Melatonin (MT), a naturally occurring compound, is found in various species worldwide. In 1958, it was first identified in the pineal gland of dairy cows. MT is an “old friend” but a “new compound” for plant biology. It brings experts and research minds from the broad field of plant sciences due to its considerable influence on plant systems. The MT production process in plants and animals is distinct, where it has been expressed explicitly in chloroplasts and mitochondria in plants. Tryptophan acts as the precursor for the formation of phyto-melatonin, along with intermediates including tryptamine, serotonin, N-acetyl serotonin, and 5-methoxy tryptamine. It plays a vital role in growth phases such as the seed germination and seedling growth of crop plants. MT significantly impacts the gas exchange, thereby improving physio-chemical functions in plant systems. During stress, the excessive generation and accumulation of reactive oxygen species (ROS) causes protein oxidation, lipid peroxidation, nucleic acid damage, and enzyme inhibition. Because it directly acts as an antioxidant compound, it awakens the plant antioxidant defense system during stress and reduces the production of ROS, which results in decreasing cellular oxidative damage. MT can enhance plant growth and development in response to various abiotic stresses such as drought, salinity, high temperature, flooding, and heavy metals by regulating the antioxidant mechanism of plants.
- melatonin
- indolamine
- abiotic stress
- antioxidant
- plant growth
1. Introduction
2. Biosynthesis of Melatonin in Plants
The MT production process in plants and animals is distinct. Many elements, including light, have a vital role in controlling its production in plants. MT is specifically expressed in chloroplasts and mitochondria in plants. Tryptophan acts as the precursor for the formation of phyto-melatonin, along with intermediates including tryptamine, serotonin, N-acetyl serotonin, and 5-methoxy tryptamine (Figure 1). According to the report from Tan and Reiter [26], the intermediates of MT production are found in several sub-cellular compartments including the cytoplasm, mitochondria, endoplasmic reticulum and chloroplasts. Tryptophan decarboxylase (TDC) first decarboxylates tryptophan to produce tryptamine in the cytoplasm, tryptamine-5-hydroxylase (T5H), and then performs an enzymatic hydroxylation to produce serotonin in the endoplasmic reticulum. N-acetyltransferase (SNAT) and acetyl serotonin methyl transferase (ASMT) convert serotonin through acetylation and methylation reactions into N-acetyl serotonin in chloroplasts and 5-methoxytryptamine in the cytoplasm. N-acetyl serotonin produced in chloroplast reacts with the ASMT in the cytoplasm and transforms into MT; meanwhile, 5-methoxytryptamine produced in cytoplasm moves into the chloroplast and reacts with SNAT to synthesize MT [27]. Alternatively, an enzyme known as caffeic acid O-methyltransferase (COMT), which regulates several substrates, can also convert N-acetyl serotonin into MT in a different route that has been studied through plants. COMT can also transform serotonin into 5-methoxytryptamine and produce MT through SNAT catalyzation [28].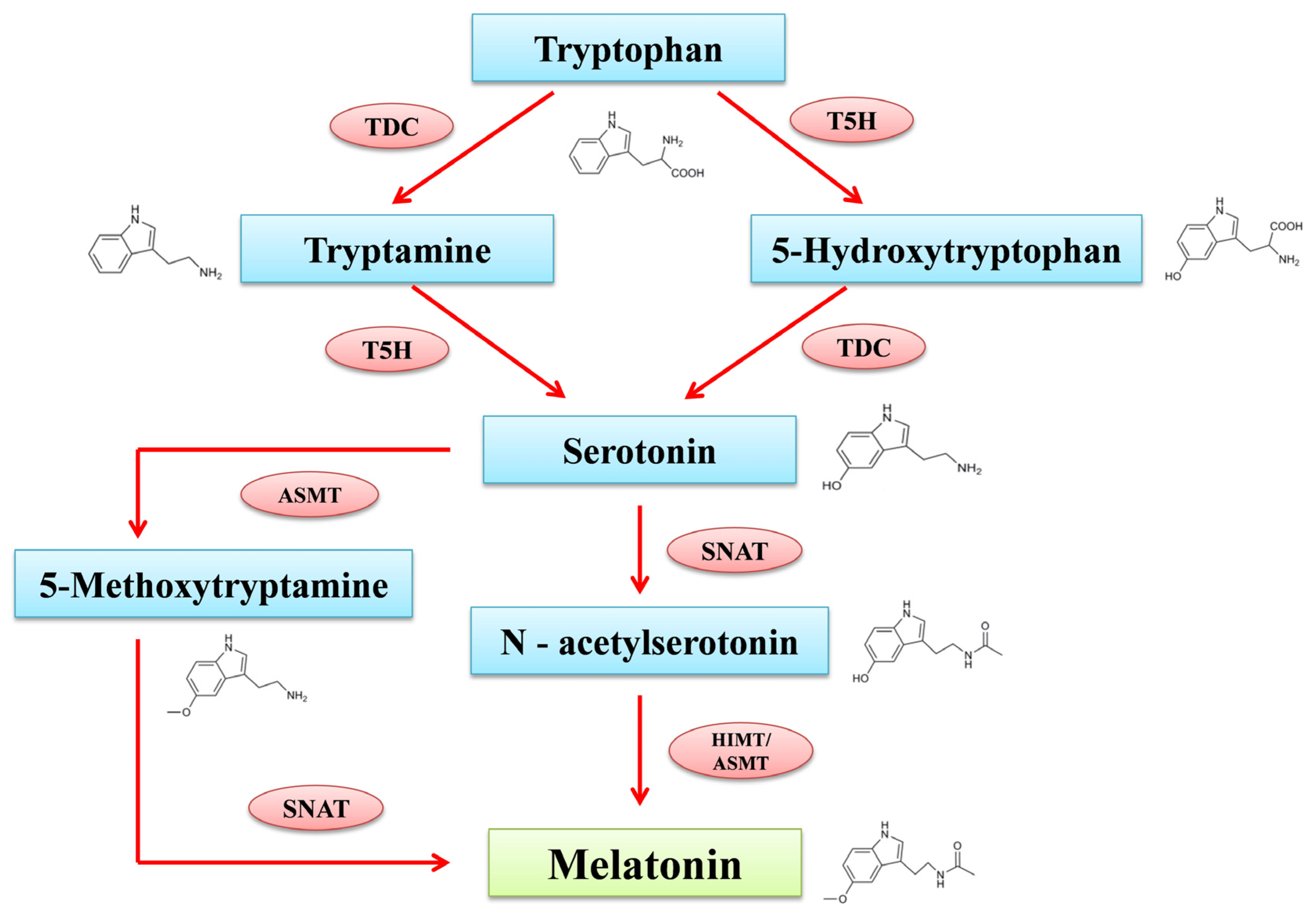
3. Melatonin’s Role in Plant Growth and Physiology
3.1. Germination
In the life cycle of higher plants, seed germination is a complicated process governed by several coordinated metabolic, cellular, and molecular activities. It is also a crucial time for the establishment of crop populations. Germination involves a number of metabolic and physical processes. This stage, which is similarly susceptible to stress and critical for determining whether plants will survive under adverse conditions, is greatly influenced by the external environment.3.2. Shoot and Root Growth
Due to the buildup of ABA, which further inactivates cell-wall-loosening enzymes under water stress in wheat, shifting the apoplastic pH from acidic to alkaline restricts the development of the plant’s shoots and roots [46][29]. The process of cell elongation involves an indoleamine molecule [47][30]. Pretreatment with MT results in a drop in intercellular pH to an acidic state and activates the enzymes responsible for loosening cell walls, which in turn triggers cell elongation like IAA [48][31]. As a consequence, seed priming with MT enhanced seed germination and seedling development through synthesizing stress-related proteins and activating signaling pathways in rice under stressful conditions [49][32]. Ahmad et al. [50][33] stated that MT along with the application of nitrogen significantly improved the shoot fresh and dry biomass in maize seedlings. Exogenous MT enhances the accumulation of soluble sugars and the protein level, which regulates osmotic adjustment under stressful conditions in cotton [51][34]. The application of MT stimulates the production of endogenous growth-inducing substances like metabolites, phytohormones, and increasing ROS and RNS scavenging systems in plants [52][35] which might lead to the production of higher shoots and denser roots. The fact that MT also causes the auxin-related genes to become active suggests that the auxin signal pathway is necessary for MT-mediated root development [53][36].3.3. Gas Exchange
Photosynthesis is the most important physiological function found in all green plants that is severely affected by abiotic stresses [61][37]. Abdulbaki et al. [62][38] explained that abiotic stresses reduce the production of assimilatory powers (ATP and NADPH) and Rubisco activity by destroying the chloroplast grana structure and photosynthetic electron transport system. The reduced diffusion and concentration of intercellular CO2 in the carboxylation site of rubisco also decreases the photosynthetic rate under stress [63][39]. Chlorophyll is a key photosynthetic pigment found in all higher plants and plays a vital function in absorption of light energy. Fu et al. [64][40] reported that the metabolite concentrations of chlorophyll a, chlorophyll b, and carotenoids were decreased under heat stress in wheat. The enhanced activity of chlorophyll-degrading enzymes like chlorophyllase, pheophytinase, and chlorophyll-degrading peroxidase catalyze the breakdown of chlorophyll