as electrochemical, optical
[3], magnetic, or piezoelectric, just to name a few
[4].
Electrochemical techniques excel among these methods by offering rapid, sensitive, selective, and cost-effective detection and monitoring of a wide range of biological molecules associated with diverse diseases. Additionally, their seamless integration into portable systems enables the implementation of point-of-care diagnostic approaches
[5]. An electrochemical biosensor is a compact device that utilizes both biorecognition processes and electrochemical transducers to convert biological information into electrical signals. This conversion provides either quantitative or semi-quantitative information about the analyte being detected
[6].
Electrochemical biosensors were recommended by the international union of pure and applied chemistry (IUPAC). Which states that an electrochemical biosensor is a self-contained integrated device that is capable of providing specific quantitative or semi-quantitative analytical information using a biological recognition element (biochemical receptor) which is kept in direct spatial contact with an electrochemical transduction element.
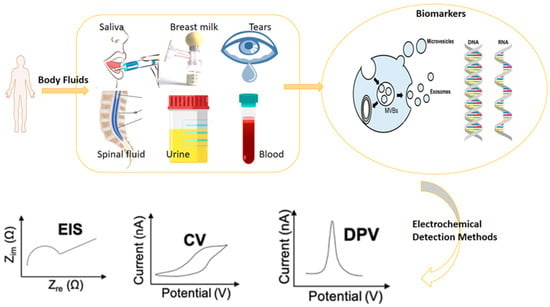
Electrochemical methods, such as electrochemical impedance spectroscopy (EIS), differential pulse voltammetry (DPV), and cyclic voltammetry (CV), play a crucial role in both the development of biosensors and the evaluation of their performance
[7]. These methods are highly valuable approaches in the field of liquid biopsy (
Figure 2). For research purposes, the CV technique is widely used in biosensor development because it provides valuable information such as the types of redox processes present in the analysis and the reversibility of reactions. A sensing system that can identify a cell’s location within a microfluidic channel was designed by Rapier et al. The results from electrochemical impedance spectroscopy (EIS) show that cells in microfluidic channels can be positioned between different pairs of electrodes at varied locations along the device’s length. Impedance spectra distinguish among confluent, sparse, and empty microfluidic channels. A huge boost to the development of this kind of sensors as well as to their application in the liquid biopsy was given by their high suitability to miniaturization. Electrodes’ architecture, indeed, can be easily achieved through micro- and nanofabrication methods, increasing the number of sensing elements per area and allowing high-throughput performances
[8].
Figure 2.
Overview of the electrochemical biosensors’ operation.
Also, lab-on-a-chip integration is directly linked to miniaturization. The possibility to perform multiple assays by lowering the dimensions of sensing elements has led to the necessity to differently functionalize and use them for the detection of different biomarkers
[9][10][9,10].
The utilization of impedance-based methods significantly facilitated the simplification of cellular assays, providing quantitative and highly sensitive results that are amenable to automation and scalability in multi-well formats. An extensive review by W. Gamal et al. shed light on their efficacy. Moreover, in the context of the real-time monitoring of a three-dimensional cell culture, dielectric spectroscopy and electrical impedance tomography emerged as promising alternatives to two-dimensional impedance sensing
[11]. These impedance-based cellular assays (IBCAs) serve as label-free phenotyping assays and are gaining increasing interest in the field of regenerative medicine applications
[12]. To determine the flow in a microfluidic chip, Evangelos S. et al. developed a strain-sensing module based on microfluidic and lab-on-a-chip systems that offers simple integration with most microfluidic systems. The sensor consists of interconnected platinum nanoparticles that self-assemble on flexible polyolefin substrates, which also serve as the sealing layer for the microfluidic channels. These nanoparticle networks are formed using a modified sputtering approach and are implemented on printed circuit board substrates (PCBs) through milling and computer numerical control machining. The resulting module exhibits a competitive limit of detection (LOD), cost-effectiveness, low power requirements, and seamless integration with existing microfluidic systems. It can be utilized as an independent unit or integrated into the sealing material, enabling the detection of flow rates as low as 5 μL/min (equivalent to a strain of 0.00337%). The sensor demonstrates a sensitivity of 0.021 μL per minute
[13].
Lucile et al. reported another novel microfluidic method that can selectively extract, preconcentrate, and fluorescently detect IL-6 directly on the chip by the fluidization of magnetic beads. The ability to switch between packed and fluidized states allowed the authors to evaluate how the physical characteristics of the beads could be altered to increase mass transport, lessen non-specific binding, and triple the detection signal. A high dynamic range (10 pg/mL to 2 ng/mL) and a twofold reduction in LOD compared to traditional approaches were demonstrated by integrating the entire ELISA protocol into a single microfluidic chamber
[14].