molecules in response to stress [65][41]. Wang et al. [66][42] suggested that a direct link was observed between MT and the concentration of photosynthetic pigment in soybean. MT reduces the rate of chlorophyll degradation by lowering the transcript levels of pheophorbide-a-oxygenase (PAO) which is involved in chlorophyll metabolism [67][43]. The expressions of genes such as Chlase, PPH, and Chl-PRX associated with degradation of chlorophyll biosynthesis were downregulated by MT in Agrostis stolonifera [68][44]. Shi et al. [69][45] stated that MT increases the Bermuda grass photosynthetic pathway by protecting the chlorophyll molecule from degradation and enhances the expression of photosynthetic proteins like LHCa and PsaG during oxidative stress. MT also protects the chloroplast ultrastructure from oxidative damage and recovers photosynthetic accessory pigments like carotenoids, chlorophyll b, xanthophyll, and anthocyanin from stress [70][46]. Liu et al. [67][43] suggested that application of MT decreases the expression level and its relative mRNA abundance of genes involved in senescence (SAG12) and the programmed cell death process. MT slows down the aging process of leaves by enhancing the ROS scavenging mechanism, which stabilizes the chloroplast structure and protects photosynthesis-related genes from deterioration [71][47] in the tomato plant. Stomata play a vital role in the regulation of photosynthesis, transpiration rate, and water status of the plant [72][48]. MT regulates the opening of stomata through upregulation of the ABA catabolism process and simultaneously downregulates ABA anabolism that results in reduced accumulation of the endogenous ABA level. The decreased ABA level by MT reduces the production of H2O2 in guard cells of stomata that makes the stomata remain open and maintains the water status of the plant [73][49]. Leaf water status and leaf temperature are positively regulated by transpiration rate. The increased transpiration rate by MT enables the plant to maintain a lower leaf temperature, thereby improving photosynthetic efficiency [74][50]. The positive effect of MT on transpiration rate and stomatal conductance through the regulation of ABA level was also noticed in tomato [75][51], rice [76][52], and pepper [77][53].3.4. Antioxidant or ROS Scavenging
The crops are more vulnerable to the several abiotic stresses with changing climate during their growth phases. During stress conditions, plants convert 1–2% of the consumed oxygen into reactive oxygen species, specifically, hydroxyl radical (•OH), hydrogen peroxide (H2O2), superoxide radical (O2•−), and singlet oxygen (1O2). Stress enhances the production of ROS that results in cellular oxidative damage. The excessive generation and accumulation of ROS causes protein oxidation, lipid peroxidation, nucleic acid damage, enzyme inhibition, early leaf senescence, and necrosis [80][54]. Plants produced various enzymatic, such as CAT, POX, APX, SOD, GPX, and GR, and non-enzymatic antioxidants, like vitamins, carotenoids, stilbenes, and flavonoids, to capture the excess ROS in the plant system and thereby protect the plants from oxidative stress. Currently, MT is an inevitable compound present in the plant system and functions as a powerful antioxidant using both direct and indirect mechanisms during abiotic stress conditions. MT scavenges free radicals produced under stressful circumstances by increasing the endogenous antioxidants such as ascorbic acid and glutathione [58][55]. The expression level of genes related to antioxidant enzyme activity like SOD, CAT, APX, and GPX was also increased by MT in response to stress [81][56]. Kaur et al. [82][57] noticed that the Asada-Halliwell pathway, a crucial antioxidant enzymatic cycle, was regulated by MT in order to enhance the ROS scavenging mechanisms in stressed plants. Zhang et al. [83][58] suggested that MT stimulates the activity of H2O2 scavenging enzymes such as CAT, POD, and APX as well as ABA-degrading enzymes. Furthermore, MT controls the AsA-GSH cycle, which is essential for ROS detoxification, and enzymes like APX, MDHAR, DHAR, and GR were involved in the regulation of this cycle [84][59]. Rehman et al. [85][60] explained that MT effectively scavenges ROS by increasing the activity of the antioxidant enzyme glutathione peroxidase (GPX), which scavenges lipid peroxides, hydroperoxides, and H2O2 under stress. MT possesses amphiphilic characteristics that enable it to diffuse and distribute readily across lipid membranes and the cytoplasm. The MT-bound hydrophilic side of the lipid bilayer prevented lipid peroxidation by directly neutralizing the damaging chemicals produced under stressful circumstances [86][61]. Lei et al. [87][62] opined that application of MT to rapeseed minimizes the free radical formation and generation of ROS like H2O2 and O2−. The integrity of the plant cell membrane was improved by MT through the increased activity of antioxidant enzymes like SOD, CAT, APX, and GPX [88][63]. MT reduces the effects of oxidative stress by directly scavenging ROS through enhanced antioxidant enzyme activity that ultimately reduces the MDA level in plants [89][64]. The increased antioxidant enzyme activity and defense system by the exogenous application of MT under stress conditions were also reported in wheat [90][65], tomato [91][66], cabbage [92][67], and rice [93][68]. The generation of superoxide anion radicals is inhibited by MT via limiting the level of O2 flux under stress conditions when ADP levels are higher [94][69]. MT functions through several methods as a mediator in many antioxidant pathways, such as the glutathione ascorbate cycle, peroxidases, superoxide dismutase, and CAT under abiotic stress responses in plants [95][70]. Talaat and Todorova et al. [96][71] also observed that the plants treated with MT have increased ascorbate (AsA) and reduced glutathione (GSH) content, thereby reducing the formation of H2O2 in plant cells. The increased non-enzymatic antioxidants like AsA and GSH production are thought to be crucial for maintaining the ROS balance in plants under stress. The positive role of MT on antioxidant enzyme activity was also reported by Ye et al. [97][72] and Yan et al. [98][73] in barley and tomato.4. Melatonin’s Role in Secondary Metabolites’ Expression
Abiotic stress downregulates the accumulation and concentration of plant metabolites, whereas foliar application of MT positively upregulates the metabolites in the plant system. At the cellular level, the concentration of several metabolites was altered by the exogenous application of MT that was both directly or indirectly involved in plant tolerance against drought stress in green gram, and the expressed metabolites were involved in the intermediates of different metabolic pathways [99][74] (Figure 2). Xie et al. [100][75] reported that the metabolites involved in the carbon metabolic pathway which includes glycolysis, the oxidative pentose phosphate pathway and the tricarboxylic acid (TCA) cycle, were upregulated by MT and showed a direct link between the carbon metabolic pathway and MT in rice. Proline is one of the compatible solutes that accumulates in plant cells in response to cadmium stress and increases the osmotic adjustment in order to retain membrane integrity. In addition, the experiment found that exogenous application of MT could significantly improve the metabolite group such as amino acids, sugar, and sugar alcohols in tomato plant [91][66] and the compounds were assigned as intermediates for plant metabolic pathways. Sheikhalipour et al. [101][76] showed that increased proline concentration by MT also increases the stabilization of protein structures from denaturation under moisture stress. Saddhe et al. [102][77] described that metabolites like proline and some sugars such as glucose, fructose, sucrose, and trehalose were involved in the regulation of osmotic adjustment under osmotic stress. MT increased the transcription level of various sucrose-related enzymes like sucrose synthase, invertase, phosphatase, and fructokinase and sucrose transporters in plant cells [103][78]. Yang et al. [104][79] explained the importance of MT between MdFRK2 and plant growth and MdFRK2 was found to be involved in the MT-mediated accumulation of sugars like glucose, fructose, and sucrose in apple leaves. Jiang et al. [105][80] found that high levels of metabolite concentration related to amino acids were observed in MT treatment that results in enhanced physiological activities. The primary function of glycolysis in the plant metabolic pathway is to supply energy in the form of ATP and synthesize precursors essential for metabolism of fatty acids and amino acids [106][81]. Zhang et al. [107][82] stated that MT improves the metabolites engaged in carbohydrate and amino acid metabolism and upregulates the glycolysis pathway in plants. MT enhances plants’ tolerance to abiotic stresses through detoxification of ROS and osmotic adjustment by synthesizing and accumulating secondary metabolites such as phenols, ascorbic acid, and carbohydrates such as mannitol and ribose which play a major role in antioxidants and osmolytes [108][83]. Foliar application of MT during drought stress expressed multifaceted metabolites in Carya cathayensis which facilitates the upregulation of biosynthetic pathways such as ABC transporters, porphyrin and chlorophyll metabolism, carotenoid biosynthesis, carbon fixation and metabolism, sugar metabolism, and the phenylpropanoid pathway in MT-treated plants [109][84]. For plants to fight against various environmental stresses, MT regulates the stress signaling pathways through the accumulation of various flavonoids, polyamines, and phenolic compounds in the plant system [110][85].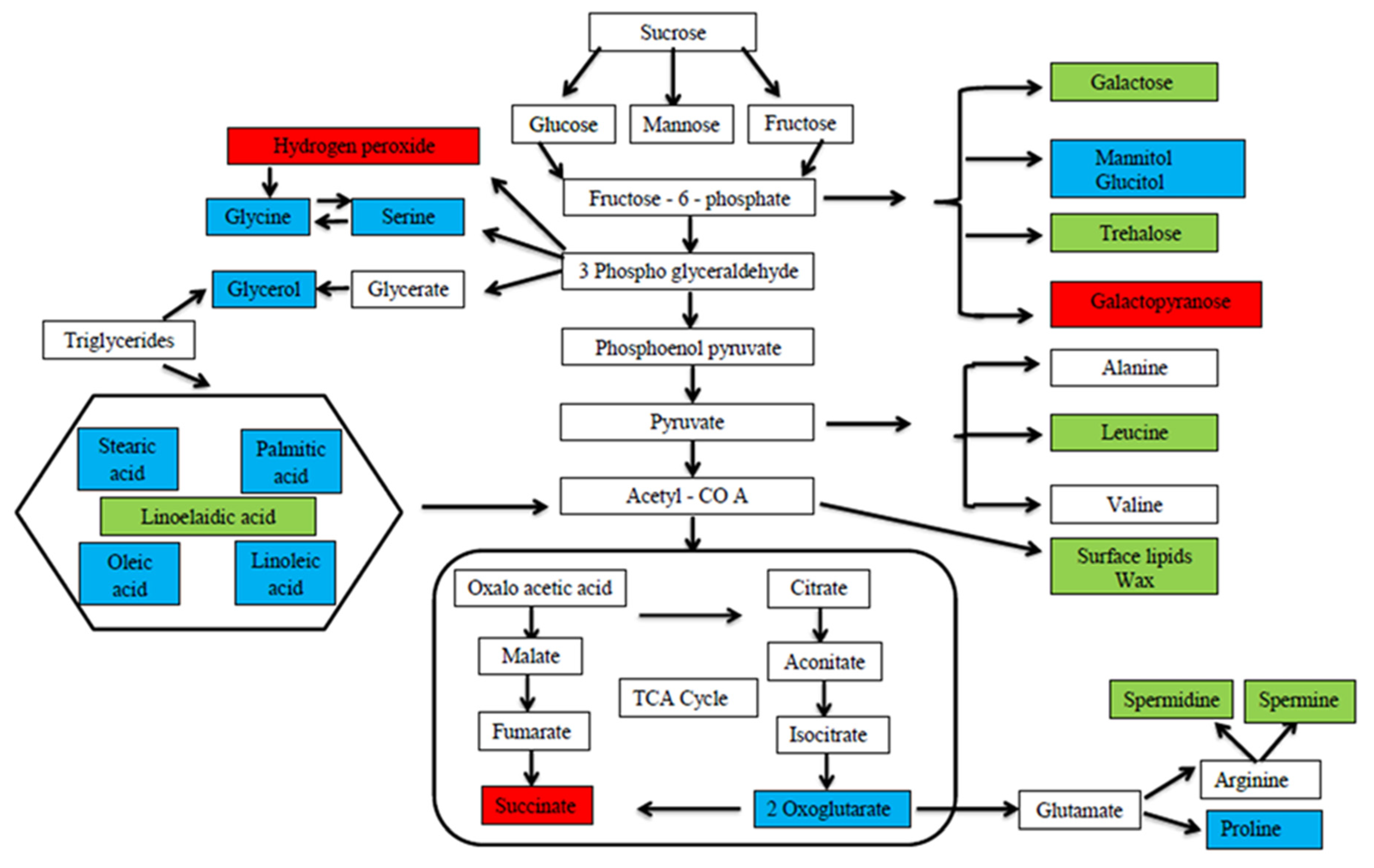
5. Melatonin’s Role in Crop Yield and Quality
MT enhances the growth-related attributes as well as the photosynthetic pigments and thus maximizes the photoassimilate production and translocation efficiency from source to sink tissues and finally the yield [114][89]. Khan et al. [115][90] mentioned that in tomato plant, the number of fruits per plant, fruit yield, and quality characters (ascorbic acid, lycopene content, and β carotene) were increased in MT-treated plants. Hassan et al. [116][91] reported that exogenous MT significantly improves the weight of the bunch, hands per bunches, total weight of hands, and finger length in banana. In addition, Hu et al. [84][59] also stated that increased photosynthetic carbon metabolism and partitioning efficiency in the MT-treated plants enhanced the boll formation and seed yield in cotton. MT regulates a variety of physiological and biochemical processes in plants, thereby improving the net photosynthetic rate and productivity of the crop [117][92]. Medina-Santamarina et al. [118][93] explained that MT showed a positive effect on the improvement of sink strength that ultimately results in improved berry size, weight, and yield of pomegranate. MT enhances the seed filling rate, seed weight, and final yield of maize crop by regulating the hormonal balance [50][33]. Jiang et al. [105][80] also observed that MT delays the early leaf senescence process and improves the photosynthetic efficiency by minimizing the production of ROS, which shows a direct impact on the improvement of quality and yield of rice grains. Liu et al. [67][43] reported that the number of fruits per plant, per fruit weight, and yield per plant were significantly improved in MT-treated cucumber plants. Application of MT showed a positive correlation between photosynthetic rate, antioxidant enzymes, and seed yield in soybean [119][94] and maize [120][95]. Ibrahim et al. [126][96] observed an enhanced fruit quality in tomato due to MT application which improved the antioxidant enzymes, lycopene, ascorbic acid, and total soluble solids. Gurjar et al. [127][97] found that exogenous MT increased the shelf life of fruits and vegetables. Medina-Santamarina et al. [118][93] described that the quality parameters of pomegranate fruits like fruit size, color, total acidity, total soluble solids, fruit number per tree, and fruit yield were improved by the application of MT. Nasser et al. [128][98] observed that the increase in transcriptome alterations during the ripening process in grape berries enhanced the quality of berries due to MT treatment. Under drought stress, foliar application of MT enhanced the yield and quality of Moringa oleifera L. in terms of amino acid composition, glutamic acid, and nutrition such as nitrogen, phosphorus, potassium, calcium, and magnesium [129][99]. In flax, total phenolic content, TSS, proline, and free amino acid contents of the seeds were increased by exogenous MT treatment [130][100]. Farouk and Al-Amri [131][101] reported that the application of MT in rosemary plants improved the essential oil content and yield under stress conditions. Foliar spray of MT in medicinal lemon verbena shrub (Lippia citriodora) enhanced the yield and essential oil content by 52% and 32%, respectively, under stress conditions [132][102].6. Melatonin’s Role in Abiotic Stress Mitigation