2. Comparison of Liquid Biopsy Electrochemical Methods: Advantages and Limitations
Nowadays, cancer is the major disease affecting human health and life, and its large diffusion requires the development of simple, practical, and facile diagnosis methods for simplifying its treatment and improving its cure rate. Compared with medical imaging and a pathological examination, which are the most common cancer diagnosis methods, the liquid biopsy represents a promising strategy for cancer biomarker detection, opening the way to direct and rapid diagnostic methods with high efficacy
[15][16][177,178]. Among several biomarkers, circulating tumor cells (CTCs) are well-established as promising targets for the detection of tumors via the liquid biopsy. Since tumor cells can be shed into the blood before the formation of visible solid tumor lesions, detecting CTCs before the imaging findings or clinical manifestations is an efficient method for the early diagnosis and monitoring of cancer
[17][179]. However, the content of CTCs in peripheral blood circulation is very small, and the techniques used for its detection (fluorescence imaging, magnetic resonance imaging, and cytological detection
[18][19][20][180,181,182]) have several shortcomings, such as high cost, a large amount of time, low sensitivity, and a lack of specificity, thus limiting their use in clinical applications
[21][183].
In the last years, electrochemical sensing technology has been widely investigated as a good alternative method for the detection of CTCs because of its advantages of high sensitivity, good selectivity, low cost, easy portability, and rapid detection. Compared with traditional detection techniques, electrochemical methods demonstrate competitive results in terms of the LOD and selectivity. CTCs can be electrochemically detected by using two common types of approaches. The first one is often related to impedimetric sensors and exploits the change in the electron transfer produced by CTCs captured on the electrode, usually conjugated to various recognitive materials including antibodies
[22][184], aptamers
[23][185], and receptors
[24][186]. However, this type of sensor usually needs just one electrode to work, thus resulting in a lack of capture efficiency. To overcome this limit, modifying electrodes with nanomaterials can be a good strategy to enhance the capture efficiency for CTCs. For example, Wang et al. conjugated gold nanostars with a high surface area, with CTCs’ specific aptamer. Owing to this design, the sensor showed a sensitive detection limit of 5 cells/mL
[25][187]. Cai et al. developed a dual-recognition electrochemical cytosensor for the detection of CTCs. The sensor, based on Cabot carbon black (BP2000)/AuNPs anchoring anti-epithelial cell adhesion molecule (anti-EpCAM) antibodies as capture probes and novel branched PtAuRh trimetallic nanospheres (b-PtAuRh TNS), linked with aptamers targeting mucin1 (MUC1) as signal probes, exhibited a wide linear range of 5–1 × 10
6 cells per mL
−1 and a low detection limit of 1 cell per mL
−1 [26][188]. In another work, Zhang et al. exploited the reaction of LiFePO
4 with sodium molybdate to generate an electrochemical signal for detecting CTCs. In particular, they captured CTCs from sample by using Fe
3O
4 magnetic nanospheres (MNs) modified with the EpCAM antibody, while gold-nanoparticle-modified LiFePO
4 (LiFePO
4/Au) was used as an electrochemical probe. The assay presented a detection range from 3 to 10,000 cells per mL
−1, with a detection limit of 1 cell per mL
−1 [27][189]. Although several studies demonstrated the advantages of electrochemical sensing technology for the detection of CTCs and the significant advances in the biosensing research area thanks to immunotechnology, microfluidics, and nanotechnology, the clinical use of such biosensors is still limited. In fact, the number of CTCs in the peripheral blood circulation is very little, and detection can be very difficult. Moreover, the existing detection techniques use nucleic acids and antibodies as target molecules, which, lacking specificity for the classification of captured CTCs, cannot be utilized to give precise information about patient-specific tumor biology. Thus, combining advanced technologies such as microfluidics and the DNA walker and exploring more cell-specific targets could be a significative strategy to improve the sensitivity and specificity of such biosensors. Recently, Ming et al. developed a new on-skin optoelectronic biosensor based on cyclic voltammetry that can measure various vital signs related to blood flow and oxygenation in a non-invasive and continuous way
[28][190].
As voltammetry is the most commonly used electrochemical technique for liquid biopsies, this research primarily focuses on the DPV technique, a subtype of voltammetry, because DPV is considered to be an important electrochemical method for liquid biopsy applications because it offers high sensitivity, selectivity, a wide dynamic range, rapid analysis, and minimal interference from other components in body fluids.
Scientists can determine which electrochemical methods are best-suited for particular applications by comparing the various electrochemical techniques used in liquid biopsy biosensing, depending on elements like the type of biomarker being analyzed, the concentration range, and the complexity of the sample matrix.