Plants experience many adverse situations throughout their lifespan. In order to survive and reproduce successfully in adverse conditions such as drought, salinity, high temperature, flooding, and heavy metal stress, plants have evolved a variety of response mechanisms. MT is a universal compound participating in the nullification of the various abiotic stress responses as a pleiotropic signaling molecule. Furthermore, it is a proficient scavenger of RNS as well as ROS. Numerous research studies have been carried out to investigate the activities of MT in plants since its discovery, indicating its protective properties against abiotic stressors (Table 1). Drought and high temperature stress reduce the permeability of water in the plants [133][103]. Stomata play a vital role in regulation of photosynthesis, transpiration rate, and plant water status in response to abiotic stresses [134][104]. Rao et al. [135][105] opined that ABA acts as a key mediator for the closure of stomata under stress conditions, which ultimately affects a cascade of physiological and molecular processes. Wang et al. [136][106] explained that exogenous MT ameliorates the oxidative stress and improves transpiration rate and stomatal conductance in sweet corn. The increase in transpiration rate and stomatal conductance might be due to the upregulation of the ABA catabolism process and the simultaneous downregulation of ABA anabolism that results in reduced accumulation of the endogenous ABA level; this fact was already reported by Hu et al. [137][107]. The decreased ABA level reduces the production of H2O2 in guard cells of stomata that makes the stomata remain open and maintains the water status of the plant under stress [29][108]. This might be the reason for the increased transpiration rate and stomatal conductance in green gram under water deficit and high temperature stress conditions. Jiang et al. [138][109] reported that MT improves the stomatal conductance by regulating the ROS-mediated stomatal closure that results in a higher transpiration rate in response to stress. Leaf water status and leaf temperature are positively regulated by transpiration rate. The increased transpiration rate by MT enables the plant to maintain lower leaf temperatures, thereby improving photosynthetic efficiency [139][110]. Supriya et al. [140][111] found that an increased stomatal conductance in MT-treated plants regulates the canopy temperature by enhancing the water loss which ultimately results in lower water use efficiency under stress. At the single-leaf level, the water use efficiency is governed by stomatal conductance and transpiration rate [136][106]. The response of water use efficiency is closely linked with physiological processes by regulating the concentration of CO2 and H2O in plant cells [27]. MT maintains better water use efficiency under stress through the control of stomatal movements; therefore, it improves the net photosynthetic rate as reported by Li et al. [141][112]. The positive effects of MT on transpiration rate and stomatal conductance through regulation of the ABA level were also noticed in tomato [142][113], rice [143][114], and barley [144][115].References
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocyteS1. J. Am. Chem. Soc. 1958, 80, 2587.
- Dubbels, R.; Reiter, R.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal Res. 1995, 18, 28–31.
- Van Tassel, D.L.; Roberts, N.; Lewy, A.; O’Neill, S.D. Melatonin in plant organs. J. Pineal Res. 2001, 31, 8–15.
- Hattori, A.; Herbert, D.C.; Vaughan, M.K.; Yaga, K.; Reiter, R. Melatonin inhibits luteinizing hormone releasing hormone (LHRH) induction of LH release from fetal rat pituitary cells. Neurosci. Lett. 1995, 184, 109–112.
- Zohar, R.; Izhaki, I.; Koplovich, A.; Ben-Shlomo, R. Phytomelatonin in the leaves and fruits of wild perennial plants. Phytochem. Lett. 2011, 4, 222–226.
- Murch, S.J.; Erland, L.A. A systematic review of melatonin in plants: An example of evolution of literature. Front. Plant Sci. 2021, 12, 683047.
- Blask, D.E.; Dauchy, R.T.; Sauer, L.A.; Krause, J.A. Melatonin uptake and growth prevention in rat hepatoma 7288CTC in response to dietary melatonin: Melatonin receptor-mediated inhibition of tumor linoleic acid metabolism to the growth signaling molecule 13-hydroxyoctadecadienoic acid and the potential role of phytomelatonin. Carcinogenesis 2004, 25, 951–960.
- Debnath, B.; Islam, W.; Li, M.; Sun, Y.; Lu, X.; Mitra, S.; Hussain, M.; Liu, S.; Qiu, D. Melatonin mediates enhancement of stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 1040.
- Mauriz, J.L.; Collado, P.S.; Veneroso, C.; Reiter, R.J.; González-Gallego, J. A review of the molecular aspects of melatonin’s anti-inflammatory actions: Recent insights and new perspectives. J. Pineal Res. 2013, 54, 1–14.
- Fan, J.; Xie, Y.; Zhang, Z.; Chen, L. Melatonin: A multifunctional factor in plants. Int. J. Mol. Sci. 2018, 19, 1528.
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin synthesis and function: Evolutionary history in animals and plants. Front. Endocrinol. 2019, 10, 249.
- Zhou, Y.; Chen, M.; Guo, J.; Wang, Y.; Min, D.; Jiang, Q.; Ji, H.; Huang, C.; Wei, W.; Xu, H. Overexpression of soybean DREB1 enhances drought stress tolerance of transgenic wheat in the field. J. Exp. Bot. 2020, 71, 1842–1857.
- Ahmad, I.; Song, X.; Hussein Ibrahim, M.E.; Jamal, Y.; Younas, M.U.; Zhu, G.; Zhou, G.; Adam Ali, A.Y. The role of melatonin in plant growth and metabolism, and its interplay with nitric oxide and auxin in plants under different types of abiotic stress. Front. Plant Sci. 2023, 14, 1108507.
- Khan, D.; Cai, N.; Zhu, W.; Li, L.; Guan, M.; Pu, X.; Chen, Q. The role of phytomelatonin receptor 1-mediated signaling in plant growth and stress response. Front. Plant Sci. 2023, 14, 1142753.
- Liu, J.; Shabala, S.; Zhang, J.; Ma, G.; Chen, D.; Shabala, L.; Zeng, F.; Chen, Z.H.; Zhou, M.; Venkataraman, G. Melatonin improves rice salinity stress tolerance by NADPH oxidase-dependent control of the plasma membrane K+ transporters and K+ homeostasis. Plant Cell Environ. 2020, 43, 2591–2605.
- Castañares, J.L.; Bouzo, C.A. Effect of exogenous melatonin on seed germination and seedling growth in melon (Cucumis melo L.) under salt stress. Hortic. Plant J. 2019, 5, 79–87.
- Li, Z.; Su, X.; Chen, Y.; Fan, X.; He, L.; Guo, J.; Wang, Y.; Yang, Q. Melatonin improves drought resistance in maize seedlings by enhancing the antioxidant system and regulating abscisic acid metabolism to maintain stomatal opening under PEG-induced drought. J. Plant Biol. 2021, 64, 299–312.
- Li, C.; Tan, D.-X.; Liang, D.; Chang, C.; Jia, D.; Ma, F. Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J. Exp. Bot. 2015, 66, 669–680.
- Hernández, I.G.; Gomez, F.J.V.; Cerutti, S.; Arana, M.V.; Silva, M.F. Melatonin in Arabidopsis thaliana acts as plant growth regulator at low concentrations and preserves seed viability at high concentrations. Plant Physiol. Biochem. 2015, 94, 191–196.
- Kołodziejczyk, I.; Dzitko, K.; Szewczyk, R.; Posmyk, M.M. Exogenous melatonin improves corn (Zea mays L.) embryo proteome in seeds subjected to chilling stress. J. Plant Physiol. 2016, 193, 47–56.
- Zhang, H.; Qiu, Y.; Ji, Y.; Wu, X.; Xu, X.; Wu, P. Melatonin promotes seed germination via regulation of ABA signaling under low temperature stress in cucumber. J. Plant Growth Regul. 2023, 42, 2232–2245.
- Dradrach, A.; Iqbal, M.; Lewińska, K.; Jędroszka, N.; Rana, M.A.K.; Tanzeem-ul-Haq, H.S. Effects of soil application of chitosan and foliar melatonin on growth, photosynthesis, and heavy metals accumulation in wheat growing on wastewater polluted soil. Sustainability 2022, 14, 8293.
- Hasan, M.K.; Ahammed, G.J.; Sun, S.; Li, M.; Yin, H.; Zhou, J. Melatonin inhibits cadmium translocation and enhances plant tolerance by regulating sulfur uptake and assimilation in Solanum lycopersicum L. J. Agric. Food Chem. 2019, 67, 10563–10576.
- Yin, X.; Bai, Y.L.; Gong, C.; Song, W.; Wu, Y.; Ye, T.; Feng, Y.Q. The phytomelatonin receptor PMTR1 regulates seed development and germination by modulating abscisic acid homeostasis in Arabidopsis thaliana. J. Pineal Res. 2022, 72, e12797.
- Jan, R.; Asif, S.; Asaf, S.; Du, X.-X.; Park, J.-R.; Nari, K.; Bhatta, D.; Lee, I.-j.; Kim, K.-M. Melatonin alleviates arsenic (As) toxicity in rice plants via modulating antioxidant defense system and secondary metabolites and reducing oxidative stress. Environ. Pollut. 2023, 318, 120868.
- Tan, D.-X.; Reiter, R.J. An evolutionary view of melatonin synthesis and metabolism related to its biological functions in plants. J. Exp. Bot. 2020, 71, 4677–4689.
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145.
- Byeon, Y.; Back, K. Melatonin synthesis in rice seedlings in vivo is enhanced at high temperatures and under dark conditions due to increased serotonin N-acetyltransferase and N-acetylserotonin methyltransferase activities. J. Pineal Res. 2014, 56, 189–195.
- Tatar, Ö.; Brück, H.; Asch, F. Atmospheric and soil water deficit induced changes in chemical and hydraulic signals in wheat (Triticum aestivum L.). J. Agron. Crop Sci. 2023, 209, 242–250.
- Liu, G.; Hu, Q.; Zhang, X.; Jiang, J.; Zhang, Y.; Zhang, Z. Melatonin biosynthesis and signal transduction in plants in response to environmental conditions. J. Exp. Bot. 2022, 73, 5818–5827.
- Yang, L.; You, J.; Li, J.; Wang, Y.; Chan, Z. Melatonin promotes Arabidopsis primary root growth in an IAA-dependent manner. J. Exp. Bot. 2021, 72, 5599–5611.
- Zeng, H.; Liu, M.; Wang, X.; Liu, L.; Wu, H.; Chen, X.; Wang, H.; Shen, Q.; Chen, G.; Wang, Y. Seed-soaking with melatonin for the improvement of seed germination, seedling growth, and the antioxidant defense system under flooding stress. Agronomy 2022, 12, 1918.
- Ahmad, S.; Wang, G.-Y.; Muhammad, I.; Chi, Y.-X.; Zeeshan, M.; Nasar, J.; Zhou, X.-B. Interactive effects of melatonin and nitrogen improve drought tolerance of maize seedlings by regulating growth and physiochemical attributes. Antioxidants 2022, 11, 359.
- Duan, W.; Lu, B.; Liu, L.; Meng, Y.; Ma, X.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H. Effects of exogenous melatonin on root physiology, transcriptome and metabolome of cotton seedlings under salt stress. Int. J. Mol. Sci. 2022, 23, 9456.
- Shafi, A.; Singh, A.K.; Zahoor, I. Melatonin: Role in abiotic stress resistance and tolerance. In Plant Growth Regulators: Signalling under Stress Conditions; Springer: Berlin/Heidelberg, Germany, 2021; pp. 239–273.
- Wen, D.; Gong, B.; Sun, S.; Liu, S.; Wang, X.; Wei, M.; Yang, F.; Li, Y.; Shi, Q. Promoting roles of melatonin in adventitious root development of Solanum lycopersicum L. by regulating auxin and nitric oxide signaling. Front. Plant Sci. 2016, 7, 718.
- Morales, F.; Ancín, M.; Fakhet, D.; González-Torralba, J.; Gámez, A.L.; Seminario, A.; Soba, D.; Ben Mariem, S.; Garriga, M.; Aranjuelo, I. Photosynthetic metabolism under stressful growth conditions as a bases for crop breeding and yield improvement. Plants 2020, 9, 88.
- Abdulbaki, A.S.; Alsamadany, H.; Alzahrani, Y.; Olayinka, B.U. Rubisco and abiotic stresses in plants: Current assessment. Turk. J. Bot. 2022, 46, 541–552.
- Ullah, A.; Al-Rajhi, R.S.; Al-Sadi, A.M.; Farooq, M. Wheat genotypes with higher intercellular CO2 concentration, rate of photosynthesis, and antioxidant potential can better tolerate drought stress. J. Soil Sci. Plant Nutr. 2021, 21, 2378–2391.
- Fu, J.; Krishna Jagadish, S.; Bowden, R.L. Effects of post-flowering heat stress on chlorophyll content and yield components of a spring wheat diversity panel. Crop Sci. 2022, 62, 1926–1936.