Such a comparison can help to guide the development of new biosensors for liquid biopsy analysis as well as to optimize existing methods for improved performance and sensitivity. Additionally, understanding the advantages and limitations of different electrochemical methods can aid in the interpretation of experimental results and can inform the selection of appropriate analytical methods for a given research question. These aspects have been summarized in
Table 1.
Table 1.
A comparison of various electrochemical methods employed in biosensing applications for liquid biopsy analysis.
| EC Method |
Advantages |
Limitations |
| Potentiometric |
- ▪
-
High selectivity for specific analytes through the use of ion-selective electrodes (ISEs)
- ▪
-
Wide range of analytes that can be detected using ISEs, including ions, gases, and molecules
- ▪
-
Simple instrumentation, with ISEs often consisting of a single electrode and a reference electrode
- ▪
-
Non-destructive, as the sample is not consumed during the analysis
|
- ▪
-
Limited sensitivity compared to other electrochemical methods
- ▪
-
Limited dynamic range, as ISEs typically have a limited linear response range
- ▪
-
Interference from other ions or molecules in the sample can affect the accuracy of the analysis
- ▪
-
Slow response time compared to other electrochemical methods
|
| Impedimetric |
- ▪
-
High sensitivity for certain analytes
- ▪
-
Can measure non-faradaic processes such as adsorption and desorption
- ▪
-
Can provide information on both the electron-transfer kinetics and the charge-transfer resistance of the system
|
- ▪
-
Requires complex instrumentation and data analysis
- ▪
-
Limited dynamic range compared to other electrochemical methods
- ▪
-
Sensitive to electrode fouling and surface defects
|
| Conductometric |
- ▪
-
High sensitivity, as changes in conductivity can be highly sensitive to analyte concentration
- ▪
-
Wide range of analytes that can be detected, including ions, gases, and molecules
- ▪
-
Simple instrumentation, with conductometric biosensors often consisting of a pair of electrodes and a transducer
- ▪
-
Non-destructive, as the sample is not consumed during the analysis
|
- ▪
-
Limited selectivity compared to other electrochemical methods
- ▪
-
Interference from other ions or molecules in the sample can affect the accuracy of the analysis
- ▪
-
May be affected by changes in temperature, humidity, and other environmental factors
- ▪
-
May require the optimization of electrode and transducer properties to achieve the desired sensitivity and selectivity
|
| Cyclic Voltammetry (CV) |
- ▪
-
High sensitivity for certain analytes
- ▪
-
Simple instrumentation and low cost
- ▪
-
Can measure both oxidation and reduction reactions
|
- ▪
-
Limited selectivity; it can be affected by interfering species
- ▪
-
Low resolution; the current signal can be difficult to interpret
- ▪
-
Slow scan rate that can limit the speed of analysis
|
| Differential Pulse Voltammetry (DPV) |
- ▪
-
High sensitivity and selectivity for certain analytes
- ▪
-
Wide dynamic range
- ▪
-
Rapid analysis
- ▪
-
Minimal interference from other components in the sample
|
- ▪
-
Limited applicability to certain types of analytes (e.g., those with weak redox activity)
- ▪
-
Requires the careful optimization of parameters such as pulse width and amplitude
- ▪
-
High background noise can be a problem in complex samples
|
| Stripping Voltammetry (SV) |
- ▪
-
High sensitivity and selectivity for analytes, like heavy metals and trace elements
- ▪
-
Wide range of analytes that can be detected, including ions, gases, and molecules
- ▪
-
Non-destructive, as the sample is not consumed during the analysis
- ▪
-
Can be used for both qualitative and quantitative analysis
|
- ▪
-
Requires pre-concentration of the analyte before measurement, which can be time-consuming and may limit the speed of analysis
- ▪
-
Can be affected by interference from other species in the sample
- ▪
-
Limited dynamic range, particularly for quantitative analysis
- ▪
-
May require specialized instrumentation, such as a mercury electrode
|
Each electrochemical technique has advantages and disadvantages of its own. The choice of method depends on the specific analyte of interest and the requirements of the analysis.
The desire to manufacture micro total analysis systems, low-cost point-of-care diagnostics, and environmental monitoring devices sparked the creation of tiny and portable biosensor devices. So, for the development of such a biosensor, it is essential to understand the electrochemical method on which this biosensor operates.
The efficient transducer surface or immobilization matrix is the most significant step in the fabrication of a miniaturized electrochemical biosensor. For optimal biosensor performance, we need to carefully select the materials, electrochemical methodology, and manufacturing process. The PoC devices used for the liquid biopsy might benefit from a wise device design and efficient detection procedures. Research needs to be conducted to create combinatorial electrochemical biosensors with a high throughput and low cost for cancer diagnosis, therapy, and monitoring, utilizing the liquid biopsy. The commercialization of biosensors will increase when an electrochemical-based biosensing platform effectively works in a real-world sample environment with excellent selectivity, sensitivity, and stability.