- Hundare, A.; Joshi, V.; Joshi, N. Salicylic acid attenuates salinity-induced growth inhibition in in vitro raised ginger (Zingiber officinale Roscoe) plantlets by regulating ionic balance and antioxidative system. Plant Stress 2022, 4, 100070.
- Wang, H.; Ren, C.; Cao, L.; Zhao, Q.; Jin, X.; Wang, M.; Zhang, M.; Yu, G.; Zhang, Y. Exogenous melatonin modulates physiological response to nitrogen and improves yield in nitrogen-deficient soybean (Glycine max L. Merr.). Front. Plant Sci. 2022, 13, 865758.
- Liu, K.; Jing, T.; Wang, Y.; Ai, X.; Bi, H. Melatonin delays leaf senescence and improves cucumber yield by modulating chlorophyll degradation and photoinhibition of PSII and PSI. Environ. Exp. Bot. 2022, 200, 104915.
- Ma, X.; Zhang, J.; Burgess, P.; Rossi, S.; Huang, B.J.E.; Botany, E. Interactive effects of melatonin and cytokinin on alleviating drought-induced leaf senescence in creeping bentgrass (Agrostis stolonifera). Environ. Exp. Bot. 2018, 145, 1–11.
- Shi, H.; Wang, X.; Tan, D.X.; Reiter, R.J.; Chan, Z. Comparative physiological and proteomic analyses reveal the actions of melatonin in the reduction of oxidative stress in Bermuda grass (Cynodon dactylon (L). Pers.). J. Pineal Res. 2015, 59, 120–131.
- Gholami, R.; Hoveizeh, N.F.; Zahedi, S.M.; Gholami, H.; Carillo, P. Melatonin alleviates the adverse effects of water stress in adult olive cultivars (Olea europea cv. Sevillana & Roughani) in field condition. Agric. Water Manag. 2022, 269, 107681.
- Yu, J.C.; Lu, J.Z.; Cui, X.Y.; Guo, L.; Wang, Z.J.; Liu, Y.D.; Wang, F.; Qi, M.F.; Liu, Y.F.; Li, T.L. Melatonin mediates reactive oxygen species homeostasis via SlCV to regulate leaf senescence in tomato plants. J. Pineal Res. 2022, 73, e12810.
- Guo, X.; Shi, Y.; Zhu, G.; Zhou, G. Melatonin Mitigated Salinity Stress on Alfalfa by Improving Antioxidant Defense and Osmoregulation. Agronomy 2023, 13, 1727.
- Kuppusamy, A.; Alagarswamy, S.; Karuppusami, K.M.; Maduraimuthu, D.; Natesan, S.; Ramalingam, K.; Muniyappan, U.; Subramanian, M.; Kanagarajan, S. Melatonin Enhances the Photosynthesis and Antioxidant Enzyme Activities of Mung Bean under Drought and High-Temperature Stress Conditions. Plants 2023, 12, 2535.
- Jahan, M.S.; Zhao, C.J.; Shi, L.B.; Liang, X.R.; Jabborova, D.; Nasar, J.; Zhou, X.B. Physiological mechanism of melatonin attenuating to osmotic stress tolerance in soybean seedlings. Front. Plant Sci. 2023, 14, 1193666.
- Jensen, N.B.; Ottosen, C.-O.; Zhou, R. Exogenous Melatonin Alters Stomatal Regulation in Tomato Seedlings Subjected to Combined Heat and Drought Stress through Mechanisms Distinct from ABA Signaling. Plants 2023, 12, 1156.
- Barman, D.; Ghimire, O.; Chinnusamy, V.; Kumar, R.; Arora, A. Amelioration of heat stress during reproductive stage in rice by melatonin. Indian J. Agric. Sci. 2019, 89, 1151–1156.
- Khosravi, S.; Haghighi, M.; Mottaghipisheh, J. Effects of melatonin foliar application on hot pepper growth and stress tolerance. Plant Stress 2023, 9, 100192.
- Garcia-Caparros, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative stress and antioxidant metabolism under adverse environmental conditions: A review. Bot. Rev. 2021, 87, 421–466.
- Liu, L.; Li, D.; Ma, Y.; Shen, H.; Zhao, S.; Wang, Y. Combined application of arbuscular mycorrhizal fungi and exogenous melatonin alleviates drought stress and improves plant growth in tobacco seedlings. J. Plant Growth Regul. 2021, 40, 1074–1087.
- Gu, Q.; Xiao, Q.; Chen, Z.; Han, Y. Crosstalk between melatonin and reactive oxygen species in plant abiotic stress responses: An update. Int. J. Mol. Sci. 2022, 23, 5666.
- Kaur, P.; Singh, D.; Rashid, F.; Kumar, A.; Kaur, H.; Kaur, K.; Singh, A.; Bedi, N.; Bedi, P.M.S.; Singh, B. Role of Melatonin-A Signaling Molecule in Modulation of Antioxidant Defense System in Plants: Amelioration of Drought and Salinity Stress. In Environmental Stress Physiology of Plants and Crop Productivity; Springer: Berlin/Heidelberg, Germany, 2021; Volume 124.
- Zhang, T.; Wang, J.; Sun, Y.; Zhang, L.; Zheng, S. Versatile roles of melatonin in growth and stress tolerance in plants. J. Plant Growth Regul. 2022, 41, 507–523.
- Hu, W.; Zhang, J.; Yan, K.; Zhou, Z.; Zhao, W.; Zhang, X.; Pu, Y.; Yu, R. Beneficial effects of abscisic acid and melatonin in overcoming drought stress in cotton (Gossypium hirsutum L.). Physiol. Plant. 2021, 173, 2041–2054.
- Rehman, R.S.; Hussain, M.; Ali, M.; Zafar, S.A.; Pasha, A.N.; Bashir, H.; Ashraf, N.A.; Javed, A.; Shah, W.A. A Comprehensive Review on Melatonin Compound and Its Functions in Different Fungi and Plants. Int. J. Pathog. Res. 2022, 10, 9–21.
- Manafi, H.; Baninasab, B.; Gholami, M.; Talebi, M.; Khanizadeh, S. Exogenous melatonin alleviates heat-induced oxidative damage in strawberry (Fragaria × ananassa Duch. cv. Ventana) Plant. J. Plant Growth Regul. 2022, 41, 52–64.
- Lei, Y.; He, H.; Raza, A.; Liu, Z.; Xiaoyu, D.; Guijuan, W.; Yan, L.; Yong, C.; Xiling, Z. Exogenous melatonin confers cold tolerance in rapeseed (Brassica napus L.) seedlings by improving antioxidants and genes expression. Plant Signal. Behav. 2022, 17, 2129289.
- Roy, R.; Sultana, S.; Begum, N.; Fornara, D.; Barmon, M.; Zhang, R.; Sarker, T.; Rabbany, M.G. Exogenous melatonin reduces water deficit-induced oxidative stress and improves growth performance of Althaea rosea grown on coal mine spoils. Environ. Sci. Pollut. Res. 2022, 29, 61550–61560.
- Wang, K.; Xing, Q.; Ahammed, G.J.; Zhou, J. Functions and prospects of melatonin in plant growth, yield, and quality. J. Exp. Bot. 2022, 73, 5928–5946.
- Buttar, Z.A.; Wu, S.N.; Arnao, M.B.; Wang, C.; Ullah, I.; Wang, C. Melatonin suppressed the heat stress-induced damage in wheat seedlings by modulating the antioxidant machinery. Plants 2020, 9, 809.
- Umapathi, M.; Kalarani, M.; Srinivasan, S.; Kalaiselvi, P. Alleviation of cadmium phytotoxicity through melatonin modulated physiological functions, antioxidants, and metabolites in tomato (Solanum lycopersicum L.). BioMetals 2022, 35, 1113–1132.
- Lee, J.; Lee, H.; Wi, S.; Yu, I.; Yeo, K.-H.; An, S.; Jang, Y.; Jang, S. Enhancement of growth and antioxidant enzyme activities on kimchi cabbage by melatonin foliar application under high temperature and drought stress conditions. Hortic. Sci. Technol. 2021, 39, 583–592.
- Yu, Y.; Deng, L.; Zhou, L.; Chen, G.; Wang, Y. Exogenous melatonin activates antioxidant systems to increase the ability of rice seeds to germinate under high temperature conditions. Plants 2022, 11, 886.
- Zhao, Q.; Chen, S.; Wang, G.; Du, Y.; Zhang, Z.; Yu, G.; Ren, C.; Zhang, Y.; Du, J. Exogenous melatonin enhances soybean (Glycine max (L.) Merr.) seedling tolerance to saline-alkali stress by regulating antioxidant response and DNA damage repair. Physiol. Plant. 2022, 174, e13731.
- Song, R.; Ritonga, F.N.; Yu, H.; Ding, C.; Zhao, X. Plant melatonin: Regulatory and protective role. Horticulturae 2022, 8, 810.
- Talaat, N.B.; Todorova, D. Antioxidant machinery and glyoxalase system regulation confers salt stress tolerance to wheat (Triticum aestivum L.) plants treated with melatonin and salicylic Acid. J. Soil Sci. Plant Nutr. 2022, 22, 3527–3540.
- Ye, F.; Jiang, M.; Zhang, P.; Liu, L.; Liu, S.; Zhao, C.; Li, X. Exogenous melatonin reprograms the rhizosphere microbial community to modulate the responses of barley to drought stress. Int. J. Mol. Sci. 2022, 23, 9665.
- Yan, R.; Li, S.; Cheng, Y.; Kebbeh, M.; Huan, C.; Zheng, X. Melatonin treatment maintains the quality of cherry tomato by regulating endogenous melatonin and ascorbate-glutathione cycle during room temperature. J. Food Biochem. 2022, 46, e14285.
- Udhayabharathi, M. Physiological and Metabolomic Studies of Drought Tolerance in Greengram (Vigna radiata L.) by Exogenous Melatonin. Master’s Thesis, Tamil Nadu Agricultural University, Coimbatore, India, 2017.
- Xie, Z.; Wang, J.; Wang, W.; Wang, Y.; Xu, J.; Li, Z.; Zhao, X.; Fu, B. Integrated analysis of the transcriptome and metabolome revealed the molecular mechanisms underlying the enhanced salt tolerance of rice due to the application of exogenous melatonin. Front. Plant Sci. 2021, 11, 618680.
- Sheikhalipour, M.; Mohammadi, S.A.; Esmaielpour, B.; Zareei, E.; Kulak, M.; Ali, S.; Nouraein, M.; Bahrami, M.K.; Gohari, G.; Fotopoulos, V. Exogenous melatonin increases salt tolerance in bitter melon by regulating ionic balance, antioxidant system and secondary metabolism-related genes. BMC Plant Biol. 2022, 22, 380.
- Saddhe, A.A.; Manuka, R.; Penna, S. Plant sugars: Homeostasis and transport under abiotic stress in plants. Physiol. Plant. 2021, 171, 739–755.
- Arnao, M.B.; Hernández-Ruiz, J.; Cano, A.; Reiter, R. Melatonin and carbohydrate metabolism in plant cells. Plants 2021, 10, 1917.
- Yang, J.; Zhang, C.; Wang, Z.; Sun, S.; Zhan, R.; Zhao, Y.; Ma, B.; Ma, F.; Li, M. Melatonin-mediated sugar accumulation and growth inhibition in apple plants involves down-regulation of fructokinase 2 expression and activity. Front. Plant Sci. 2019, 10, 150.
- Jiang, Y.; Huang, S.; Ma, L.; Kong, L.; Pan, S.; Tang, X.; Tian, H.; Duan, M.; Mo, Z. Effect of exogenous melatonin application on the grain yield and antioxidant capacity in aromatic rice under combined lead–cadmium stress. Antioxidants 2022, 11, 776.
- Peixoto, B.; Baena-González, E. Management of plant central metabolism by SnRK1 protein kinases. J. Exp. Bot. 2022, 73, 7068–7082.
- Zhang, G.; Yan, Y.; Zeng, X.; Wang, Y.; Zhang, Y. Quantitative proteomics analysis reveals proteins associated with high melatonin content in barley seeds under NaCl-induced salt stress. J. Agric. Food Chem. 2022, 70, 8492–8510.
- Khalid, M.; Rehman, H.M.; Ahmed, N.; Nawaz, S.; Saleem, F.; Ahmad, S.; Uzair, M.; Rana, I.A.; Atif, R.M.; Zaman, Q.U. Using exogenous melatonin, glutathione, proline, and glycine betaine treatments to combat abiotic stresses in crops. Int. J. Mol. Sci. 2022, 23, 12913.
- Sharma, A.; Wang, J.; Xu, D.; Tao, S.; Chong, S.; Yan, D.; Li, Z.; Yuan, H.; Zheng, B. Melatonin regulates the functional components of photosynthesis, antioxidant system, gene expression, and metabolic pathways to induce drought resistance in grafted Carya cathayensis plants. Sci. Total Environ. 2020, 713, 136675.
- Pan, Y.; Xu, X.; Li, L.; Sun, Q.; Wang, Q.; Huang, H.; Tong, Z.; Zhang, J. Melatonin-mediated development and abiotic stress tolerance in plants. Front. Plant Sci. 2023, 14, 1100827.
- Bhavithra, S. Mitigation of Salt Stress in Cassava (Manihot esculenta Crantz) by Exogenous Melatonin. Master’s Thesis, Tamil Nadu Agricultural University, Coimbatore, India, 2021.
- Shah, A.A.; Ahmed, S.; Ali, A.; Yasin, N.A. 2-Hydroxymelatonin mitigates cadmium stress in cucumis sativus seedlings: Modulation of antioxidant enzymes and polyamines. Chemosphere 2020, 243, 125308.
- Vafadar, F.; Amooaghaie, R.; Ehsanzadeh, P.; Ghanadian, M.; Talebi, M.; Ghanati, F. Melatonin and calcium modulate the production of rosmarinic acid, luteolin, and apigenin in Dracocephalum kotschyi under salinity stress. Phytochemistry 2020, 177, 112422.
- Behera, B.; Kancheti, M.; Raza, M.B.; Shiv, A.; Mangal, V.; Rathod, G.; Altaf, M.A.; Kumar, A.; Aftab, T.; Kumar, R. Mechanistic insight on boron-mediated toxicity in plant vis-a-vis its mitigation strategies: A review. Int. J. Phytoremediation 2023, 25, 9–26.
- Khan, T.A.; Saleem, M.; Fariduddin, Q. Melatonin influences stomatal behavior, root morphology, cell viability, photosynthetic responses, fruit yield, and fruit quality of tomato plants exposed to salt stress. J. Plant Growth Regul. 2023, 42, 2408–2432.
- Hassan, I.F.; Gaballah, M.S.; Ogbaga, C.C.; Murad, S.A.; Brysiewicz, A.; Bakr, B.M.; Mira, A.; Alam-Eldein, S.M. Does melatonin improve the yield attributes of field-droughted banana under Egyptian semi-arid conditions? J. Water Land Dev. 2022, 52, 221–231.
- Hu, C.-h.; Zheng, Y.; Tong, C.-l.; Zhang, D.-j. Effects of exogenous melatonin on plant growth, root hormones and photosynthetic characteristics of trifoliate orange subjected to salt stress. Plant Growth Regul. 2022, 97, 551–558.
- Medina-Santamarina, J.; Serrano, M.; Lorente-Mento, J.M.; García-Pastor, M.E.; Zapata, P.J.; Valero, D.; Guillén, F. Melatonin treatment of pomegranate trees increases crop yield and quality parameters at harvest and during storage. Agronomy 2021, 11, 861.
- Oliveira-Spolaor, B.; Chiari-Bertoli, S.; Silva-Sukert, D.; Sala, H.R.; Picoli de Oliveira, B.F.; de Freitas, Í.R.; Lima-Moro, A. Exogenous melatonin induces tolerance to drought stress damage in seedlings and soybean plants. Chil. J. Agric. Res. 2022, 82, 515–526.
- Muhammad, I.; Yang, L.; Ahmad, S.; Mosaad, I.S.; Al-Ghamdi, A.A.; Abbasi, A.M.; Zhou, X.-B. Melatonin application alleviates stress-induced photosynthetic inhibition and oxidative damage by regulating antioxidant defense system of maize: A meta-analysis. Antioxidants 2022, 11, 512.
- Ibrahim, M.F.; Elbar, O.H.A.; Farag, R.; Hikal, M.; El-Kelish, A.; El-Yazied, A.A.; Alkahtani, J.; El-Gawad, H.G.A. Melatonin counteracts drought induced oxidative damage and stimulates growth, productivity and fruit quality properties of tomato plants. Plants 2020, 9, 1276.
- Gurjar, P.; Killadi, B.; Pareek, P.K.; Hada, T. Application of melatonin in maintaining post harvest quality of fruits and vegetables: A review. Agric. Rev. 2022, 43, 193–198.
- Nasser, M.A.; El-Mogy, M.M.; Samaan, M.S.; Hassan, K.M.; El-Sayed, S.M.; Alsubeie, M.S.; Darwish, D.B.E.; Mahmoud, S.F.; Al-Harbi, N.A.; Al-Qahtani, S.M. Postharvest exogenous melatonin treatment of table grape berry enhances quality and maintains bioactive compounds during refrigerated storage. Horticulturae 2022, 8, 860.
- Sadak, M.S.; Abdalla, A.M.; Abd Elhamid, E.M.; Ezzo, M. Role of melatonin in improving growth, yield quantity and quality of Moringa oleifera L. plant under drought stress. Bull. Natl. Res. Cent. 2020, 44, 18.
- Sadak, M.S.; Bakry, B.A. Alleviation of drought stress by melatonin foliar treatment on two flax varieties under sandy soil. Physiol. Mol. Biol. Plants 2020, 26, 907–919.
- Farouk, S.; Al-Amri, S. Ameliorative roles of melatonin and/or zeolite on chromium-induced leaf senescence in marjoram plants by activating antioxidant defense, osmolyte accumulation, and ultrastructural modification. Ind. Crops Prod. 2019, 142, 111823.
- Hosseini, M.S.; Samsampour, D.; Zahedi, S.M.; Zamanian, K.; Rahman, M.M.; Mostofa, M.G.; Tran, L.S.P. Melatonin alleviates drought impact on growth and essential oil yield of lemon verbena by enhancing antioxidant responses, mineral balance, and abscisic acid content. Physiol. Plant. 2021, 172, 1363–1375.
- Prasad, V.R.; Govindaraj, M.; Djanaguiraman, M.; Djalovic, I.; Shailani, A.; Rawat, N.; Singla-Pareek, S.L.; Pareek, A.; Prasad, P.V. Drought and high temperature stress in sorghum: Physiological, genetic, and molecular insights and breeding approaches. Int. J. Mol. Sci. 2021, 22, 9826.
- Peng, P.; Li, R.; Chen, Z.-H.; Wang, Y. Stomata at the crossroad of molecular interaction between biotic and abiotic stress responses in plants. Front. Plant Sci. 2022, 13, 1031891.
- Rao, S.; Tian, Y.; Zhang, C.; Qin, Y.; Liu, M.; Niu, S.; Li, Y.; Chen, J. The JASMONATE ZIM-domain–OPEN STOMATA1 cascade integrates jasmonic acid and abscisic acid signaling to regulate drought tolerance by mediating stomatal closure in poplar. J. Exp. Bot. 2023, 74, 443–457.
- Wang, D.; Wang, J.; Shi, S.; Huang, L.; Zhu, M.; Li, F. Exogenous melatonin ameliorates salinity-induced oxidative stress and improves photosynthetic capacity in sweet corn seedlings. Photosynthetica 2021, 59, 815769.
- Hu, W.; Zhang, J.; Wu, Z.; Loka, D.A.; Zhao, W.; Chen, B.; Wang, Y.; Meng, Y.; Zhou, Z.; Gao, L. Effects of single and combined exogenous application of abscisic acid and melatonin on cotton carbohydrate metabolism and yield under drought stress. Ind. Crops Prod. 2022, 176, 114302.
- Li, H.; Guo, Y.; Lan, Z.; Zhang, Z.; Ahammed, G.J.; Chang, J.; Zhang, Y.; Wei, C.; Zhang, X. Melatonin antagonizes ABA action to promote seed germination by regulating Ca2+ efflux and H2O2 accumulation. Plant Sci. 2021, 303, 110761.
- Jiang, D.; Lu, B.; Liu, L.; Duan, W.; Meng, Y.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H.; et al. Exogenous melatonin improves the salt tolerance of cotton by removing active oxygen and protecting photosynthetic organs. BMC Plant Biol. 2021, 21, 331.
- Teng, Z.; Zheng, W.; Jiang, S.; Hong, S.-B.; Zhu, Z.; Zang, Y. Role of melatonin in promoting plant growth by regulating carbon assimilation and ATP accumulation. Plant Sci. 2022, 319, 111276.
- Supriya, L.; Durgeshwar, P.; Muthamilarasan, M.; Padmaja, G. Melatonin mediated differential regulation of drought tolerance in sensitive and tolerant varieties of upland cotton (Gossypium hirsutum L.). Front. Plant Sci. 2022, 13, 821353.
- Li, Y.; Zhang, L.; Yu, Y.; Zeng, H.; Deng, L.; Zhu, L.; Chen, G.; Wang, Y. Melatonin-induced resilience strategies against the damaging impacts of drought stress in rice. Agronomy 2022, 12, 813.
- Hu, E.; Liu, M.; Zhou, R.; Jiang, F.; Sun, M.; Wen, J.; Zhu, Z.; Wu, Z. Relationship between melatonin and abscisic acid in response to salt stress of tomato. Sci. Hortic. 2021, 285, 110176.
- Li, R.; Yang, R.; Zheng, W.; Wu, L.; Zhang, C.; Zhang, H. Melatonin promotes SGT1-involved signals to ameliorate drought stress adaption in rice. Int. J. Mol. Sci. 2022, 23, 599.
- Yang, X.; Chen, J.; Ma, Y.; Huang, M.; Qiu, T.; Bian, H.; Han, N.; Wang, J. Function, mechanism, and application of plant melatonin: An update with a focus on the cereal crop, barley (Hordeum vulgare L.). Antioxidants 2022, 11, 634.
